Abstract
Involvement of reactive oxygen species in regulation of plant growth and development is recently being demonstrated with various results depending on the experimental system and plant species. Role of superoxide and its metabolism in germination and axis growth was investigated in case of Vigna radiata seeds, a non-endospermous leguminous species having epigeal germination, by studying the effect of different reactive oxygen species (ROS) inhibitors, distribution of O2•ˉ and H2O2 and ROS enzyme profile in axes. Germination percentage and axis growth were determined under treatment with ROS inhibitors and scavengers. Localization of O2•ˉ and H2O2 was done using nitroblue tetrazolium (NBT) and 3,3′,5,5′-tetramethyl benzidine dihydrochloride hydrate (TMB), respectively. Apoplastic level of O2•ˉ was monitored by spectrophotometric analysis of bathing medium of axes. Profiles of NADPH oxidase and superoxide dismutase (SOD) were studied by in-gel assay. Germination was retarded by treatments affecting ROS level except H2O2 scavengers, while axis growth was retarded by all. Superoxide synthesis inhibitor and scavenger prevented H2O2 accumulation in axes in later phase as revealed from TMB staining. Activity of Cu/Zn SOD1 was initially high and declined thereafter. Superoxide being produced in apoplast possibly by NADPH oxidase activity is further metabolized to •OH via H2O2. Germination process depends possibly on •OH production in the axes. Post-germinative axis growth requires O2•ˉ while the differentiating zone of axis (radicle) requires H2O2 for cell wall stiffening.
Keywords: Superoxide, germination, axis growth, Vigna radiata, reactive oxygen species, hydrogen peroxide, hydroxyl radical
Introduction
Independent existence of a plant starts with seed germination, a complex stage of development involving drastic morphogenetic and physiological changes under tight regulation of hormones and other signals. The event of seed germination, in strict sense, is spread between imbibition of seed and the emergence of radicle after rupturing the testa.1 Thus the final phase is closely associated with the initiation of growth of the embryonic axes (Phase I) and continuation of such axis growth beyond emergence may be considered as Phase II seedling growth. This early growth usually results from cell elongation rather than cell division2 in the lower part of the axes3 although the exact zone (radicle, hypocotyl, or the transition zone) where the first sign of growth occurs is much debatable.
Cell elongation growth depends on both turgidity development due to water uptake and cell wall relaxation, the latter being an important process involved in several plant developmental processes. Recently, reactive oxygen species (ROS) have been considered to have an important role in growth and development, apart from their involvement in cellular damages under biotic and abiotic stresses. Among ROS, superoxide (O2•ˉ) and hydroxyl radicals (•OH) are very short-lived molecules that can only act close to the site where they are produced, while hydrogen peroxide (H2O2) can easily diffuse between cells and tissues.4 Thereby, ROS have been attributed to play important physiological roles in plant growth through their mechanism of non-specific cleavage of polymers resulting in wall relaxation5,6 and as signaling molecules.7 Because of relatively longer stability H2O2 can act as signaling molecule that can enter into the cells through aquaporins.8 On the other hand, apoplastic •OH may participate in the scission of cell wall polysaccharides effecting wall loosening and extension growth.6 Apoplastic •OH formation may result from apoplastically produced O2•ˉ and H2O2. Dedicated apoplastic sources of O2•ˉ in plants include plasma membrane NADPH oxidases9 and peroxidases.10,11 It has been reported that O2•ˉ is an agent for root hair development.12 However, the early phase of germination when no root hair development takes place, the presence of O2•ˉ would mean their possible role in growth associated processes. O2•ˉ is one of the main precursor for other ROS such as H2O2 and •OH that may form in the apoplastic space. H2O2 may be produced from O2•ˉ spontaneously or via the action of superoxide dismutase (SOD), whereas •OH may form either through non-enzymatic Fenton reaction or through enzymatic Haber-Weiss process.5,6 Similar ROS metabolism in apoplast has also been implicated for seed germination13,14 which is hormonally and developmentally regulated.14
Present work on the verification and characterization of ROS metabolism of embryonic axes in relation to germination-associated axis growth has been performed with the seeds of Vigna radiata (Leguminosae), which is non-endospermic and non-dormant. Thus, unlike Brassicaceae seeds (Arabidopsis and Lepidium) where radicle emergence occurs following rupture of both testa and endosperm, here germination requires only the rupture of testa. We have already reported about the involvement of ROS and oxidative metabolism in case of germination of Vigna radiata seeds.15,16 However, the exact role of ROS, particularly superoxide and its metabolism in the process of germination and associated growth is not clear. Therefore, attempts have been made to elucidate the mechanism of ROS involvement in seed germination and associated axis growth in case of Vigna radiata.
