Abstract
Purpose
To evaluate the neuroprotective and neurite outgrowth effects of maltol, a natural aroma compound, on retinal ganglion cells (RGCs) under oxidative stress in vitro.
Methods
Mouse primary RGCs were isolated using immunopanning–magnetic separation and exposed to H2O2 in the presence of maltol. The cell viability and apoptosis were determined by using adenosine 5′-triphosphate (ATP) assay and terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL), respectively. Neurite outgrowth was assessed by immunofluorescence for α-tubulin. The activation of nuclear factor-κB (NF-κB) was also evaluated using immunofluorescence.
Results
When the RGCs were exposed to 20 μM of H2O2 for 16 h, their viability dropped to 40.3±3.4%. However, the maltol treatment restored the cells in a dose-dependent manner. The viability recovered to 73.9±5.1% with 10 μM of maltol and even reached 175.1±11.3% with 2 mM of maltol, as measured by ATP assay. This oxidative stress significantly increased the number of TUNEL-positive RGCs, but the maltol drastically reduced the proportion of those apoptotic cells. The oxidative stress hampered the neurite outgrowth of the RGCs, whereas maltol restored their ability to sprout neurites. Regarding NF-κB, the active form of phosphorylated NF-κB (pNF-κB) increased the oxidative stress level but the maltol treatment again reduced it to an unstressful level.
Conclusions
Our data revealed that maltol attenuated the oxidative stress–induced injury in the primary mouse RGCs. Its neuroprotective and neurite outgrowth effects seemed to be related to NF-κB signaling. Maltol has potential as a new neuroprotective therapeutic agent for oxidative stress–related ocular diseases, including glaucoma.
Introduction
Glaucoma is the second leading cause of blindness globally. In fact, about 8% of cases of blindness are caused by glaucoma worldwide [1]. Elevated intraocular pressure (IOP) is the best known risk factor for the development and progression of glaucoma; methods of lowering IOP represent the cynosure of all glaucoma research. However, after it was discovered that oxidative stress injury to retinal ganglion cells (RGCs) is a leading pathophysiology of glaucoma [2], researchers’ attention has turned to how to directly rescue the damaged RGCs from fatal oxidative stress.
Maltol (3-hydroxy-2-methyl-4-pyrone) is a naturally occurring aroma compound which is found in beans [3] and primarily used as a flavor enhancer. It is also a product of the Maillard reaction of Korean red ginseng [4], and it is reported to have a strong free radical scavenging activity [3,4]. Exogenous maltol administration protects liver cells from lipopolysaccharide-induced hepatic damage and kidney cells from streptozotocin-induced diabetic renal injuries by suppressing thiobarbituric acid reactive substance (TBA-RS), nuclear factor-κB (NF-κB), and inducible nitric oxide synthase (iNOS) [5]. In the nervous system, maltol rescues the hippocampal neurons from kainic acid–induced neurotoxicity by returning the level of glutathione and TBA-RS to that of undamaged animals [6]. RGC axons form the optic nerve that transit visual information from the retina to the brain; there is no definitive study which has shown that maltol effectively protects them from the devastating effects of oxidative stress.
With this in mind, the present study investigates whether maltol attenuates oxidative stress injury in primary RGCs in vitro. Under oxidative stress, the effects of maltol on cell viability, apoptosis, and neurite outgrowth were assessed. To better understand its intracellular working mechanism, the change in activity of NF-κB was also evaluated.
Methods
Animals
A total of 48 pregnant Crl:CD-1 mice were purchased from Orientbio (Seongnam, South Korea). In terms of the mice pups, 676 newborn mice were euthanized by decapitation. All animals were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Institutional Animal Care and Use Committee of Yonsei University College of Medicine, Seoul, Republic of Korea. Great effort was made to minimize the number of animals euthanized and their suffering. Each following experiment was conducted in quadruplicate and it was repeated at least three times from different cell harvests.
