Abstract
Bacterial wilt phytopathogen Ralstonia solanacearum is a serious soil-borne disease that attacks several economically important plants worldwide, including Brassicaceae. Previous studies indicate that recognition of avirulence (Avr)-effector PopP2 by resistance (R) protein, RRS1-R, and physical interaction between RRS1-R and PopP2 in the nucleus are required for resistance. Of late, we showed that a pair of Arabidopsis thaliana TIR-NLR proteins, RRS1 and RPS4, function together in disease resistance against multiple pathogen isolates. Here, we report that dual R proteins, RRS1 and RPS4, from A. thaliana ecotype Wassilewskija confer resistance to bacterial wilt in transgenic Brassica crops. For practical applications, this finding may provide a new strategy for developing disease resistant plants that express R genes from other plants.
Keywords: R gene, RPS4, RRS1, Ralstonia solanacearum, Brassicaceae
Plant diseases cause serious agricultural losses worldwide. Breeding disease resistant crops help increase the world food supply. Among the various plant disease control strategies, the introgression of disease resistance (R) genes has been used in traditional plant breeding for decades. R proteins mostly carry a C-terminal leucine-rich repeats and a central the nucleotide-binding adaptor shared by Apaf-1, Resistance proteins, and CED-4 domain (NLR).1 R proteins detect avirulence (Avr) proteins secreted by the pathogen and confer resistance to various pathogens. In addition, the hypersensitive reaction mediated by the R protein is the most powerful defense system in plants. Therefore, cloning of R genes from a variety of crops or their wild relatives and transferring them into susceptible cultivars is being attempted.2 However, the transfer of NLR type R genes into crops is limited because the R genes often fail to function, i.e., either no responses or inappropriate auto-immune responses are obtained, when they are transferred between different plant families or even between related species in the same family. For example, a single NLR-type R protein, Mi-1.2, in tomato confers resistance to the following 3 different pathogens: root-knot nematodes, potato aphid, and sweet potato whitefly.3 Goggin et al.3 indicated that Mi-1.2-mediated resistance could not completely function in a heterologous background. Therefore, this suggests that the signaling components in NLR-type R protein-mediated defense system are highly conserved in family or genus.4
Of late, it was reported that some pairs of R genes, such as RPP2A/RPP2B, N/NRG1, RPM1/TAO1, Pikm1-TS/Pikm2-TS, Lr10/RGA2, and Pi5–1/Pi5–2,5-11 may function in concert. Previously, we also demonstrated that a pair of Toll/interleukin-1 receptor (TIR)-NLR genes, RRS1 and RPS4, localize in Arabidopsis thaliana accession Wassilewskija Chromosome V (Fig. 1), and function together in disease resistance against 3 different pathogens: anthracnose (Colletotrichum higginsianum), bacterial speck (Pseudomonas syringae pv. tomato strain DC3000 expressing avrRps4), and bacterial wilt (Ralstonia solanacearum).12,13 Thus, both R proteins, RRS1 and RPS4, are required for effector-triggered immunity in A. thaliana.