Results
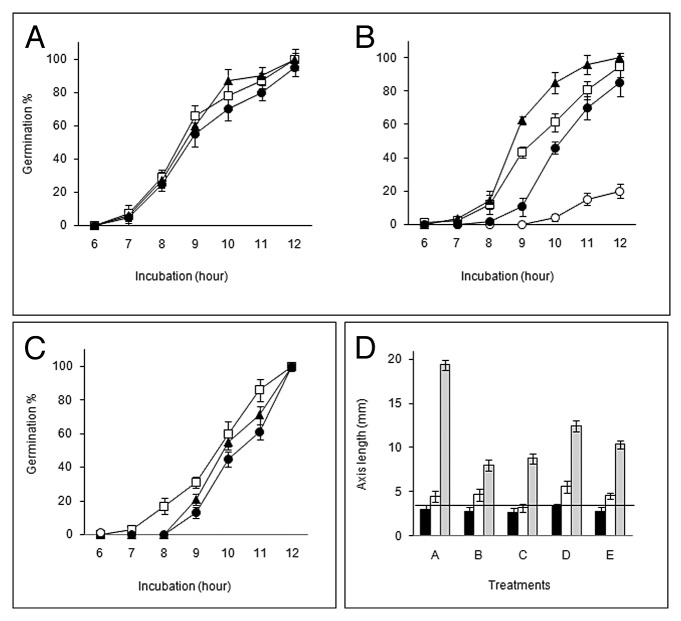
Visible radicle protrusion in V. radiata seeds usually occurs during 8–12 h during incubation at 30 ± 2 °C in darkness. The embryonic axes at this stage protrude out from the seed coat approximately by 2 mm. To elucidate and establish the possible role of ROS in seed germination and axis growth seeds were treated with different ROS scavenging agents or ROS enzyme inhibitors as summarized in Table 1. Treatment of the seeds with NADPH oxidase inhibitor, ZnCl219,20 (10, 50 mM) or O2•ˉ scavengers like CuCl219 (5, 10 mM), Tiron21 (10, 50 mM) and DABCO22 (10, 50 mM) showed differential inhibition of germination which was also dose dependent (Fig. 1A, B). Thus, ZnCl2 was effective to retard germination significantly only at 50 mM concentration, while CuCl2 was most effective in decreasing germination to a minimum even at 10 mM concentration (Fig. 1A). However, other O2•ˉ scavengers (Tiron and DABCO) could slow down germination only at high (50 mM) concentration (Fig. 1B). On the other hand, DPI (Diphenylene iodonium chloride), a potent inhibitor of NADPH oxidase,6,12,17,18 showed marginal inhibition at lower concentration (0.2 mM), but also showed similar inhibition by 0.8% DMSO, the concentration which was required to dissolve DPI of 0.2 mM (Fig. 1C). Further, higher concentration of DPI (0.5 mM), which was dissolved in 2% DMSO, inhibited germination significantly. Again, almost similar inhibition was also showed by 2% DMSO alone up to a certain time during incubation, beyond which germination reached to maximum of the level of control in case of 2% DMSO while germination percentage remained low in DPI (Fig. 1C). Therefore, among superoxide scavengers CuCl2 was found to be most effective in retarding germination while NADPH oxidase inhibitors were only effective at high concentrations.
Table 1. Summary of findings to address the purpose, effects, dose and references of the treatments used for study.
| Treatments | Purpose | Concentration (mM) | Effect(s) | |||
|---|---|---|---|---|---|---|
| DPI | NAD(P)H oxidase inhibitor6,12,17,18 | 0.2 | Retarded axis growth in Phase II only (Fig. 1Dand Table 2) | |||
| Partial inhibition of NBT stain in axis (Fig. 2D) | ||||||
| Could not inhibit TMB stain in axis (Fig. 5E) | ||||||
| 0.5 | Inhibited germination (Fig. 1C) | |||||
| ZnCl2 | NAD(P)H oxidase inhibitor19,20 | 10 | Retarded axis growth in Phase II only (Table 2) | |||
| 50 | Retarded germination (Fig. 1A) | |||||
| Inhibited axis growth in both Phase I and II (Fig. 1D and Table 2) | ||||||
| Inhibited NBT stain in axis (Fig. 2C) | ||||||
| Inhibited TMB stain in axis (Fig. 5D) | ||||||
| CuCl2 | superoxide scavenger19 | 5 | Retarded germination (Fig. 1A) | |||
| Inhibited axis growth in both Phase I and II (Fig. 1Dand Table 2) | ||||||
| Inhibited NBT stain in axis (Fig. 2E) | ||||||
| Inhibited TMB stain in axis (Fig. 5C) | ||||||
| 10 | Inhibited germination (Fig. 1A) | |||||
| Inhibited axis growth almost completely (Fig. 1A, Dand Table 2) | ||||||
| DABCO | superoxide scavenger22 | 10 | Partial inhibition of NBT stain in axis (Fig. 2F) | |||
| 50 | Retarded Germination (Fig. 1B) | |||||
| Inhibited Phase II axis growth (Fig. 1D and Table 2) | ||||||
| KI | hydrogen peroxide scavenger23 | 10 | No significant inhibition or promotion in any observations | |||
| 50 | Marginal retardation of germination (Fig. 4C) | |||||
| Retardation of Phase II axis growth (Fig. 4D and Table 3) | ||||||
| DMTU | hydrogen peroxide scavenger23 | 10 | No significant inhibition or promotion in any observations | |||
| 50 | Marginal retardation of germination (Fig. 4C) | |||||
| Retardation of Phase II axis growth (Fig. 4D and Table 3) | ||||||
| DEDTC | superoxide dismutase inhibitor24 | 10 | Enhanced apoplastic O2•¯ production in axis (Fig. 3B) | |||
| Inhibited Germination (Fig. 4B) | ||||||
| Inhibited axis growth both in Phase I and II (Fig. 4D and Table 3) | ||||||
| Inhibited TMB staining in axis (Fig. 5B) | ||||||
| SHAM | peroxidase inhibitor23 | 5 | Intensified TMB stain in axis (Fig. 5F) | |||
| H2O2 | itself a ROS or ROS generator4,23 | 10 | Promoted germination (Fig. 4B) | |||
| Recovered inhibition of germination caused by DEDTC (Fig. 4B) | ||||||
Figure 1. Germination percentage (A,B, C) and axis growth (D) of Vigna radiata seeds incubated in darkness in a seed germinator under different treatments. (A) Open rectangle, control; open triangle, zinc chloride (10 mM); closed triangle, zinc chloride (50 mM); open circle, copper chloride (5 mM); closed circle, copper chloride (10 mM). (B) Open rectangle, control; open triangle, tiron (10 mM); closed triangle, tiron (50 mM); open circle, DABCO (10 mM); closed circle, DABCO (50 mM). (C) Open rectangle, control; open triangle, diphenylene iodonium (DPI, 0.2 mM) in DMSO (0.8%); closed triangle, DMSO (0.8%); open circle, DPI (0.5 mM) in DMSO (2%); closed circle, DMSO (2%). (D) Black bar, 6 h incubation; white bar, 12 h incubation; gray bar, 24 h incubation; (A), control; (B), DPI (0.2 mM); (C), copper chloride (5 mM); (D), copper chloride (10 mM); (E), zinc chloride (10 mM); (F), zinc chloride (50 mM); (G), tiron (10 mM); (H), tiron (50 mM); (I), DABCO (10 mM); (J), DABCO (50 mM). In all cases data were mean of 3 replicates and ± SE shown as vertical bars. The horizontal line in figure (D) represents an axis length of 3.5 mm when the seeds have attained germination.