Cell culture
Primary mouse RGCs were harvested from three- or four-day-old newborn mice using the immunopanning–magnetic separation method, as previously described [7]. Briefly, the retinal tissue was separated from the enucleated eyeballs and the mixed retinal cells were collected as a suspension. The retinal cell suspension was incubated with a rabbit anti-mouse macrophage antibody (Fitzgerald Industries International, Concord, MA) and distributed over a Petri dish coated with a goat anti-rabbit immunoglobulin G (IgG) antibody (Southern Biotechnology Associates, Birmingham, AL). Nonadherent cells were then treated with a biotinylated anti-mouse Thy 1.2 antibody (Abcam, Cambridge, MA) and interacted with MACSTM anti-biotin MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The magnetically labeled RGCs were finally collected using a magnetic separating unit (Miltenyi Biotec). The isolated cells were cultured in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12; Catalog no. SH30023.01; Hyclone, Logan, UT) containing 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin (Life Technologies, Grand Island, NY). The cultures were incubated at 37 °C in humidified 5% CO2 and 95% air. For all of the experiments conducted, the cells were used at 70–80% confluence. At 24 h after harvesting, the RGCs were exposed to 20 μM of H2O2 (Sigma-Aldrich, St. Louis, MO) for 16 h. In the maltol treatment group, various concentrations of maltol (Sigma-Aldrich) were added to culture media at the time of injury.
Cell viability
The number of viable cells in the culture was determined by quantification of the adenosine 5′-triphosphate (ATP) present, which signals the presence of metabolically active cells (CellTiter-Glo® Luminescent Cell Viability Assay; Promega, Madison, WI). For each well of an opaque-walled 96-well plate, a volume of CellTiter-Glo® Reagent (100 μl) was added to the same volume of cell culture medium (100 μl) containing the cells. To induce cell lysis, the contents were mixed for 2 min in an orbital shaker. The solution was allowed to stabilize for 10 min at room temperature and the luminescent signal was then recorded.
Nuclear DNA fragmentation
Apoptotic cells were evaluated using the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL), which detects nuclear DNA fragmentation by labeling the terminal end of nucleic acids (Apo-BrdU In situ DNA Fragmentation Assay Kit; BioVision, Mountain View, CA). In brief, the cells were fixed with 1% paraformaldehyde for 15 min on ice and 70% ethanol for at least 30 min at -20 °C. They were incubated with TdT reaction buffer, TdT enzyme, and bromolated dUTP (Br-dUTP) for 60 min at 37 °C and subsequently reacted with the anti-BrdU-fluorescein isothiocyanate (FITC) antibody in the dark for 30 min at room temperature. In addition, the cells were counterstained with propidium iodide (PI) in the dark for 30 min at room temperature. The cells were finally assessed under a fluorescence microscope; the Br-dUTP transferred to the free3'-hydroxyl (3'-OH) groups of cleaved DNA and TdT was detected. Apoptotic cells showed green fluorescence over PI counterstained cells of orange-red fluorescence.
Neurite outgrowth
The cells were fixed with 4% paraformaldehyde (Yakuri Pure Chemicals Co., LTD., Kyoto, Japan) for 30 min, treated with 0.1% Triton X-100 (Sigma-Aldrich) in 0.1% Na-Citrate (Sigma-Aldrich) for 10 min, and then blocked with 2% bovine serum albumin (BSA; Sigma-Aldrich) for 1 h. They were incubated with an anti-mouse α-tubulin (1:50 dilution; Abcam) antibody overnight at 4 °C, and then exposed to the FITC-conjugated secondary antibody (1:100 dilution; Life Technologies) for 60 min at room temperature. A mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI; Santa Cruz Biotechnology, Dallas, TX) was applied, and four random fields were examined under a confocal microscope. α-tubulin was finally detected with FITC, which appeared as green fluorescence in neurites, and all nuclei were counterstained with DAPI, which appeared as blue fluorescence.
Activity of nuclear factor-κB
The activity of NF-κB was evaluated by immunofluorescence as described above (see neurite outgrowth section). Specifically, the NF-κB p50 (1:50 dilution; Santa Cruz Biotechnology) and pNF-κB p50 (1:50 dilution; Santa Cruz Biotechnology) primary antibodies were used. The original form of NF-κB was labeled with Alexa594 (Life Technologies) with red fluorescence in the cytosolic space, and the active form of pNF-κB was labeled with FITC with green fluorescence in the nucleus.