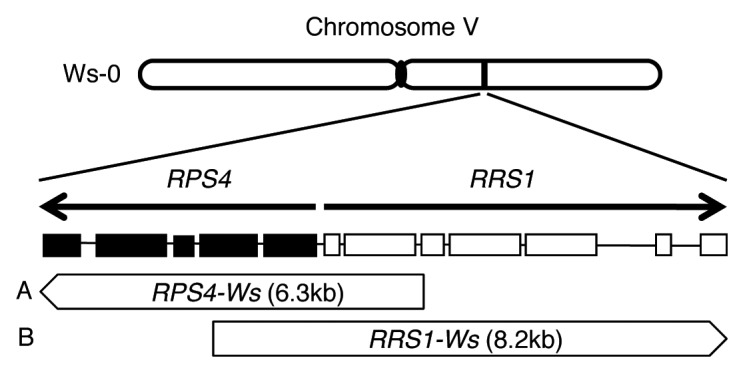
Figure 1. Construction of the R gene plasmid. RPS4 and RRS1 genes are arranged in a head-to-head configuration in Arabidopsis thaliana ecotype Wassilewskija (Ws-0) chromosome V. The genomic fragments containing RPS4 or RRS1 were amplified from Ws-0 genomic DNA using PCR. (A) The 6.3 kbp genomic RPS4 fragment, including approximately 2.1 kbp upstream and 109 bp downstream regions, was cloned into pBI101-SK+.12 (B) The 8.2 kbp genomic RRS1 fragment, including approximately 1.8 kbp upstream and 176 bp downstream regions, was subcloned into the destination vector pGWB1 using the LR cloning reaction.12Brassica rapa var. perviridis (Japanese Mustard Spinach, Komatsuna cv. Osome, Takii and Co. Ltd, Kyoto, Japan) was transformed by inoculating hypocotyl sections with Agrobacterium tumefaciens (Rhizobium radiobactor) strain EHA101, harboring the binary vector with the fragments, i.e., both (A) and (B) (RPS4 + RRS1), either (A) (RPS4) or (B) (RRS1) alone, as described above.26
The bacterial wilt caused by R. solanacearum is an serious soilborne disease affecting important agricultural crops. R. solanacearum infects more than 200 plant species belonging to 50 plant families, including Brassicaceae.14,15 Previous studies have shown that RRS1-R in A. thaliana accession Nd-1 recognized Avr-protein secreted by R. solanacearum and conferred resistance to the pathogen.16,17 Linden et al.18 suggested that RRS1, but not RPS4, in A. thaliana Kil-0 is the key factor of a tolerant phenotype against R. solanacearum BCCF402. However, the bacterial wilt resistance mechanism is poorly understood at the molecular level.
The primary aim of present study was to clarify whether a pair of NLR-type R proteins, RRS1 and RPS4, from A. thaliana confer resistance to bacterial wilt in a heterologous background, i.e., Brassica crops, to facilitate molecular breeding using the Arabidopsis dual R gene. Therefore, we transferred genomic fragments of Arabidopsis RRS1 and RPS4 under the control of their native promoters into Brassica rapa plants, which belong to the same family (Brassicaceae). Furthermore, we also determined whether both RRS1 and RPS4 are essentially required for resistance to bacterial wilt in Brassica crops, i.e., Brassica rapa.
To determine whether Arabidopsis RRS1 and RPS4 function as a pair in Brassicaceae plants other than A. thaliana, we transformed Arabidopsis RRS1 and RPS4 genes into Brassica rapa var. perviridis (Japanese Mustard Spinach, Komatsuna). The transgenes, RRS1 and RPS4, were then segregated in a 1:1 ratio in the T3 generation. We also generated B. rapa transformants that expressed only RRS1 or RPS4 genes. Under the normal condition, these transformants grew normally and did not constitutively induce the expression of defense-related marker gene Br-PR1. This suggests that a inappropriate auto-immune responses was not induced by transformation with both RRS1 and RPS4 genes (Fig. 2).

Figure 2. Growth and expression of defense-related genes (Br-PR1) in transgenic Brassica rapa plants under normal growth condition. (A) Each image shows 25-d-old T3 transgenic B. rapa that carried both RPS4 and RRS1 (R + R), RPS4 alone, RRS1 alone, and wild type control plants (WT). (B) Five leaf disks were cut from the leaves of WT and transgenic B. rapa plants, and total RNA was isolated for qRT-PCR analysis. Expression level of Br-CBP20 in B. rapa was used for normalization.27Br-PR1 expression is shown relative values.27 Bars indicate SE. The data indicate no significant differences compared with the wild type controls (Dunnett’s method, P < 0.05).28 The experiment was repeated twice with similar results.