Data on growth studies of axes of germinating seeds during incubation under treatments have been presented in Figure 1D. Again CuCl2 (both 5 mM and 10 mM concentrations) was most effective in retarding growth drastically during early (up to 12 h) and late (beyond 12 h) incubation period. NADPH oxidase inhibitors, DPI (0.2 mM) and ZnCl2 (10 and 50 mM) were relatively less effective in retarding growth, though ZnCl2 of particularly higher concentration (50 mM) was more inhibitory than DPI during late phase of growth. Both Tiron and DABCO inhibited axis growth mainly in the late phase at higher concentration (50 mM). In another experiment, effect of the above mentioned inhibitors on axis growth was verified in the phase II (late phase) separately by transferring seeds to these inhibitor solutions after germination in distilled water for 12 h. At the end of 24 h incubation growth data (Table 2) shows similar pattern of growth inhibition by these agents. The severity of the inhibition was observed at higher concentrations of Tiron (50 mM), ZnCl2 (50 mM) and DABCO (50 mM) while CuCl2 at low concentration (5 mM) was sufficient to produce the similar effect. Thus axis growth at later phase was more sensitive to these pharmacological agents.
Table 2. Axis growth of germinating seeds of Vigna radiata incubated in darkness in seed germinator. Seeds were incubated in distilled water for 12 h followed by transfer to the treatments and growth was recorded after 24 h of total incubation. Data were mean of 10 readings ± SE.
| Treatments | Concentration (mM) | Axis growth (% of control) ± SE |
|---|---|---|
| control | 100.00 ± 0 | |
| DPI | 0.2 | 75.70 ± 2.50 |
| CuCl2 | 5.0 | 45.45 ± 4.65 |
| CuCl2 | 10.0 | 33.33 ± 2.30 |
| ZnCl2 | 10.0 | 62.50 ± 4.55 |
| ZnCl2 | 50.0 | 43.75 ± 5.10 |
| Tiron | 10.0 | 82.47 ± 6.22 |
| Tiron | 50.0 | 44.15 ± 4.50 |
| DABCO | 10.0 | 83.88 ± 5.30 |
| DABCO | 50.0 | 37.18 ± 2.34 |
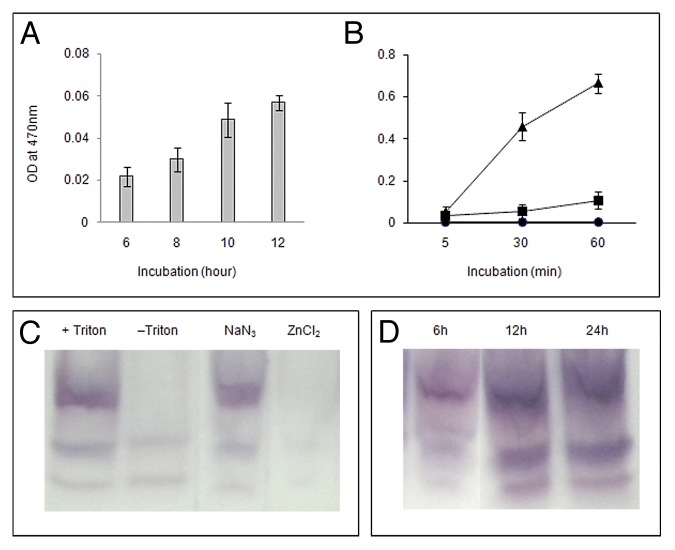
The accumulation of O2•ˉ during germination was localized in the axis by using superoxide-specific stain NBT. Time course study of the localization showed that the increase in growth of the axis during germination is well correlated with gradual intensification of NBT stain due to accumulation of blue formazan after reacting with O2•ˉ (Fig. 2A). The accumulation was effectively inhibited by treatment with ZnCl2 of higher concentration (50 mM) and lower concentration of CuCl2 (5 mM), while DPI treatment (0.2 mM) only partially inhibited such accumulation of stain (Figs. 2B, C, D, E). DABCO (10 mM) and Tiron (10 mM) treatments were least effective in inhibiting NBT staining of axes during germination (Fig. 2F, G). Differential accumulation of superoxide in the axes under treatments correlates with their effect on germination and axis growth. Production of apoplastic O2•ˉ during germination was monitored spectrophotometrically by analyzing the bathing medium of intact axes using XTT. The production of O2•ˉ in the apoplast was in compliance with NBT staining of axes, i.e., O2•ˉ release in the apoplast increases with germination (Fig. 3A). In another experiment it was shown that O2•ˉ accumulation in the bathing medium increased with the time of incubation of axes in the medium containing XTT and such accumulation increased sharply in presence of DEDTC (10 mM), an SOD inhibitor,24 in the bathing medium (Fig. 3B). In-gel assay for NADPH oxidase activity has been performed with the axes of germinating Vigna radiata seeds (Fig. 3C, D). Three NBT-sensitive bands were found in presence of NADPH and the uppermost band corresponding to highest molecular weight enzyme disappeared in absence of Triton X-100. Bands were unaffected by the treatment with NaN3 (1 mM), an inhibitor of peroxidase while ZnCl2 (10 mM) treatment totally abolished these bands (Fig. 3C). Time course study for NADPH oxidase in-gel assay (Fig. 3D) showed gradual intensification of bands corresponding to increased production of O2•ˉ. It appears that NADPH oxidase activity initiates superoxide production in the apoplastic space.
