Abstract
Filopodia are highly dynamic, rod-like protrusions that are found in abundance at the leading edge of migrating cells such as endothelial tip cells and at axonal growth cones of developing neurons. One proposed function of filopodia is that of an environmental probe, which serves to sense guidance cues during neuronal pathfinding and blood vessel patterning. However, recent studies show that tissue guidance occurs unhindered in the absence of filopodia, suggesting a dispensability of filopodia in this process. Here, we discuss evidence that support as well as dispute the role of filopodia in guiding the formation of stereotypic neuronal and blood vessel patterns.
Keywords: cell guidance, cell migration, endothelial tip cell, filopodia, neuronal pathfinding, vascular patterning
Many biological processes depend on the guided migration of single cells or cell collectives.1 Directed migration is based on gradients of attractive or repulsive molecular cues that are either diffusible or surface bound.2,3 Guidance mechanisms share some characteristic hallmarks that distinguish them from tissue patterns generated by random processes. One of the most striking hallmarks resulting from directed migration is stereotypic tissue patterns such as that observed in neuronal and blood vascular networks. However, the final pattern is highly adapted to function, where neuronal activity fine-tunes synaptic connectivity and blood flow shapes the blood vessel pattern.
Axonal growth cones navigate to their target over large distances with a precision and timing that is highly conserved from animal to animal.3 Modulation of attractive and repulsive gradients leads to erroneous pathfinding with misprojections. For example, Netrin is an axonal guidance cue acting from the neural tube midline that, if presented from an ectopic source, causes axonal growth cone turning toward the ectopic site of presentation within explant cultures.4 Furthermore, its genetic deletion induces misguidance in vivo.5 Although it is difficult to experimentally show protein gradients of diffusible cues, a tissue gradient of Netrin in the developing neural tube has been described.6
The blood vascular network is also highly organized and displays branching patterns that are very similar between animals. This is particularly true for major arteries and veins. Live imaging of intersegmental vessel (ISV) development in the zebrafish embryo reveals that directed migration of endothelial cells lay the foundation of the vascular network (Fig. 1A).7 Ectopic expression of the vascular cue vascular endothelial growth factor, VEGF-A, in muscle cells leads to altered ISV trajectory from one side of the trunk to the other (Fig. 1B), suggesting misguidance of the vessels. This observation suggests that physical barriers provided by tissues are insufficient for steering vessel patterning and that guidance cues may direct endothelial cell migration. Indeed, the VEGF,8-11 Netrin1/Unc5B,11 and Semaphorin/PlexinD112-14 signaling pathways regulate ISV patterning.
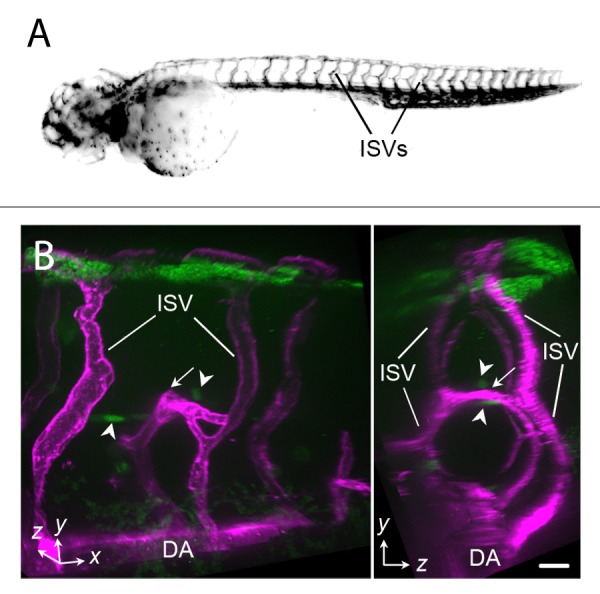
Figure 1. Intersegmental vessels form a highly stereotypic vessel pattern in the zebrafish embryo (A). Misexpression of Vegfa165 in the embryonic trunk leads to misguidance of an ISV (arrows) toward cells expressing ectopic Vegfa165 (green, arrowheads) (B). DA, dorsal aorta; ISV, intersegmental vessel. Scale bars, 20µm. Plasmid encoding Vegfa165 is a gift from Nathan Lawson.
