Abstract
As a consequence of the endosymbiotic gene transfer, most mitochondrial and chloroplastic proteins are nuclear encoded and synthesized in the cytosol as precursor proteins with transit peptides mediating transport to their subcellular destination. It is often assumed that these transit peptides are strictly monospecific for a single organelle. But in recent years more and more proteins have been identified which carry transit peptides that are capable of mediating transport into both mitochondria and chloroplasts. In a recent study we showed with a combination of in silico, in organello, and in vivo approaches that the frequency of such proteins is apparently much higher than usually anticipated.1 Here we demonstrate with in organello competition experiments that the import of 2 of these dually targeted proteins (GrpE and EF-Tu) takes place by the same import pathways that are used by organelle proteins with “typical” monospecific targeting properties.
Keywords: dual targeting, mitochondria, chloroplasts, competition, in organello import, transport pathway
Plant cells harbor 2 types of endosymbiotic organelles: mitochondria and plastids. Their establishment as organelles was accompanied by a massive gene transfer, predominantly from the endosymbiont to the host nucleus.2,3 Consequently, the majority of organelle proteins is nuclear encoded and synthesized in the cytosol as precursor proteins carrying N-terminal transit peptides that mediate transport “back” into the respective organelle. The precursor proteins are guided by cytosolic factors like chaperones to the target organelles and imported by translocases of the outer and inner membranes of mitochondria (TOM/TIM) and chloroplasts (TOC/TIC) (for a review see ref. 4).
The majority of such nuclear encoded organelle proteins is specifically targeted and imported into a single type of organelle. However, a number of precursor proteins show dual targeting, i.e., they are imported into both mitochondria and chloroplasts. The number of proteins that were found to possess dual localization has continually increased. In the past years, approximately 100 organelle proteins with such properties were found (summarized in refs. 5,6). However, the actual number of such dually targeted proteins is probably in the range of several hundred as deduced from both prediction of proteins with dual targeting properties from Physcomitrella patens and a combination of in silico, in organello, and in vivo approaches analyzing the targeting behavior of selected proteins from Arabidopsis thaliana.1,7
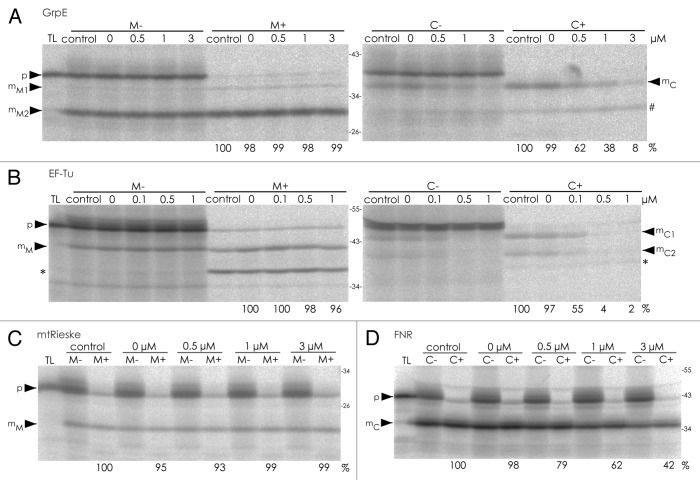
Here, we have analyzed the transport pathways of 2 proteins from Arabidopsis thaliana, notably GrpE (At5g55200) and the elongation factor EF-Tu (At4g02930), that were both recently shown to posses dual targeting properties in transient transformations assays as well as in in organello experiments.1 Our aim was to investigate whether these proteins are imported along the general import pathways into the 2 endosymbiotic organelles or whether they use transport routes that are different from those of the monospecific organelle proteins. To address this question in organello transport experiments were performed in the presence of increasing amounts of competitor proteins. As competitor for chloroplast import we used pre-OEC33 (Fig. 1), the precursor of the 33 kDa subunit of the oxygen evolving system associated with photosystem II, which was shown earlier to be well suited for chloroplast-specific import competition.9 In the presence of increasing amounts of this competitor, the import of both GrpE and EF-Tu into chloroplasts is gradually inhibited (Fig. 1A and B) leading to < 10 % residual transport of GrpE at 3 µM competitor concentration and almost completely abolished transport of EF-Tu in the presence of ≥□0.5 µM competitor. Thus, both proteins show a behavior comparable to that of FNR (ferredoxin-NADP+-oxidoreductase), the chloroplast protein that was analyzed in parallel as control (Fig. 1D). In contrast, protein import of GrpE and EF-Tu as well as of the mitochondrial Rieske protein (mtRieske) into mitochondria (Fig. 1A-C), which was analyzed in parallel, is unaffected under these conditions confirming that the competitive effect of pre-OEC33 is specific for chloroplasts.