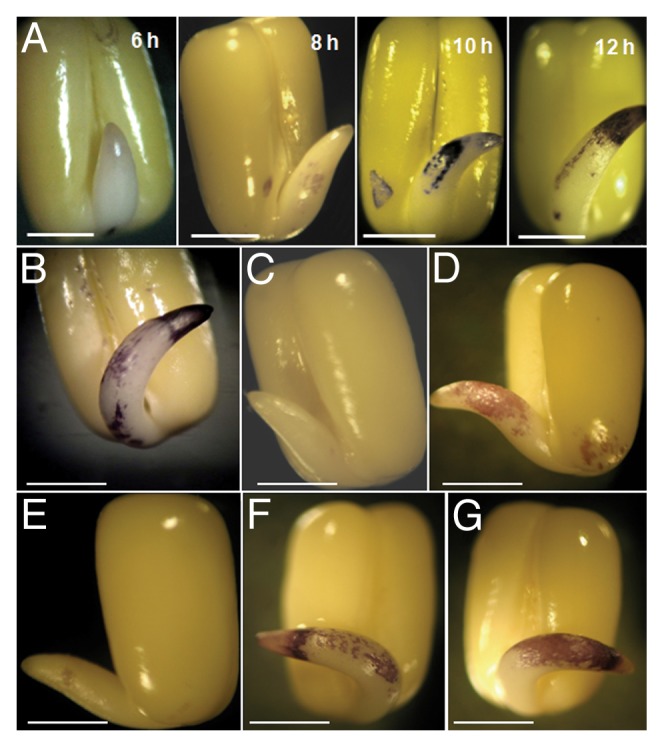
Figure 2. Localization of superoxide using NBT staining in case of Vigna radiata seeds (A) incubated for different durations (6, 8, 10, 12 h) and after 12 h of treatments by different agents [(B), control; (C), zinc chloride (50 mM); (D), diphenylene iodonium (0.2 mM); (E), copper chloride (5 mM); (F), DABCO (10 mM); (G), tiron (10 mM)]. Photographs are representatives of at least 10 replicates. Bars in all cases, 2 mm.
Figure 3. Superoxide production and NADPH oxidase activity (in-gel assay) by axes during germination of Vigna radiata seeds. (A) Superoxide production by axes in the bathing medium assessed by reacting with XTT from seeds incubated for different durations (6, 8, 10, 12 h). (B) Superoxide production by axes (from 12 h incubated seeds) in the bathing medium where the axes were incubated with XTT for different durations (5, 30, 60 min) in presence (closed triangle) or absence (closed rectangle) of DEDTC (10 mM), with a background control (closed circle) without axes in the medium. In all cases data were mean of 3 replicates and ± SE shown as vertical bars. (C) In-gel assay of NADPH oxidase in the axes from 12 h incubated seeds. First lane (from left), extraction with triton X-100 (0.05%); second lane, extraction without triton X-100; third lane, gel assay in presence of sodium azide (1 mM); fourth lane, gel assay in presence of zinc chloride (10 mM). (D) In-gel assay of NADPH oxidase in axes from seeds incubated for different durations (6, 12, 24 h).
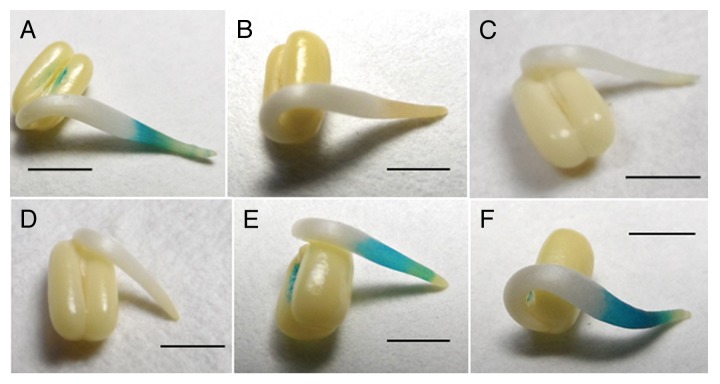
MnCl2 (50 mM), a potent dismutator of O2•ˉ,21 could not inhibit germination even at higher concentration (Fig. 4A). On the other hand, treatment of seeds with DEDTC (10 mM) decreased germination percentage to less than 20% at the end of 12 h incubation (Fig. 4B). Germination percentage improved almost to the level of control when DEDTC was combined with H2O2 (10 mM). However, treatment with KI (50 mM) and DMTU (50 mM), both H2O2 specific scavengers,23 could not inhibit germination (Fig. 4C). Comparative growth kinetics of axes during germination under continuous treatments with these agents was studied to understand the importance of dismutation of O2•ˉ into H2O2 and scavenging of H2O2 (Fig. 4D). Axis growth in the early phase was affected only in case of treatment with DEDTC (10 mM), (also supported by ANOVA where P value < 0.05) but in the late phase it was retarded by on an average of 50% by all these agents (MnCl2, 50 mM; DEDTC, 10 mM; KI, 50 mM and DMTU, 50 mM), MnCl2 being the most effective. Almost similar inhibition of axis growth was noted with these treatments when applied on the seeds germinated in distilled water for 12 h (Table 3). Therefore germination was dependent on H2O2 while continuation of axis growth requires both superoxide as well as H2O2. The accumulation of H2O2 in the growing axes after 18 h of incubation was monitored by TMB staining (Fig. 5). Intense stain due to H2O2 accumulation was observed in the subapical region of the axes (1–2 mm behind the tip) of the germinated seeds. This accumulation was totally inhibited by DEDTC (10 mM). TMB staining of axes was also inhibited by CuCl2 (5 mM) and ZnCl2 (10 mM) whereas DPI (0.2 mM) could not inhibit it. On the contrary, treatment with SHAM (5 mM), an inhibitor of peroxidases,23 rather intensified the stain. Thus superoxide produced by NADPH oxidase is converted to H2O2 that may further metabolized by peroxidases. SOD activity pattern of the germinating seeds was studied using in-gel assay where NBT was used to detect the SOD bands as non-colored zones (Fig. 6). Three bands were detected in the axes of germinating seeds; these correspond to Mn SOD (the uppermost), Cu/Zn SOD1 (middle) and Cu/Zn SOD2 (lowermost). SOD types were characterized by sensitivity to H2O2 and NaN3 (data not shown). Among the bands, width of the band corresponding to Cu/Zn SOD1 was initially thick up to 12 h followed by gradual thinning, whereas other bands were almost unaltered.