In mouse hindbrain and retina, VEGF-A mRNA production shows a tissue gradient and there is some evidence for graded protein distribution in the hindbrain.15,16 The c-terminal heparin-binding domain of VEGF-A is important for VEGF-A protein distribution such that its deletion results in more diffused VEGF-A distribution, perturbed vessel morphology and mispatterning of the vasculature.15,16 These data suggest that extracellular VEGF-A resembles axonal guidance cues by steering vascular sprouts by means of a protein gradient.17 This concept is further supported by the presence of a gradient of internalized VEGF-A and VEGF-C in the developing retina vasculature, with endothelial cells at the migrating front displaying the highest level of endocytosed VEGF protein.18 Although a gradient of VEGF-A protein has not been demonstrated in the zebrafish embryo, indirect evidence exists. For instance, loss of soluble decoy receptor sFlt1 (VEGFR1) expression in Plexin-D1 mutants leads to ISV misprojections, presumably via the modulation of VEGF-A availability and therefore gradient formation.14 sFlt1 has also been proposed to locally shape the VEGF gradient in the mouse retina and thereby ensures appropriate angles of vascular sprouting.19 In addition, the CXCL12-CXCR4 chemokine signaling pathway plays an essential role in the patterning of arteries and nerve-artery alignment in the skin of the embryonic mouse limb.20 Hence, it seems likely that vascular sprouts, like axonal growth cones, are guided by gradients of diffusible cues. However, the mechanism of how blood vessels integrate signaling cues to form a hierarchical network of vessels is as yet unknown.
Is There a Role for Filopodia in Cell Guidance?
Guided cell migration requires the generation of polarized plasma membrane protrusions such as filopodia, lamellipodia, blebs and invadopodia. Although these protrusions differ structurally and in their composition, they each contribute to cell movement by pushing the leading edge forward. Both axonal growth cones and endothelial tip cells display abundant filopodia protrusions (Fig. 2). Their dynamic behavior of extension and retraction is reminiscent of antennae probing their environment and are generally thought to serve as environmental sensors that integrate extracellular signals during directed migration. Growth cone morphology, reflected for instance by filopodia number, has been correlated with guidance decisions at choice points21-24 and filopodia preferentially contact target cells, sometimes over long distance.23,25 In vivo experiments with titrated doses of an inhibitor of F-actin polymerization, cytochalasin B, blocked growth cone filopodia formation in grasshopper Ti1 neurons and in Xenopus retinal ganglion cell neurons.26,27 While pathfinding was disrupted in conditions without filopodia, only minor effects were seen on axonal extension, suggesting that filopodia are dispensable for growth but essential for guidance. Similarly, in vitro treatment with cytochalasin B abolished glutamate-induced growth cone turning with only slight effects on neurite extension.28 However, a little known study on retinal ganglion cells suggests that axon guidance can occur without filopodia.29 Here, the authors demonstrate that growth cones extend few or no filopodia after inhibiting Ena/VASP function.29 While axonal elongation was slowed down, retinal ganglion cell axons devoid of filopodia showed normal trajectories in vivo and growth cone navigation across several choice points was unaffected. Thus, the role of filopodia in mediating growth cone navigation is unclear.
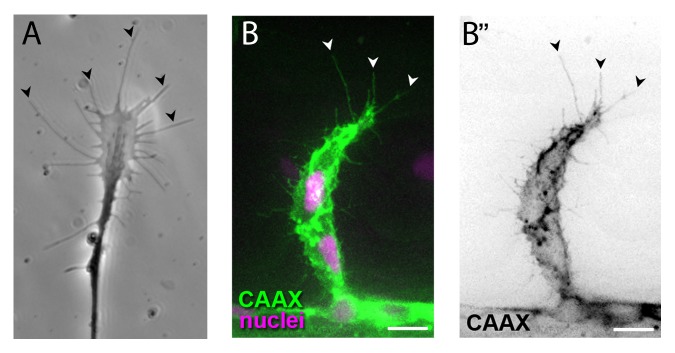
Figure 2. Axonal growth cones (A, image courtesy of Isabelle Brunet) and endothelial tip cells (B) extend long filopodia (arrowheads) in the direction of migration. Scale bars, 10µm.
Similar to growth cones, endothelial tip cells at the leading edge of vascular sprouts produce many long filopodia that extend toward the direction of vascular growth during angiogenesis. These filopodia express the VEGF-A receptor, VEGFR2, and become misdirected and shorter upon disruption of VEGF-A distribution in the mouse retina.16 As filopodia have been proposed to act as sensors of the extracellular milieu and endothelial cells respond to axon guidance molecules such as Slits and Roundabouts, Netrins and Unc5 receptors, Semaphorins, Plexins and Neuropilins, and Ephrins and Eph receptors,30 it has been widely assumed that they sense and integrate pro-angiogenic and repulsive cues in tip cells to enable guided migration and stereotypic vessel patterning. However, the role of filopodia in vessel guidance has never been proven nor questioned.