Statistical analysis
Cell viability data were expressed with a mean ± standard error of the mean (SEM) and compared with the Kruskal–Wallis one-way analysis of variance (ANOVA) using the PASW Statistics 18 for Windows, version 18.0.0 (SPSS, Chicago, IL). A p value of less than 0.05 was considered statistically significant.
Results
Maltol attenuates oxidative stress–induced cytotoxicity of retinal ganglion cells
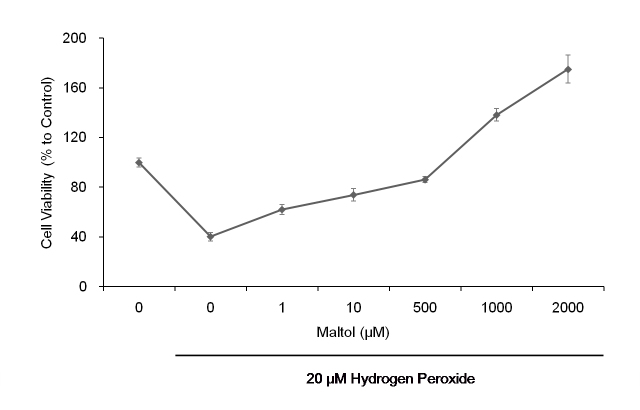
When the primary mouse RGCs were exposed to 20 μM of H2O2 for 16 h, the cell viability dropped to 40.3±3.4% compared to the undamaged control cells, as measured by ATP assay (Figure 1). Maltol treatment improved the cell viability in a dose-dependent manner. It recovered to 73.9±5.1% of that of the control with 10 μM of maltol, and even reached 175.1±11.3% of that of the control with 2 mM of maltol.
Figure 1.
Cell viability was determined by adenosine 5′-triphosphate (ATP) assay. Primary mouse retinal ganglion cells (RGCs) were exposed to H2O2 for up to 16 h. In the presence of maltol, the oxidative stress-induced cytotoxicity was significantly decreased in a dose-dependent manner. At concentrations of maltol higher than 1 mM, the cell viability became even greater compared to the untreated control. This ATP assay was conducted in quadruplicate and repeated at least three times from different cell harvests; cell viability data were expressed as mean ± standard error of the mean (SEM).
Maltol reduces apoptosis in retinal ganglion cells under oxidative stress
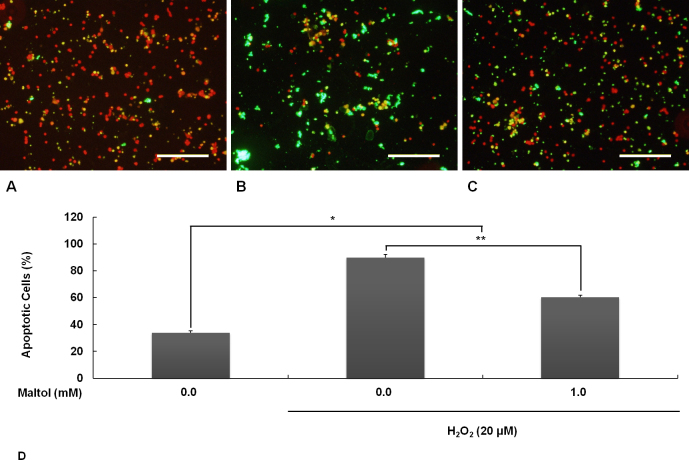
Oxidative stress from H2O2 (20 μM, 16 h) attacked the primary RGCs in a devastating manner. In the undamaged control group, the TUNEL-positive cells with green fluorescence were seldom observed (Figure 2A). In the oxidative stress only group, the greater part of the cells were TUNEL-positive apoptotic cells (Figure 2B). In the maltol treatment group, when 1 mM of maltol was added to the culture medium at the time of injury, the number of apoptotic cells was significantly reduced (Figure 2C). The proportion of TUNEL-positive apoptotic cells is quantified and shown in Figure 2D.