To analyze whether RRS1 and RPS4 could provide resistance to R. solanacearum strain 1002 (Rs1002), which produces the PopP2 effector that can be recognized by RRS1,16 we performed a root inoculation assay with the pathogen. Wilting was observed 3 to 4 d after the inoculation (dai) in the wild type (WT), followed by complete wilting within 7 to 10 dai (Fig. 3A). In contrast, the transgenic B. rapa plants that expressed RRS1 and RPS4 were healthy after inoculation with the pathogen. The bacterial growth in the WT was approximately 10-fold higher than that in the dual R gene-transformed plants (Fig. 3B). To assess whether RRS1 and RPS4 function in concert, the B. rapa transformants that expressed only RRS1 or RPS4 were inoculated with Rs1002. As shown in Figure 3B, there are no significant difference in bacterial growth between the WT and the single R gene transformants. Therefore, these results show that both RRS1 and RPS4 are required for resistance to Rs1002 and function cooperatively. Rs1002 contains an Avr-gene popP2, which belongs to a YopJ-like family of effectors.16 To determine whether PopP2 plays an important role in the interaction with RRS1, we used an Rs1002-ΔpopP2 where popP2 homolog was disrupted.12 On the root inoculation assay with Rs1002-ΔpopP2, the level of susceptibility in the dual R gene transformed plants was similar to that in the WT (Fig. 3C). These results indicate that PopP2 specifically elicits RRS1/RPS4-mediated resistance in the dual R gene-transformed B. rapa plants.
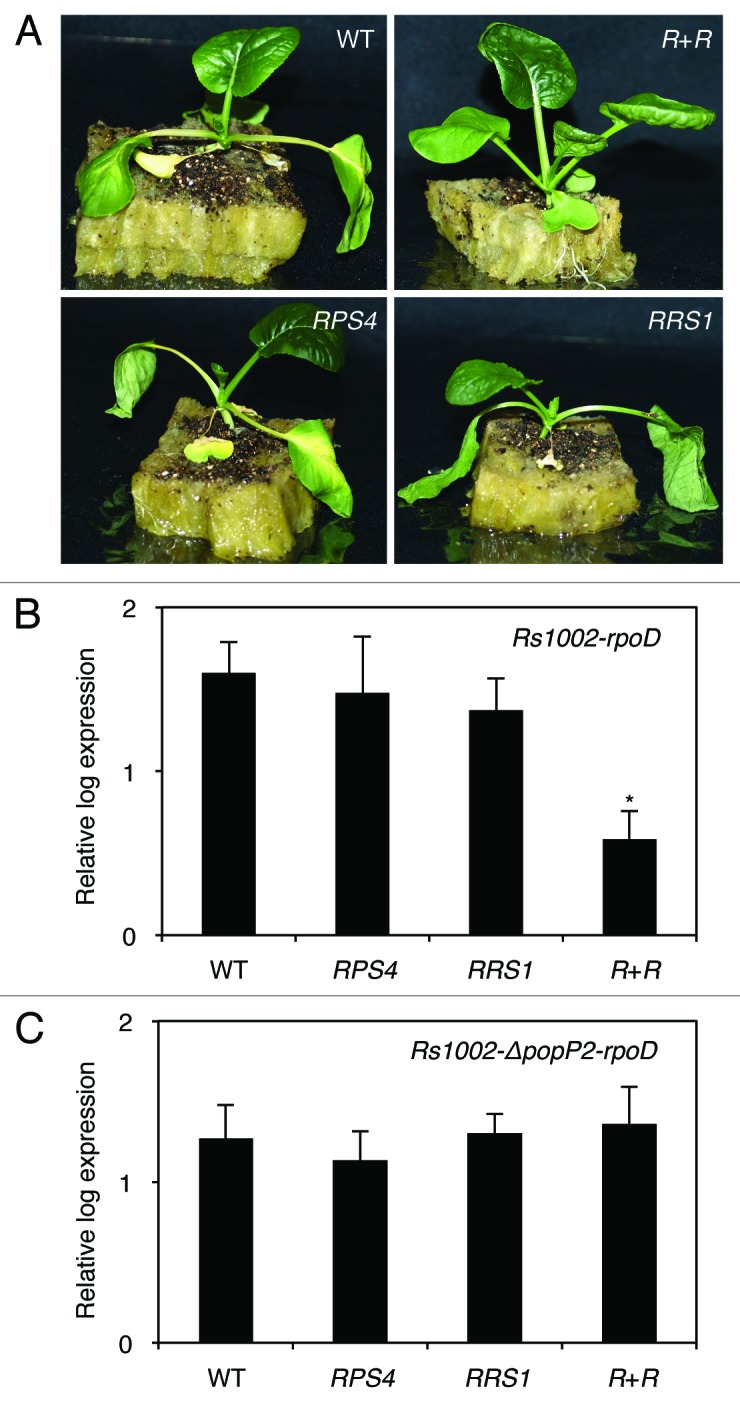
Figure 3.Ralstonia solanacearum resistance analysis in RPS4 and RRS1 dual R gene-transformed Brassica rapa. R. solanacearum strains 1002 (Rs1002) and 1002-ΔpopP2 (Rs1002-ΔpopP2) were described previously.12,29 T3 transgenic B. rapa lines that carried both RPS4 and RRS1 (R + R), either RPS4 or RRS1 alone, and wild type control plants (WT) were tested. B. rapa plants were grown in rock wool for 17 d (12 h light cycle) at 22 °C. Root inoculations were performed by cutting approximately 2 cm from the bottom of the rock wool, and the exposed roots of the plants were immersed in a bacterial suspension at a concentration of 5 × 108 colony-forming units (cfu) ml−1 for 24 h. The plants were then transferred to a growth chamber at 25 °C (12 h light cycle). (A) Infection phenotypes of plants inoculated with Rs1002. Images were taken 4 d after inoculation. Each image shows a representative example from 3 independent experiments. Quantification of Rs1002 (B) and Rs1002-ΔpopP2 (C) in planta was performed using qRT-PCR, as described by Narusaka et al.30 Inoculated plants were harvested at 4 dpi and total RNA was isolated. qRT-PCR data for R. solanacearum rpoD (Rs-rpoD) and Br-CBP20 expression from B. rapa were obtained from a standard curve of cycle times as a function of copy number. Abundance of Rs-rpoD was normalized with Br-CBP20 in infected samples.27,30 Bars indicate standard error (SE). Asterisks indicate significant differences compared with the wild type controls (Dunnett’s method, P < 0.05).28 This experiment was repeated at least twice with similar results.