Figure 4. Germination percentage (A, B, C) and axis growth (D) of Vigna radiata seeds incubated in darkness in a seed germinator under different treatments. (A) Open rectangle, control; closed triangle, manganese chloride (10 mM); closed circle, manganese chloride (50 mM). (B) Open rectangle, control; open circle, DEDTC (10 mM); closed triangle, hydrogen peroxide (10 mM); closed circle, DEDTC (10 mM) and hydrogen peroxide (10 mM) combined. (C) Open rectangle, control; closed circle, dimethyl thiourea (50 mM); closed triangle, potassium iodide (50 mM). (D) Black bar, 6 h incubation; white bar, 12 h incubation; gray bar, 24 h incubation; (A), control; (B), manganese chloride (50 mM); (C), DEDTC (10 mM); (D), potassium iodide (50 mM); (E), dimethyl thiourea (50 mM). In all cases data were mean of 3 replicates and ± SE shown as vertical bars. The horizontal line in figure (D) represents an axis length of 3.5 mm when the seeds have attained germination.
Table 3. Axis growth of germinating seeds of Vigna radiata incubated in darkness in seed germinator. Seeds were incubated in distilled water for 12 h followed by transfer to the treatments and growth was recorded after 24 h of total incubation. Data were mean of 10 readings ± SE.
| Treatments | Concentration (mM) | Axis growth (% of control) ± SE |
|---|---|---|
| Control | 100.00 ± 0 | |
| KI | 50 | 58.70 ± 7.32 |
| DMTU | 50 | 53.33 ± 3.2 |
| MnCl2 | 50 | 32.30 ± 5.51 |
| DEDTC | 10 | 39.37 ± 4.27 |
Figure 5. Localization of hydrogen peroxide accumulation using TMB in presence of different agents in case of Vigna radiata seeds incubated for 18 h. [(A), control; (B), DEDTC (10 mM); (C), copper chloride (5 mM); (D), zinc chloride (10 mM); (E), diphenylene iodonium (0.2 mM); (F), SHAM (5 mM). Photographs are representatives of at least 10 replicates. Bars in all cases, 5 mm.
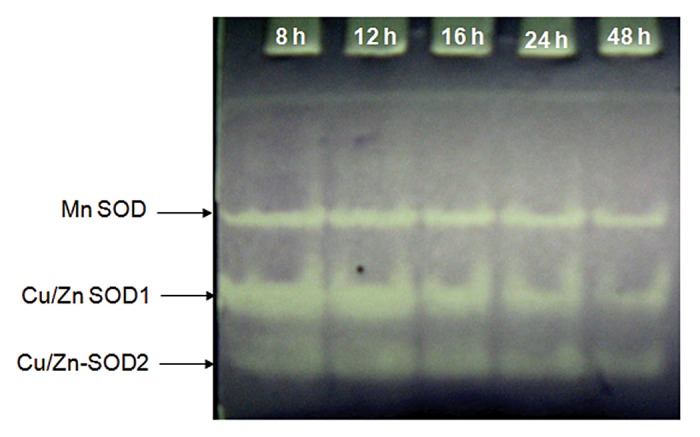
Figure 6. In-gel assay of superoxide dismutase (SOD) activity in the axes from Vigna radiata seeds incubated for different hours (8, 12, 16, 24, 48 h).
Discussion
The process of seed germination begins with the hydration of the embryonic axes and surrounding tissues (cotyledons in this case) and ends with the initiation of extension growth of axes which is revealed by radicle emergence. Thus the seed germination capacity depends on the ability of resuming the growth potential of the axes and weakening of the surrounding tissue, if any. In case of endospermic seeds, like Arabidopsis, germination requires both increase in growth potential of the embryo and weakening of endospermic cap.14,25 However, seeds of Vigna radiata lack any endosperm at maturity, as a result of which germination occurs quickly (around 8–10 h) possibly in absence of such constraint. Moreover, being a leguminous seed usually having hard seed coat it imbibes very slowly for initial 4 h because of seed coat impermeability followed by rapid uptake.26 Thus it appears that in case of V. radiata seeds, axes develop growth potential required for radicle emergence within few hours.
Seed germination-associated growth of axes is a result of cell extension rather than cell division3 and such growth continues during seedling growth. Extension growth of plant cells depends to a great extent on the cell wall loosening, a process induced by cell wall remodeling proteins (expansions) and apoplastic ROS generation.14,17,27 Accordingly we have tested pharmacologically the effects of different ROS scavengers or inhibitors on seed germination and growth of axes at different stages. Among the superoxide scavengers, CuCl2 was most effective in inhibiting germination while Tiron and DABCO had little effect (Figs. 1A, B). This was also revealed from their effect during growth of the axes when treated from the beginning (Fig. 1D). The early spell of axis growth (usually up to 12 h), which is corresponding to radicle emergence (about 3.5 mm considered since out of this length about 2 mm comes out through ruptured testa), showed a similar inhibition, i.e., more pronounced in case of CuCl2 compared with Tiron and DABCO. Same was true for growth of axes beyond 12 h incubation and higher concentration of Tiron and DABCO retarded growth (Table 2). Leymarie et al.13 also showed Tiron as not much effective in retarding germination of non-dormant Arabidopsis seeds. On the other hand, treatment with DPI and ZnCl2, inhibitors of NADPH oxidase activity were also not much effective in preventing germination and retarding axis growth unlike observations of others.12,13,23 An inhibition of germination particularly at higher concentration (0.5 mM) of DPI was partially due to the solvent DMSO (2%) used to dissolve DPI (Fig. 1C). Indeed, Demidchik et al.28 explained inhibition of root elongation in Arabidopsis by DMSO (0.1%) as due to •OH scavenging. ZnCl2 was also effective in retarding germination significantly only at high concentration (50 mM). It is possible that either the superoxide synthesizing enzyme (likely to be NADPH oxidase) was not accessible to these chemicals (DPI and ZnCl2) during this early stage or the enzyme itself was not much sensitive to these agents. However, as regard to the retardation of subsequent axis growth both DPI and ZnCl2 were more or less effective (Fig. 1D, Table 2). The overall observation support the view that superoxide generation is essential for seed germination and associated growth as demonstrated in several species.11,29,30 A direct evidence for involvement of superoxide in seed germination is the histolocalization of superoxide accumulation in growing region of axes by NBT staining (Fig. 2A) what we have also observed earlier.31 Thus superoxide accumulated predominantly in the elongating regions and on the convex side of the curved axes (in response to gravity) which took intense stain earlier around 8–10 h. Such asymmetric accumulation of superoxide by germinating axes was also observed in case of Arabidopsis.14 Following curvature growth axes started growing by radicle tip that also corresponded with superoxide accumulation as revealed from NBT staining gradually intensified at about 12 h (Fig. 2A). Moreover, localization of this stain on the surface is indicative of apoplastic production of superoxide which is related to the putative role of ROS in cell wall loosening associated with growth.13 Treatment of seeds with CuCl2 (Fig. 2E) totally prevented while Tiron and DABCO (Fig. 2F, G) partially reduced such staining of axes indicating that the latter compounds could not scavenge superoxide well. Interestingly, DPI (Fig. 2D) also could not inhibit totally superoxide accumulation in axes while treatment with ZnCl2 (Fig. 2C) at high concentration only resulted in complete inhibition of superoxide accumulation. It appears that superoxide generating system, possibly NAD(P)H oxidase, is not much sensitive to DPI and ZnCl2 during germination and associated axis growth as mentioned previously. Apoplastic production of superoxide can be proved again by measuring superoxide level in the bathing medium of axes from germinating seeds by XTT assay (Fig. 3A) correlating with increased NBT staining with time of incubation of mung bean seeds for germination. However, such superoxide is under rapid turnover, since inhibition of SOD (dismutating superoxide into H2O2) by DEDTC resulted in dramatic accumulation of superoxide in the bathing medium (Fig. 3B).