Figure 2.
Apoptosis was evaluated by terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL). Oxidative stress was induced by exposing the retinal ganglion cells (RGCs) to 20 μM of H2O2 for 16 h. In the maltol treatment group, 1 mM of maltol was added to culture medium at the time of injury. A: Undamaged control; B: Oxidative stress only; C: Oxidative stress with maltol treatment. Apoptotic cells showed broken nuclei with green fluorescence over counterstained, unfragmented nuclei with orange-red fluorescence. Scale bar is 200 μm. D: The proportion of apoptotic cells was expressed as mean ± standard error of the mean (SEM). Asterisks, p<0.05, n=12, *Significant difference between control versus oxidative stress with/without maltol treatment, **Significant difference between oxidative stress without versus with maltol treatment.
Maltol restores neurite outgrowth ability of retinal ganglion cells damaged by oxidative stress
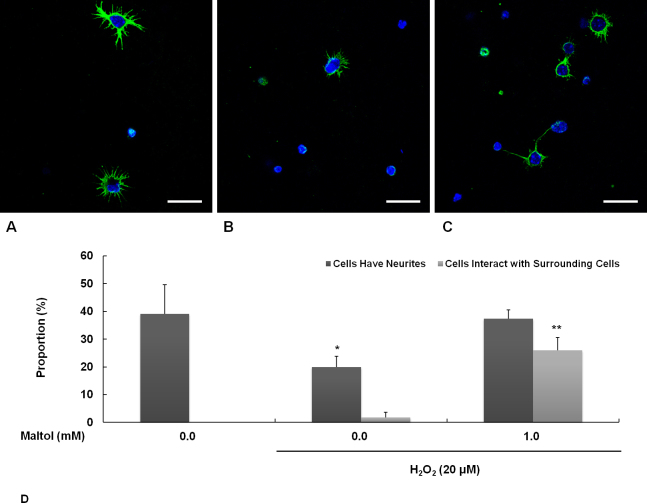
Primary mouse RGCs sprouted many neurites in an unstressful environment (Figure 3A). These neurites contained a high amount of α-tubulin, which assembles the microtubules with β-tubulin. Oxidative stress was induced by exposing the cells to 20 μM of H2O2 for 16 h. Under stress, most of the cells were hampered and reached fewer neurites (Figure 3B). However, when 1 mM of maltol was added to the culture media during the experiment, the RGCs were fairly restored and had the ability to stretch their neurites out (Figure 3C). Interestingly, in the presence of maltol, the number of RGCs that came into contact and interacted with the surrounding cells increased vastly even under oxidative stress (Figure 3D).
Figure 3.
Neurite outgrowth from retinal ganglion cells (RGCs) was investigated by immunoreactivity to α-tubulin. Oxidative stress was induced by exposure to 20 μM of H2O2 for 16 h. In the maltol treatment group, 1 mM of maltol was added to the culture medium at the time of injury. A: Undamaged control; B: Oxidative stress only; C: Oxidative stress with maltol treatment. Neurites were labeled with green fluorescence and nuclei were counterstained with blue fluorescence. Scale bar is 25 μm. D: The proportion of cells having neurites and interacting with surrounding cells was expressed as mean ± standard error of the mean (SEM). Asterisks, p<0.05, n=12, *Significant difference between control & oxidative stress with maltol treatment vs. oxidative stress only, **Significant difference between control & oxidative stress only vs. oxidative stress with maltol treatment.
Maltol suppresses the activation of nuclear factor-κB in retinal ganglion cells under oxidative stress
For unstressed RGCs, the inactive form of NF-κB was dominant and reacted to the anti-NF-κB p50-specific antibody tagged by red fluorescence (Figure 4A). When the RGCs were placed under oxidative stress due to exposure to 20 μM of H2O2 for 16 h, the active form of pNF-κB increased dramatically and was labeled with the anti-pNF-κB p50-specific antibody tagged by green fluorescence (Figure 4B). In the presence of 1 mM of maltol, the oxidative stress did not significantly influence the NF-κB activation. The immunoreactive pattern to NF-κB p50/pNF-κB p50 looked similar to that of undamaged control cells (Figure 4C).