In plant biology, A. thaliana is the most popular and important model species in the Brassicaceae (mustards or crucifers) family. A. thaliana is an excellent tool for the research into genetics, molecular basis of disease resistance, and molecular breeding of Brassica crops. A. thaliana is also an important model plant for the investigation of plant immune systems.19 The reference Arabidopsis accession Col-0 genome sequence has been used to generate a list of defense-related genes against pathogens and subsequently identified 149 NB-LRR-type R genes.20 If these R genes can function in a heterologous background, they will be useful for breeding disease resistant plants. Despite the discovery of R genes against Brassica crop pathogens, the direct use of Arabidopsis R genes has not progressed in other Brassicaceae plants.21-25
The present study showed that the NLR type R proteins, RRS1 and RPS4, from A. thaliana functioned as a pair in another Brassicaceae species. According to the guard hypothesis, the dual R protein pair, RRS1 and RPS4, may function as a guard–guardee pair where the R protein can interact with signaling components downstream of R protein in a heterologous background. If the functional expression of the R gene pair is required for R gene-mediated resistance in a heterologous background, each partner of the previously reported R genes will soon be discovered and R genes could be exploited in molecular breeding to enhance resistance to crop diseases. This suggests that all the necessary components of R gene-mediated signaling must be highly conserved among plant species. This solution will increase the range of potential resistance resources. For practical applications, this novel finding will provide strategies for development of disease-resistant transgenic plants and novel approaches for plant genome manipulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the science and technology research promotion program for agriculture, forestry, fisheries and food industry to K.S. and Y.N. and by a Grant-in-Aid for Scientific Research (KAKENHI) (25450523 to M.N.). We thank Mses. Yasuyo Katayama, Shoko Miyashita, Yukiko Kurosaki, Chiaki Watanabe, and Masami Miyamoto for their excellent technical assistance, and Tsuyoshi Nakagawa (Shimane University) for kindly providing pGWB1.