Figure 1. In organello protein transport experiments in the presence of increasing amounts of pre-OEC33. Radiolabelled precursor proteins of (A) GrpE, (B) EF-Tu, both from Arabidopsis thaliana, (C) mtRieske from potato, and (D) FNR from spinach, were obtained by coupled in vitro transcription/translation of the corresponding cDNA clones and incubated for 20 min at 25 °C with freshly isolated intact mitochondria (lanes M) or chloroplasts (lanes C) from pea in the presence or absence of competitor protein dissolved in urea.8 The concentration of competitor protein present in each assay (given in µM) is indicated above the lanes. Standard import reactions (lanes control) were performed in the absence of both competitor and urea. After the import reaction, the organelles were recovered by centrifugation and either treated with thermolysin (lanes M+, C+) or mock-treated (lanes M-, C-). Stoichiometric amounts of each fraction corresponding to 50 μg protein (mitochondria) or 12.5 μg chlorophyll (chloroplasts) were separated on 7.5–15 % SDS polyacrylamide gradient gels and visualized by phosphorimaging. Aliquots of the in vitro translation assays (corresponding to 10 % of the protein added to each import reaction) were loaded in lanes TL. The position of precursor proteins (p) and terminal processing products accumulating in mitochondria (mM) and chloroplasts (mC) are indicated by arrowheads. The relative amounts of translocated protein (in terms of percentage of transported protein in the standard reaction) were quantified and are given below the corresponding lanes. In cases with more than a single transport product the main processing band was quantified (for GrpE mM2; for EF-Tu mC1). The hash mark points to a product of approximately 30 kDa, which might correspond to the thermolysin-resistant domain of the GrpE translation product observed earlier (see ref. 1) or which might also be the result for a slight cross contamination of the chloroplast fraction with mitochondria. The asterisk marks a degradation product of approximately 38 kDa, which corresponds to a thermolysin-resistant domain of the EF-Tu translation product (see ref. 1). The size of molecular marker proteins is given in kDa.
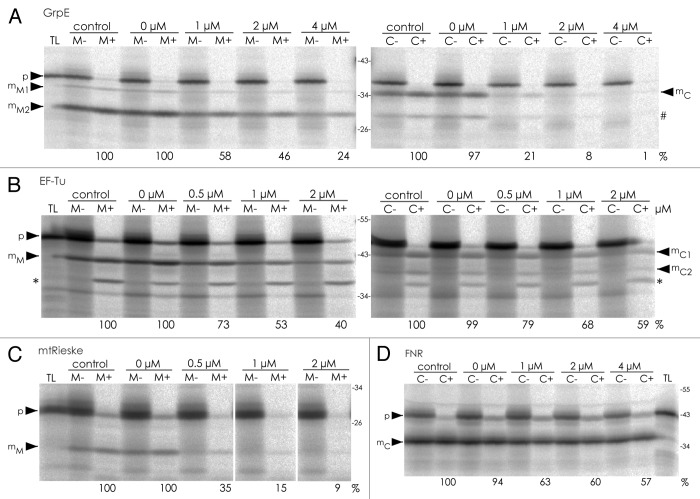
For the corresponding experiments with mitochondria the precursor of cytochrome c1 (pre-Cytc1), which is an integral subunit of complex III of the respiratory electron transport chain, was used as competitor protein (Fig. 2). Pre-Cytc1 was shown to possess dual targeting properties10 and, consequently, both monospecific control proteins, mtRieske and FNR, show reduced import into the respective target organelle in the presence of increasing amounts of pre-Cytc1 (Fig. 2C, D). The competitive effect is stronger for the mitochondrial control (mtRieske) suggesting that pre-Cytc1 possesses higher affinity, and thus more efficient saturation, for mitochondrial receptors than for chloroplast receptors. In case of GrpE and EF-Tu, the import into both endosymbiotic organelles is affected with increasing amounts of pre-Cytc1 (Fig. 2A, B). While both proteins show similar inhibition of their mitochondrial transport (approx. 40–50 % residual import in the presence of 2 µM pre-Cytc1), import into chloroplasts is affected to different degrees: GrpE transport is almost abolished in the presence of 2 µM pre-Cytc1 (< 10 %), whereas the import of EF-Tu shows still 60 % import at this competitor concentration suggesting that the substrates have different affinities for chloroplasts. Nevertheless, the results demonstrate that dually targeted as well as monospecific substrates utilize common components of the import pathways suggesting that they are transported by the same route. These experiments fit well to earlier observations10-12 and reconfirm the conclusion that proteins with dual targeting properties do not use distinct import pathways that were specifically developed for this purpose. This observation can be taken as further hint for the hypothesis that dual targeting is an evolutionary remnant in the development of transit peptides of both endosymbiotic organelles.