Figure 4.
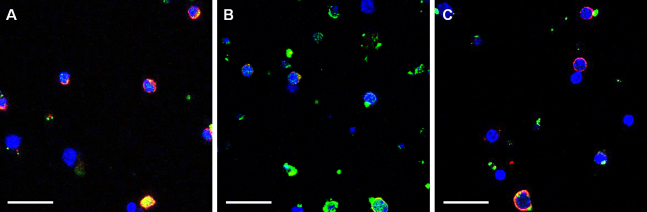
The activity of nuclear factor-κB (NF-κB) was assessed by immunofluorescence for NF-κB p50 and phosphorylated NF-κB p50 (pNF-κB p50). Oxidative stress was induced by exposure to 20 μM of H2O2 for 16 h. In the maltol treatment group, 1 mM of maltol was added to the culture medium at the time of injury. A: Undamaged control; B: Oxidative stress only; C: Oxidative stress with maltol treatment. Unphosphorylated inactive NF-κB was labeled with red fluorescence and phosphorylated active pNF-κB was labeled with green fluorescence. Scale bar represents 25 μm.
Discussion
Maltol, a natural organic compound, can act as a free radical scavenger [3,4], and protects the neuronal/non-neuronal cells from various fatal injuries [5,6]. It saves hippocampal neurons from oxidative stress generated by kainic acid, hepatocytes from lipopolysaccharide-induced inflammatory damage, and renal cells from streptozotocin-induced diabetic injury. Although its intracellular working mechanism is still not well understood, NF-κB signaling has come to the fore as a strong candidate for generating maltol’s cytoprotective signaling cascade [5,8]. In the present study, maltol was applied on RGCs under oxidative stress in vitro and significantly reduced the cytotoxicity caused by H2O2. In higher concentrations than 1 mM, maltol raised the cell viability to much greater levels than that of undamaged control cells. It decreased the proportion of apoptotic cells and restored the neurite outgrowth ability of stressed RGCs. These neuroprotective and neurite outgrowth effects of maltol seemed to be related to the NF-κB inactivation. When the RGCs were exposed to H2O2, NF-κB was phosphorylated and activated. However, in the presence of maltol, the oxidative stress could not induce NF-κB phosphorylation. Our results are somewhat similar to those of the other previous findings concerning maltol’s antiapoptotic effect on human neuroblastoma cells [8]; when those cells were exposed to H2O2, maltol drastically reduced apoptosis/DNA fragmentation and NF-κB activation.
NF-κB is a transcription factor and plays a key role in regulating cellular responses to diverse noxious stimuli [9]. While in the latent state, NF-κB is sequestered in the cytosol as a complex with inhibitor of κB (IκB), its inhibitory protein. When a certain extracellular signal turns on the cascade, IκB is phosphorylated and NF-κB cut loose from the inhibitory complex. Subsequently, the activated NF-κB translocates into the nucleus and binds the target DNA sequences, which then finally triggers the target gene transcription. In the nervous system, NF-κB plays a complicated dual role [10]. According to the stimuli, it turns on the death as well as the survival of signaling pathways. For RGCs, pressure- or hypoxia-induced activation of NF-κB seems to contribute to the cytotoxicity [11-14], and its inactivation appears to protect the retina from any damage [13-15]. Our data also suggest that maltol protects RGCs from oxidative stress injuries via the suppression of NF-κB activation. As a matter of fact, a definite conclusion cannot be drawn from this investigation that maltol directly suppresses the NF-κB activation and it is the only mechanism by which maltol rescues the oxidative stressed RGCs. Maltol, as an antioxidant, possibly decreases the oxidative stress itself, and consequently reduces the stress response of NF-κB. In this sense, it seems like a chicken-and- egg question. However, the direct downregulation of NF-κB rescues neurons against acute DNA damage [16], and our data are in accordance with this. In addition, even though its precise working mechanism is still unclear, it seems to be apparent that maltol does indeed diminish RGCs’ oxidative damage in vitro.