Further characterization of NADPH oxidase (NOX), supposed to be involved in superoxide generation in germinating axes, by in-gel assay showed 3 NBT-sensitive bands (also observed earlier,31) of which the uppermost band was probably membrane bound, since it did not appear in absence of triton X-100. These bands were Zn2+ -sensitive but not sodium azide-sensitive indicating that these belong to NOX family (Fig. 3C). Time-course study revealed that membrane-bound NOX activity (corresponding to the upper most band) as well as the activity of the NOXs corresponding to the other bands increased in growing axes during seed germination (Fig. 3D).
Next we looked for further metabolism of superoxide, since it is reported that superoxide is converted to H2O2 and subsequently to •OH in the apoplast of elongating cells.6,18 Apparently, the role of H2O2 in germination of mung bean seeds is questionable, as treatment with KI and DMTU, recognized scavenger of H2O2, could not inhibit germination (Fig. 4C). On the contrary, generation of H2O2 appears to be important for germination as revealed from significant inhibition of germination by DEDTC, potent inhibitor of SOD, which is likely to be involved in rapid dismutation of O2•ˉ into H2O2. This view is further strengthened when exogenous treatment with H2O2 simultaneously with DEDTC recovered germination to a great extent (Fig. 4B). MnCl2, a superoxide dismutating agent, also did not inhibit germination (Fig. 4A). That DEDTC effectively inhibited the O2•ˉ dismutation process in the apoplast of axis tissue of germinating seeds was indicated by increased accumulation of XTT-derived soluble formazan in the bathing medium of axes in presence of DEDTC (Fig. 3B). Interestingly, axis growth was retarded to different extent by scavenging (KI or DMTU treatment) as well as by inhibiting generation of H2O2 (DEDTC treatment) either from the beginning or after radicle emergence (Fig. 4D and Table 3, respectively) suggesting a definite role of H2O2 in the process of axis growth. Surprisingly MnCl2 also retarded axis growth significantly pointing probably to the role of superoxide in axis growth as well.
As the generation of H2O2 appears to be essential for germination and axis growth, the activity of SODs in the axes of Vigna radiata was followed. Based on metal requirement, different SODs are present in the different cellular compartments. In case of germinating mung bean seeds, Mn SOD and Cu/Zn SOD activity was detected while Fe SOD was absent (Fig. 6), as was also observed in lupine seeds,32 while only Mn SOD was reported in case of germinating seeds of Chenopodium murale.33 Besides cytosol, Cu/Zn SOD is also localized in cell wall34 and may play an important role in ROS metabolism in connection with cell elongation process. Here increasing activity of one of the isoforms of Cu/Zn SOD (Cu/Zn SOD1) during germination (up to 12 h) may be ascribed for initiation of axis growth requiring a supply of H2O2 in the apoplastic space. Interestingly, there was no corresponding accumulation of H2O2 in the axes in this phase, as revealed from TMB staining of axes (data not shown). This may be due to rapid conversion of H2O2 further to •OH radical that plays a direct role in cell wall loosening associated with axis growth.6 However, H2O2 accumulated later (18 h incubation) during post-germinative axis growth in the differentiation zone, little behind the elongation zone (Fig. 5A), which may be associated with cell wall stiffening due to increased cross-linking of polymers in the differentiating cells.35 Such accumulation of H2O2 was totally relied upon SOD activity as it was inhibited by DEDTC treatment (Fig. 5B). On the other hand, in the elongating zone of axes peroxidase remained active possibly in producing •OH from H2O2, as the axes showed TMB stain extended up to this zone when peroxidase was inhibited by SHAM treatment (Fig. 5F). Even DPI treatment also gave the same result, i.e., could not affect TMB staining similarly (Fig. 5E) that may be explained by less inhibitory effect of DPI on O2•ˉ producing activity (as mentioned earlier) and also probable inhibition of peroxidase by DPI.36 Moreover, the ultimate source of this H2O2 was O2•ˉ, as suggested by the observation that treatment with CuCl2 and ZnCl2 blocked accumulation (Fig. 5C, D).
In summary, the present investigation on seed germination and early axis growth of Vigna radiata reveals the involvement of ROS in the growth process associated with germination and post-germination stage. Superoxide produced by plasma membrane-bound NADPH oxidase activity in the apoplastic space is readily metabolized further to H2O2 and subsequently to •OH radical by the activity of Cu/Zn SOD and peroxidases, respectively leading to cell extension through cell wall relaxation. But the relative importance of each of these ROS molecules varies with stage of germination and zone of axes. Thus during germination proper (phase I) when the axis is not differentiated much, axis growth is brought about by rapid generation and subsequent conversion of O2•ˉ through •OH required for cell wall extension. However, post-germinative axis growth requires O2•ˉ in addition to other ROS while the differentiating zone of axis (radicle) requires H2O2 for cell wall stiffening. Such preferential requirement of particular ROS molecules for axis growth is possibly fulfilled by intricate regulation of ROS metabolism in the apoplast.