Figure 2. In organello protein transport experiments in the presence of increasing amounts of pre-Cytc1. For details, see the legend to Figure 1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Baudisch B, Langner U, Garz I, Klösgen RB. The exception proves the rule? Dual targeting of nuclear-encoded proteins into endosymbiotic organelles. New Phytol. 2014;201:80–90. doi: 10.1111/nph.12482. [DOI] [PubMed] [Google Scholar]
- 2.Martin W, Herrmann RG. Gene transfer from organelles to the nucleus: how much, what happens, and Why? Plant Physiol. 1998;118:9–17. doi: 10.1104/pp.118.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock R, Timmis JN. Reconstructing evolution: gene transfer from plastids to the nucleus. Bioessays. 2008;30:556–66. doi: 10.1002/bies.20761. [DOI] [PubMed] [Google Scholar]
- 4.Schleiff E, Becker T. Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat Rev Mol Cell Biol. 2011;12:48–59. doi: 10.1038/nrm3027. [DOI] [PubMed] [Google Scholar]
- 5.Morgante CV, Rodrigues RA, Marbach PA, Borgonovi CM, Moura DS, Silva-Filho MC. Conservation of dual-targeted proteins in Arabidopsis and rice points to a similar pattern of gene-family evolution. Mol Genet Genomics. 2009;281:525–38. doi: 10.1007/s00438-009-0429-7. [DOI] [PubMed] [Google Scholar]
- 6.Carrie C, Small I. A reevaluation of dual-targeting of proteins to mitochondria and chloroplasts. Biochim Biophys Acta. 2013;1833:253–9. doi: 10.1016/j.bbamcr.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Mitschke J, Fuss J, Blum T, Höglund A, Reski R, Kohlbacher O, Rensing SA. Prediction of dual protein targeting to plant organelles. New Phytol. 2009;183:224–35. doi: 10.1111/j.1469-8137.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 8.Rödiger A, Baudisch B, Klösgen RB. Simultaneous isolation of intact mitochondria and chloroplasts from a single pulping of plant tissue. J Plant Physiol. 2010;167:620–4. doi: 10.1016/j.jplph.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Molik S, Karnauchov I, Weidlich C, Herrmann RG, Klösgen RB. The Rieske Fe/S protein of the cytochrome b6/f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts? J Biol Chem. 2001;276:42761–6. doi: 10.1074/jbc.M106690200. [DOI] [PubMed] [Google Scholar]
- 10.Rödiger A, Baudisch B, Langner U, Klösgen RB. Dual targeting of a mitochondrial protein: the case study of cytochrome c1. Mol Plant. 2011;4:679–87. doi: 10.1093/mp/ssr001. [DOI] [PubMed] [Google Scholar]
- 11.Berglund AK, Spånning E, Biverståhl H, Maddalo G, Tellgren-Roth C, Mäler L, Glaser E. Dual targeting to mitochondria and chloroplasts: characterization of Thr-tRNA synthetase targeting peptide. Mol Plant. 2009;2:1298–309. doi: 10.1093/mp/ssp048. [DOI] [PubMed] [Google Scholar]
- 12.Baudisch B, Klösgen RB. Dual targeting of a processing peptidase into both endosymbiotic organelles mediated by a transport signal of unusual architecture. Mol Plant. 2012;5:494–503. doi: 10.1093/mp/ssr092. [DOI] [PubMed] [Google Scholar]