Glaucoma is a neurodegenerative disease of the optic nerve. The retinal nerve fiber layer, which consists of RGC axons, is progressively atrophied and the RGC soma gradually vanishes. Oxidative stress is known to be one of the major causes of glaucomatous optic nerve degeneration [2], and many researchers are eager to determine how it rescues the damaged RGCs from fatal oxidative stress. Several molecules, including α2-adrenergic agonist [17,18], N-methyl-D-aspartate receptor antagonist [19,20], NOS inhibitor [21,22], caspase inhibitor [23,24], and others [25-28], have been reported to have neuroprotective effects on experimental glaucoma models. However, the fruits of all these studies are not yet satisfactory, and we still have to dig further to attain more conclusive results. Our data suggest that maltol may be another candidate for a novel neuroprotective therapeutic strategy for oxidative neuronal damage in glaucoma. Apart from the influence on the viability of damaged RGCs, the ability of maltol to stretch the neurites out from the RGCs even in a stressful environment is another outstanding merit. Regarding cell-to-cell interaction, maltol treatment apparently increased the proportion of RGCs that came into direct contact with the other surrounding cells, even in an environment of oxidative stress. This phenomenon is supposed to be due to the maltol treatment, which subsequently increased the cell density or total neurite length. The initial glaucomatous changes usually begin in RGC axons; thus, how to promote axonal regrowth from damaged RGCs is one of the major concerns in the glaucoma field. To confirm maltol’s neuroprotective and neurite outgrowth effects on RGCs under oxidative stress, further in vivo studies are needed, and these are currently in progress.
As axonal degeneration precedes cell death, glaucoma shares common features with major central nervous system (CNS) diseases, including amyotrophic lateral sclerosis, Alzheimer’s disease, and Parkinson’s disease [29]. Maltol is supposed to be a prospect for the novel neuroprotective/regenerative therapeutic strategy in these common obstinate CNS neurodegenerative diseases related to oxidative stress injuries in addition to glaucoma.
In summary, our data revealed that maltol effectively attenuates the RGC injury caused by oxidative stress. Maltol not only significantly reduced cytotoxicity/apoptosis, but also restored the neurite outgrowth ability of damaged RGCs. Its capability to protect RGCs and to stretch out neurites seemed to be related to the NF-κB signaling pathway. Our data imply that maltol may offer a new powerful neuroprotective or neuroregenerative therapeutic agent for oxidative stress–related diseases, including glaucoma.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2010–0008721 and 2011–0013288) and a grant from Yuyu Pharma, Inc. (2011) in Republic of Korea.
References
- 1.Mariotti SP. Global data on visual impairment 2010. Geneva: World Health Organization; 2012, p. 3–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KG, Shibamoto T. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J Agric Food Chem. 2000;48:4290–3. doi: 10.1021/jf000442u. [DOI] [PubMed] [Google Scholar]
- 4.Kang KS, Kim HY, Pyo JS, Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–4. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 5.Yokozawa T, Kang KS, Yamabe N, Kim HY. Therapeutic potential of heat-processed Panax ginseng with respect to oxidative tissue damage. Drug Discov Ther. 2007;1:30–44. [PubMed] [Google Scholar]
- 6.Kim YB, Oh SH, Sok DE, Kim MR. Neuroprotective effect of maltol against oxidative stress in brain of mice challenged with kainic acid. Nutr Neurosci. 2004;7:33–9. doi: 10.1080/10284150310001653604. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Iizuka Y, Kim CY, Seong GJ. Isolation of primary mouse retinal ganglion cells using immunopanning-magnetic separation. Mol Vis. 2012;18:2922–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Wang J, Xu C, Pan H, Zhang Z. Maltol inhibits apoptosis of human neuroblastoma cells induced by hydrogen peroxide. J Biochem Mol Biol. 2006;39:145–9. doi: 10.5483/bmbrep.2006.39.2.145. [DOI] [PubMed] [Google Scholar]