Materials and Methods
Plant material and experimental set up
Surface-sterilized seeds of mung bean [Vigna radiata (L.) Wilczek var B1] were incubated for germination in petri dishes on Whatman no.1 filter paper moistened with 3 ml of distilled water or test solutions under controlled temperature (30 ± 2 °C) and darkness in a seed germinator.
Pharmacological experiments
Germination study of seeds under different treatments was performed in replicates of 3 sets each containing 50 seeds. Number of seeds germinated was counted at hourly intervals right from 6 h of incubation up to 12 h (unless otherwise mentioned). The average of percentages from the 3 replicates in each case was then presented as percentage of seeds germinated.
Growth of the axis in continuous treatments was measured at 6, 12 and 24 h of incubation. To determine Phase II growth of the axis separately, 12 h germinated seeds (in distilled water) were transferred to different sets of petri dishes and soaked with test solutions for 24 h. The result was presented as an average of readings from 15 seeds.
Localization of O2•ˉ
The accumulation of O2•ˉ was detected using 0.5 mM NBT (nitro blue tetrazolium chloride) in 50 mM phosphate buffer, pH 6.8 as modified from Liszkay et al.19 Seeds, both treated and control were kept in the staining solution for 25 min at 30 °C. The reaction was terminated by keeping the seeds in distilled water and photographs of the representative seeds corresponding to respective treatments were taken under a stereo zoom microscope (Hund, Germany).
Localization of H2O2
H2O2 production in vivo was detected by staining with 1 mM TMB (3,3′,5,5′-Tetramethyl benzidine dihydrochloride hydrate) in 10 mM potassium-citrate buffer, pH 4.0.19 Seeds germinated for 18 h were incubated in the staining solution with or without treatments for 60 min.
Spectrophotometric analysis of apoplastic O2•ˉ
Determination of apoplastic O2•ˉ level was done following the method of Able et al.37 Intact axes were incubated in the medium containing 0.5 mL each of phosphate buffer (50 mM, pH 6.8) or buffer containing inhibitor and 1 mM XTT [2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt]. After 5, 30 and 60 min of incubation under shaking, OD of the bathing medium was measured at 470nm. To avoid anomalies caused by O2•ˉ produced by injury during excision of embryo, an initial reading was taken after 5 min of incubation for each sets. O2•ˉ production in the germinating axes was also monitored with increasing incubation period of the axes in the XTT containing medium.
In-gel native PAGE assay
For determination of NADPH oxidase activity, axes of germinated seeds (6, 8 and 12 h germinated seeds for time course study and 12 h germinated seeds for in-gel treatments) were homogenized in pre-chilled 50 mM phosphate buffer, pH 6.8 with 0.05% Triton X-100 (unless mentioned otherwise). The enzyme extract was then centrifuged at 9000 g for 15 min at 4 °C and the supernatant was collected for determination of enzyme activity. Protein content of the sample was quantified using Bradford reagent. Accordingly protein samples (corresponding to 50 μg protein) from respective extracts were loaded with loading buffer and run in a vertical native PAGE at 4 °C.
The activity of NADPH oxidase enzyme was detected by immersing the gel in O2•ˉ specific NBT dye using NADPH as substrate. For in-gel treatments the gel slabs were pre-incubated for 30 min in the respective test solutions followed by NBT staining.
For determination of Superoxide dismutase activity, axes tissue from seeds germinated for different periods (8, 12, 16, 24, 48 h) was homogenized in Tris buffer (0.15 M, pH 7.5) under chilling condition.38 The samples were centrifuged at 9000 g at 4 °C for 10 min and the supernatants were used as sample for in gel assay of SOD. Protein content of the sample was quantified using Bradford reagent. Accordingly protein samples (corresponding to 100 μg protein) from respective extracts were loaded with loading buffer and run in a vertical native PAGE at 4 °C.
The activity of SOD enzyme was detected by a modified photochemical method of Beauchamp and Fridovich.39 The gel was first soaked in 25 ml of NBT (1.23 mM) in darkness for 15 min, briefly washed, then soaked in the dark in 30ml of potassium phosphate buffer (100 mM, pH 7.0) containing TEMED (28 mM) and riboflavin (2.8 x10−2 mM) for another 15 min in darkness. The gel was briefly washed again and then illuminated on a light box with a light intensity of 30 μEm−2s−1 for 15 min to initiate the photochemical reaction. All the procedures were performed at 25 °C and the gel was shaken gently in 2 soaking steps at regular intervals.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
Authors acknowledge the funding for the present investigation from research grants by University Grants Commission, New Delhi under the scheme of major research project [No. 32–406/2006(SR) and No. 39–375/2010 (SR)].