References
- 1.McHale L, Tan X, Koehl P, Michelmore RW. Plant NBS-LRR proteins: adaptable guards. Genome Biol. 2006;7:212. doi: 10.1186/gb-2006-7-4-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pink D, Puddephat I. Deployment of disease resistance genes by plant transformation - a ‘mix and match’ approach. Trends Plant Sci. 1999;4:71–5. doi: 10.1016/S1360-1385(98)01372-7. [DOI] [PubMed] [Google Scholar]
- 3.Goggin FL, Jia L, Shah G, Hebert S, Williamson VM, Ullman DE. Heterologous expression of the Mi-1.2 gene from tomato confers resistance against nematodes but not aphids in eggplant. Mol Plant Microbe Interact. 2006;19:383–8. doi: 10.1094/MPMI-19-0383. [DOI] [PubMed] [Google Scholar]
- 4.Brutus A, He SY. Broad-spectrum defense against plant pathogens. Nat Biotechnol. 2010;28:330–1. doi: 10.1038/nbt0410-330. [DOI] [PubMed] [Google Scholar]
- 5.Sinapidou E, Williams K, Nott L, Bahkt S, Tör M, Crute I, Bittner-Eddy P, Beynon J. Two TIR:NB:LRR genes are required to specify resistance to Peronospora parasitica isolate Cala2 in Arabidopsis. Plant J. 2004;38:898–909. doi: 10.1111/j.1365-313X.2004.02099.x. [DOI] [PubMed] [Google Scholar]
- 6.Peart JR, Mestre P, Lu R, Malcuit I, Baulcombe DC. NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr Biol. 2005;15:968–73. doi: 10.1016/j.cub.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 7.Eitas TK, Nimchuk ZL, Dangl JL. Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc Natl Acad Sci U S A. 2008;105:6475–80. doi: 10.1073/pnas.0802157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, Ono K, Yano M. Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics. 2008;180:2267–76. doi: 10.1534/genetics.108.095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loutre C, Wicker T, Travella S, Galli P, Scofield S, Fahima T, Feuillet C, Keller B. Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J. 2009;60:1043–54. doi: 10.1111/j.1365-313X.2009.04024.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Song MY, Seo YS, Kim HK, Ko S, Cao PJ, Suh JP, Yi G, Roh JH, Lee S, et al. Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics. 2009;181:1627–38. doi: 10.1534/genetics.108.099226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eitas TK, Dangl JL. NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Curr Opin Plant Biol. 2010;13:472–7. doi: 10.1016/j.pbi.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narusaka M, Shirasu K, Noutoshi Y, Kubo Y, Shiraishi T, Iwabuchi M, Narusaka Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. 2009;60:218–26. doi: 10.1111/j.1365-313X.2009.03949.x. [DOI] [PubMed] [Google Scholar]
- 13.Narusaka M, Kubo Y, Shiraishi T, Iwabuchi M, Narusaka Y. A dual resistance gene system prevents infection by three distinct pathogens. Plant Signal Behav. 2009;4:954–5. doi: 10.4161/psb.4.10.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward AC. Biology and epidemiology of bacterial wilt caused by pseudomonas solanacearum. Annu Rev Phytopathol. 1991;29:65–87. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- 15.Hayward AC. The hosts of Pseudomonas solanacearum, In: Hayward AC, Hartman GL, eds. Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum Wallingford, United Kingdom: CAB International, 1994: 9-24. [Google Scholar]