Recently, work from our laboratory demonstrated that filopodia are not essential for mediating endothelial tip cell guidance.31 By using low concentrations of Latrunculin B (Lat. B), which prevents F-actin polymerization, endothelial filopodia formation was abolished in the zebrafish embryo. Live microscopy revealed that endothelial tip cells of ISVs without filopodia continued to migrate along normal trajectories to form the stereotypic ISV pattern and to anastomose with other tip cells. At the low concentrations of Lat. B used, tip cells were able to generate lamellipodia that provided the driving force for cell movement although at a decreased velocity. Furthermore, the induction of new vascular sprouts toward sources of ectopic Vegfa165 ensued in the absence of filopodia.
Filopodia are Dispensable for Tip Cell Guidance
In summary, our study shows that during angiogenesis, endothelial filopodia are dispensable for tip cell guidance. This finding complements that of Dwivedy et al.,29 who showed that filopodia are also not essential for axonal growth cone navigation and challenges the long-standing notion that filopodia are required for guided migration. In fact, filopodia or filopodia-like structures have been ascribed many other functions. These include facilitating cell-cell matching and epithelial sheet adherence during dorsal closure in Drosophila,32,33 transmitting signals such as Delta-Notch and Sonic Hedgehog signaling between non-neighboring cells,34,35 inducing cell shape changes required for preimplantation embryonic development by providing tension36 and positioning nuclei in nurse cells during oogenesis in Drosophila.37 In endothelial cells, we propose that filopodia serve as templates from which lamellipodia emerge and that both protrusive structures coordinate to allow efficient migration and expansion of new vascular sprouts.31 In addition, tip cell filopodia facilitate the process of anastomosis, a process whereby tip cells meet, fuse and establish new junctions to form a connected vascular network. Thus, the mechanism(s) by which blood vessels are guided by extracellular cues is still unresolved.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 2.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–64. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 3.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 5.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/S0092-8674(00)81795-X. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–74. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–90. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 8.Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci U S A. 2006;103:6554–9. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan BM, Herpers R, Witte M, Heloterä H, Alitalo K, Duckers HJ, Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–9. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- 10.Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, et al. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–20. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Bréant C, Claes F, De Smet F, Thomas J-L, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 12.Childs S, Chen J-N, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–82. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Vázquez J, Gitler AD, Fraser SD, Berk JD, Fishman MC, Childs S, Epstein JA, Weinstein BM, Weinstein BM, Van N Pham Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–23. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting H-G, Affolter M, et al. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell. 2011;21:301–14. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–98. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–6. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HCA, Itoh N, Hirose T, Breier G, Vestweber D, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–60. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–86. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou Y-R, Nagasawa T, Mukouyama YS. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013;24:359–71. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raper JA, Bastiani M, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. I. Divergent choices made by the growth cones of sibling neurons. J Neurosci. 1983;3:20–30. doi: 10.1523/JNEUROSCI.03-01-00020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosney KW, Landmesser LT. Growth cone morphology and trajectory in the lumbosacral region of the chick embryo. J Neurosci. 1985;5:2345–58. doi: 10.1523/JNEUROSCI.05-09-02345.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bovolenta P, Mason C. Growth cone morphology varies with position in the developing mouse visual pathway from retina to first targets. J Neurosci. 1987;7:1447–60. doi: 10.1523/JNEUROSCI.07-05-01447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt CE. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J Neurosci. 1989;9:3123–45. doi: 10.1523/JNEUROSCI.09-09-03123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastiani MJ, Raper JA, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. III. Selective affinity of the G growth cone for the P cells within the A/P fascicle. J Neurosci. 1984;4:2311–28. doi: 10.1523/JNEUROSCI.04-09-02311.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–5. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 27.Chien CB, Rosenthal DE, Harris WA, Holt CE. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11:237–51. doi: 10.1016/0896-6273(93)90181-P. [DOI] [PubMed] [Google Scholar]
- 28.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–9. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134:2137–46. doi: 10.1242/dev.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development. 2013;140:4031–40. doi: 10.1242/dev.097352. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–6. doi: 10.1016/S0960-9822(00)00796-X. [DOI] [PubMed] [Google Scholar]
- 33.Millard TH, Martin P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development. 2008;135:621–6. doi: 10.1242/dev.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen M, Georgiou M, Stevenson NL, Miodownik M, Baum B. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev Cell. 2010;19:78–89. doi: 10.1016/j.devcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–32. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fierro-González JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1424–33. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- 37.Huelsmann S, Ylänne J, Brown NH. Filopodia-like actin cables position nuclei in association with perinuclear actin in Drosophila nurse cells. Dev Cell. 2013;26:604–15. doi: 10.1016/j.devcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