Glossary
Abbreviations:
- DABCO
1,4-diazabicyclo[2,2,2]octane
- DEDTC
diethyl dithiocarbamate
- DMSO
dimethyl sulphoxide
- DMTU
dimethyl thiourea
- DPI
diphenylene iodonium chloride
- NBT
nitroblue tetrazolium chloride
- ROS
reactive oxygen species
- SHAM
salicyl hydroxamic acid
- SOD
superoxide dismutase
- TMB
3,3′,5,5′-tetramethyl benzidine dihydrochloride hydrate
- XTT
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt
References
- 1.BewleyJD, BlackM. Seeds: Physiology of Development and Germination, 2nd Edition. New York: Plenum Press, 1994. [Google Scholar]
- 2.Weitbrecht K, Müller K, Leubner-Metzger G. First off the mark: early seed germination. J Exp Bot. 2011;62:3289–309. doi: 10.1093/jxb/err030. [DOI] [PubMed] [Google Scholar]
- 3.Sliwinska E, Bassel GW, Bewley JD. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J Exp Bot. 2009;60:3587–94. doi: 10.1093/jxb/erp203. [DOI] [PubMed] [Google Scholar]
- 4.HalliwellB, GutteridgeJMC. Free radicals in biology and medicine, 4th Edition. Oxford: Clarendon Press, 2007. [Google Scholar]
- 5.Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 2001;28:679–88. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- 6.Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009;150:1855–65. doi: 10.1104/pp.109.139204. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BroschéM, OvermyerK, WrzaczekM, KangasjärviJ, KangasjärviS. Stress signaling III: Reactive oxygen species. In: Abiotic stress adaptation in plants: Physiological, molecular and genomic foundation. Pareek A, Sopory SK, Bohnert HJ, Govindjee, eds. Dordrecht: Springer, 2010: 91-102. [Google Scholar]
- 8.Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–92. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 9.Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–40. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 11.Kranner I, Roach T, Beckett RP, Whitaker C, Minibayeva FV. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J Plant Physiol. 2010;167:805–11. doi: 10.1016/j.jplph.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 13.Leymarie J, Vitkauskaité G, Hoang HH, Gendreau E, Chazoule V, Meimoun P, Corbineau F, El-Maarouf-Bouteau H, Bailly C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012;53:96–106. doi: 10.1093/pcp/pcr129. [DOI] [PubMed] [Google Scholar]
- 14.Oracz K, Voegele A, Tarkowská D, Jacquemoud D, Turecková V, Urbanová T, Strnad M, Sliwinska E, Leubner-Metzger G. Myrigalone A inhibits Lepidium sativum seed germination by interference with gibberellin metabolism and apoplastic superoxide production required for embryo extension growth and endosperm rupture. Plant Cell Physiol. 2012;53:81–95. doi: 10.1093/pcp/pcr124. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhuri A, Kar RK. Effect of ethylene synthesis and perception inhibitor and ABA on seed germination of Vigna radiata. World J Agri Sci. 2008;4:914–21. [Google Scholar]
- 16.Chaudhuri A, Kar RK. Involvement of reactive oxygen species and oxidative metabolism in regulation of seed germination in Vigna radiata. Indian J Plant Physiol. 2012;17:286–91. [Google Scholar]
- 17.Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol. 2009;184:885–97. doi: 10.1111/j.1469-8137.2009.03005.x. b. [DOI] [PubMed] [Google Scholar]
- 18.Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O(2)(.-), H(2)O(2), and (.)OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004;136:3114–23, discussion 3001. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta. 2002;214:821–8. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- 21.Bustos D, Lascano R, Villasuso AL, Machado E, Senn ME, Córdoba A, Taleisnik E. Reductions in maize root-tip elongation by salt and osmotic stress do not correlate with apoplastic O2*- levels. Ann Bot. 2008;102:551–9. doi: 10.1093/aob/mcn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke D, Sun G. The effect of reactive oxygen species on ethylene production induced by osmotic stress in etiolated mungbean seedling. Plant Growth Regul. 2004;44:199–206. doi: 10.1007/s10725-004-5575-7. [DOI] [Google Scholar]
- 23.Causin HF, Roqueiro G, Petrillo E, Láinez V, Pena LB, Marchetti CF, Gallego SM, Maldonado SI. The control of root growth by reactive oxygen species in Salix nigra Marsh. seedlings. Plant Sci. 2012;183:197–205. doi: 10.1016/j.plantsci.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Sairam RK, Dharmar K, Lekshmy S, Chinnusamy V. Expression of antioxidant defense genes in mung bean (Vigna radiata L.) roots under water-logging is associated with hypoxia tolerance. Acta Physiol Plant. 2011;33:735–44. doi: 10.1007/s11738-010-0598-3. [DOI] [Google Scholar]
- 25.Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, et al. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell. 2009;21:3803–22. doi: 10.1105/tpc.109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty R, Kar RK. Differential water uptake kinetics in axes and cotyledons during seed germination of Vigna radiata under chilling temperature and cycloheximide treatment. Braz J Plant Physiol. 2008;20:277–84. doi: 10.1590/S1677-04202008000400003. [DOI] [Google Scholar]
- 27.Schopfer P. Biomechanics of plant growth. Am J Bot. 2006;93:1415–25. doi: 10.3732/ajb.93.10.1415. [DOI] [PubMed] [Google Scholar]
- 28.Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci. 2003;116:81–8. doi: 10.1242/jcs.00201. [DOI] [PubMed] [Google Scholar]
- 29.Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, Zheng SH, Yuasa T, Iwaya-Inoue M. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012;158:1705–14. doi: 10.1104/pp.111.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishibashi Y, Tawaratsumida T, Zheng S, Yuasa T, Iwaya-inoue M. NADPH oxidases act as key enzyme on germination and seedling growth in barley (Hordeum vulgare L.) Plant Prod Sci. 2010;13:45–52. doi: 10.1626/pps.13.45. [DOI] [Google Scholar]
- 31.Chaudhuri A, Singh KL, Kar RK. Interaction of hormones with reactive oxygen species in regulating seed germination of Vigna radiata (L.) Wilczek. J Plant Biochem Physiol. 2013;1:1. doi: 10.4172/jpbp.1000103. [DOI] [Google Scholar]
- 32.Garnczarska M, Wojtyla L. Differential response of antioxidative enzymes in embryonic axes and cotyledons of germinating lupine seeds. Acta Physiol Plant. 2008;30:427–32. doi: 10.1007/s11738-008-0138-6. [DOI] [Google Scholar]
- 33.Bogdanović J, Radotić K, Mitrović A. Changes in activities of antioxidant enzymes during Chenopodium murale seed germination. Biol Plant. 2008;52:396–400. doi: 10.1007/s10535-008-0083-7. [DOI] [Google Scholar]
- 34.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331–41. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- 35.Schopfer P. Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta. 1996;199:43–9. doi: 10.1007/BF00196879. [DOI] [Google Scholar]
- 36.Frahry G, Schopfer P. Inhibition of O2-reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry. 1998;48:223–7. doi: 10.1016/S0031-9422(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 37.Able AJ, Guest DI, Sutherland MW. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of phytophthora parasitica var nicotianae. Plant Physiol. 1998;117:491–9. doi: 10.1104/pp.117.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Pan S. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot Bull Acad Sin. 1996;37:107–11. [Google Scholar]
- 39.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]