- 16.Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, Boucher C, Somssich I, Genin S, Marco Y. Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci U S A. 2003;100:8024–9. doi: 10.1073/pnas.1230660100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasset C, Bernoux M, Jauneau A, Pouzet C, Brière C, Kieffer-Jacquinod S, Rivas S, Marco Y, Deslandes L. Autoacetylation of the Ralstonia solanacearum effector PopP2 targets a lysine residue essential for RRS1-R-mediated immunity in Arabidopsis. PLoS Pathog. 2010;6:e1001202. doi: 10.1371/journal.ppat.1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Linden L, Bredenkamp J, Naidoo S, Fouché-Weich J, Denby KJ, Genin S, Marco Y, Berger DK. Gene-for-gene tolerance to bacterial wilt in Arabidopsis. Mol Plant Microbe Interact. 2013;26:398–406. doi: 10.1094/MPMI-07-12-0188-R. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura MT, Dangl JL. Arabidopsis and the plant immune system. Plant J. 2010;61:1053–66. doi: 10.1111/j.1365-313X.2010.04131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell. 2003;15:809–34. doi: 10.1105/tpc.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science. 1995;269:843–6. doi: 10.1126/science.7638602. [DOI] [PubMed] [Google Scholar]
- 22.Cooley MB, Pathirana S, Wu HJ, Kachroo P, Klessig DF. Members of the Arabidopsis HRT/RPP8 family of resistance genes confer resistance to both viral and oomycete pathogens. Plant Cell. 2000;12:663–76. doi: 10.1105/tpc.12.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao S, Ellwood S, Calis O, Patrick E, Li T, Coleman M, Turner JG. Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science. 2001;291:118–20. doi: 10.1126/science.291.5501.118. [DOI] [PubMed] [Google Scholar]
- 24.Staal J, Kaliff M, Bohman S, Dixelius C. Transgressive segregation reveals two Arabidopsis TIR-NB-LRR resistance genes effective against Leptosphaeria maculans, causal agent of blackleg disease. Plant J. 2006;46:218–30. doi: 10.1111/j.1365-313X.2006.02688.x. [DOI] [PubMed] [Google Scholar]
- 25.Staal J, Kaliff M, Dewaele E, Persson M, Dixelius C. RLM3, a TIR domain encoding gene involved in broad-range immunity of Arabidopsis to necrotrophic fungal pathogens. Plant J. 2008;55:188–200. doi: 10.1111/j.1365-313X.2008.03503.x. [DOI] [PubMed] [Google Scholar]
- 26.Takasaki T, Hatakeyama K, Ojima K, Watanabe M, Toriyama K, Hinata K. Factors influencing Agrobacterium-mediated transformation of Brassica rapa L. Breed Sci. 1997;47:127–34. [Google Scholar]
- 27.Narusaka M, Kubo Y, Hatakeyama K, Imamura J, Ezura H, Nanasato Y, Tabei Y, Takano Y, Shirasu K, Narusaka Y. Interfamily transfer of dual NB-LRR genes confers resistance to multiple pathogens. PLoS One. 2013;8:e55954. doi: 10.1371/journal.pone.0055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–211. doi: 10.1080/01621459.1955.10501294. [DOI] [Google Scholar]
- 29.Mukaihara T, Tamura N, Murata Y, Iwabuchi M. Genetic screening of Hrp type III-related pathogenicity genes controlled by the HrpB transcriptional activator in Ralstonia solanacearum. Mol Microbiol. 2004;54:863–75. doi: 10.1111/j.1365-2958.2004.04328.x. [DOI] [PubMed] [Google Scholar]
- 30.Narusaka M, Shiraishi T, Iwabuchi M, Narusaka Y. rpoD gene expression as an indicator of bacterial pathogens in host plants. J Gen Plant Pathol. 2011;77:75–80. doi: 10.1007/s10327-011-0298-x. [DOI] [Google Scholar]