- 9.Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–68. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mincheva-Tasheva S, Soler RMNF. -κB signaling pathways: role in nervous system physiology and pathology. Neuroscientist. 2013;19:175–94. doi: 10.1177/1073858412444007. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J Biol Chem. 2005;280:31240–8. doi: 10.1074/jbc.M502641200. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Lee JE, Kim CY, Seong GJ. Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci. 2007;8:81. doi: 10.1186/1471-2202-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Sun MH, Pang JH, Chen SL, Han WH, Ho TC, Chen KJ, Kao LY, Lin KK, Tsao YP. Retinal protection from acute glaucoma-induced ischemia-reperfusion injury through pharmacologic induction of heme oxygenase-1. Invest Ophthalmol Vis Sci. 2010;51:4798–808. doi: 10.1167/iovs.09-4086. [DOI] [PubMed] [Google Scholar]
- 14.Tulsawani R, Kelly LS, Fatma N, Chhunchha B, Kubo E, Kumar A, Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci. 2010;11:125. doi: 10.1186/1471-2202-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Li F, Ge J, Sarkisian SR, Jr, Tomita H, Zaharia A, Chodosh J, Cao W. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol. 2007;67:603–16. doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- 16.Aleyasin H, Cregan SP, Iyirhiaro G, O'Hare MJ, Callaghan SM, Slack RS, Park DS. Nuclear factor-(kappa)B modulates the p53 response in neurons exposed to DNA damage. J Neurosci. 2004;24:2963–73. doi: 10.1523/JNEUROSCI.0155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler LA, Gil DW, WoldeMussie E. Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv Ophthalmol. 2001;45(Suppl 3):S290–4. doi: 10.1016/s0039-6257(01)00206-5. [DOI] [PubMed] [Google Scholar]
- 18.Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol Neurodegener. 2011;6:4. doi: 10.1186/1750-1326-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol. 2003;48:S38–46. doi: 10.1016/s0039-6257(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Nakamura S, Tanaka H, Shimazawa M, Araie M, Hara H. Memantine protects against secondary neuronal degeneration in lateral geniculate nucleus and superior colliculus after retinal damage in mice. CNS Neurosci Ther. 2008;14:192–202. doi: 10.1111/j.1755-5949.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neufeld AH. Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthalmol. 1999;43:S129–35. doi: 10.1016/s0039-6257(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 22.Neufeld AH. Pharmacologic neuroprotection with an inhibitor of nitric oxide synthase for the treatment of glaucoma. Brain Res Bull. 2004;62:455–9. doi: 10.1016/j.brainresbull.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Patil K, Sharma SC. Broad spectrum caspase inhibitor rescues retinal ganglion cells after ischemia. Neuroreport. 2004;15:981–4. doi: 10.1097/00001756-200404290-00010. [DOI] [PubMed] [Google Scholar]
- 24.Schuettauf F, Stein T, Choragiewicz TJ, Rejdak R, Bolz S, Zurakowski D, Varde MA, Laties AM, Thaler S. Caspase inhibitors protect against NMDA-mediated retinal ganglion cell death. Clin Experiment Ophthalmol. 2011;39:545–54. doi: 10.1111/j.1442-9071.2010.02486.x. [DOI] [PubMed] [Google Scholar]
- 25.Fu QL, Wu W, Wang H, Li X, Lee VW, So KF. Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension. Cell Mol Neurobiol. 2008;28:317–29. doi: 10.1007/s10571-007-9155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belforte NA, Moreno MC, de Zavalía N, Sande PH, Chianelli MS, Keller Sarmiento MI, Rosenstein RE. Melatonin: a novel neuroprotectant for the treatment of glaucoma. J Pineal Res. 2010;48:353–64. doi: 10.1111/j.1600-079X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 27.Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI, Miller JW. Etanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE. 2012;7:e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen FT, Yang CM, Yang CH. The protective effects of the proteasome inhibitor bortezomib (velcade) on ischemia-reperfusion injury in the rat retina. PLoS ONE. 2013;8:e64262. doi: 10.1371/journal.pone.0064262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman MP. The challenges of axon survival: introduction to the special issue on axonal degeneration. Exp Neurol. 2013;246:1–5. doi: 10.1016/j.expneurol.2013.06.007. [DOI] [PubMed] [Google Scholar]