Abstract
Non-malignant cells found within neoplastic lesions express alanyl (membrane) aminopeptidase (ANPEP, best known as CD13), and CD13-null mice exhibit limited tumor growth and angiogenesis. We have recently demonstrated that a subset of bone marrow-derived CD11b+CD13+ myeloid cells accumulate within neoplastic lesions in several murine models of transplantable cancer to promote angiogenesis. If these findings were confirmed in clinical settings, CD11b+CD13+ myeloid cells could become a non-malignant target for the development of novel anticancer regimens.
Keywords: angiogenesis, tumor, CD13, mouse models, bone marrow-derived cells
Angiogenesis, the formation of new blood vessels from pre-existing ones, is essential for the development of most solid tumors. While angiogenesis in normal tissues is tightly coordinated by the activity of various anti- and pro-angiogenic factors, the formation of new blood vessels within neoplastic lesions is sustained by the imbalanced expression of pro-angiogenic mediators.1 This generally involves a functional interplay between malignant cells and several of their non-malignant counterparts that populate the tumor microenvironment, including endothelial cells, pericytes, and fibroblasts. Tumor-infiltrating bone marrow-derived cells (BMDCs), such as tumor-associated macrophages (TAMs), Tie2-expressing monocytes (TEMs), mast cells, granulocytes, dendritic cells, and myeloid suppressor cells, may also promote angiogenesis in the tumor microenvironment by producing soluble mediators that stimulate the proliferation, migration, and functional differentiation of endothelial cells.2,3 In addition, angiogenesis critically relies on several extracellular proteases that activate growth factors, inhibit suppressive mediators, and/or promote extracellular matrix (ECM) degradation along with tissue remodeling.
We have previously shown that alanyl (membrane) aminopeptidase (ANPEP, best known as CD13) is one of the proteolytic enzymes involved in angiogenesis and tumor growth.4 CD13 is a membrane-bound metallopeptidase expressed in tumors by endothelial cells, pericytes and fibroblasts, as well as by some neoplastic cells. Different immunoreactive forms of CD13 are also expressed by many non-malignant cells of normal tissues, including some types of epithelial cells, keratinocytes, mast cells, myeloid cells, and antigen-presenting cells.5 In physiological conditions, CD13 operates quite broadly in protein degradation, antigen presentation, signal transduction, differentiation, proliferation, adhesion and migration, and also regulates various hormones and cytokines.5 In addition, CD13 has been involved in the epithelial-to-mesenchymal transition, hence promoting tumor progression.5 Our group has shown CD13 to have a functional role in angiogenesis both in normal and neoplastic tissues.4,6 In line with this notion, newborn CD13-null mice exhibit a reduced angiogenic response to hypoxia in a model of oxygen-induced retinopathy of prematurity.6 Furthermore, adult CD13-null mice display reduced angiogenesis and limited tumor growth rates than their wild-type (WT) counterparts.4 Although these results indicated that CD13 participates in pathological angiogenesis, the specific cell types involved were not identified in these studies.
To dissect the relative contributions of different CD13+ cells to abnormal angiogenesis, we have recently investigated the growth of B16F10 melanomas, TSA mammary adenocarcinomas and Lewis lung carcinomas (LLC) subcutaneously implanted into 4 different syngeneic murine models: (1) WT mice transplanted with WT BMDCs recovered from isogenic donors (WTwt); (2) CD13-null (KO) mice transplanted with WT BMDCs (KOwt); (3) WT mice transplanted with CD13-null BMDCs (WTko); and (4) CD13-null mice transplanted with CD13-null BMDCs recovered from isogenic donors (KOko) (Fig. 1).7 Tumor growth was severely impaired in mice lacking CD13+ BMDCs (WTko and KOko), correlating with a reduction in both vascular density and pericyte coverage within residual neoplastic lesions. Notably, the growth of TSA and LLC cells was restored in KOwt mice, which fail to express CD13 on pericytes, endothelial cells and fibroblasts but are reconstituted with CD13+ BMDCs. Of note, the absence of CD13+ BMDCs in KOko and WTko mice markedly reduced the metastatic dissemination to the lung of cancer cells administered intravenously, suggesting that CD13+ BMDCs promote angiogenesis at both primary and secondary tumor sites. Moreover, the administration of CD45+CD11b+CD13+ myeloid cells isolated from TSA tumors grown in WT mice could by itself increase the number of blood vessels (as well as their pericyte coverage) that serving tumors implanted in KO mice. These CD45+CD11b+CD13+ cells, which consisted mainly of macrophages, specifically localized to the tumor microenvironment and produced soluble pro-angiogenic factors such as matrix metallopeptidase 9 (MMP9) and chemokine (C-C motif) ligand 2 (CCL2, also known as MCP1). Given that both MMP9 and CCL2 are known to recruit pericytes, hence consolidating vascular architecture, these findings indicate that CD13+ myeloid BMDCs are likely to sustain tumor progression by attracting pericytes and thus promoting angiogenesis and vascular maturation.
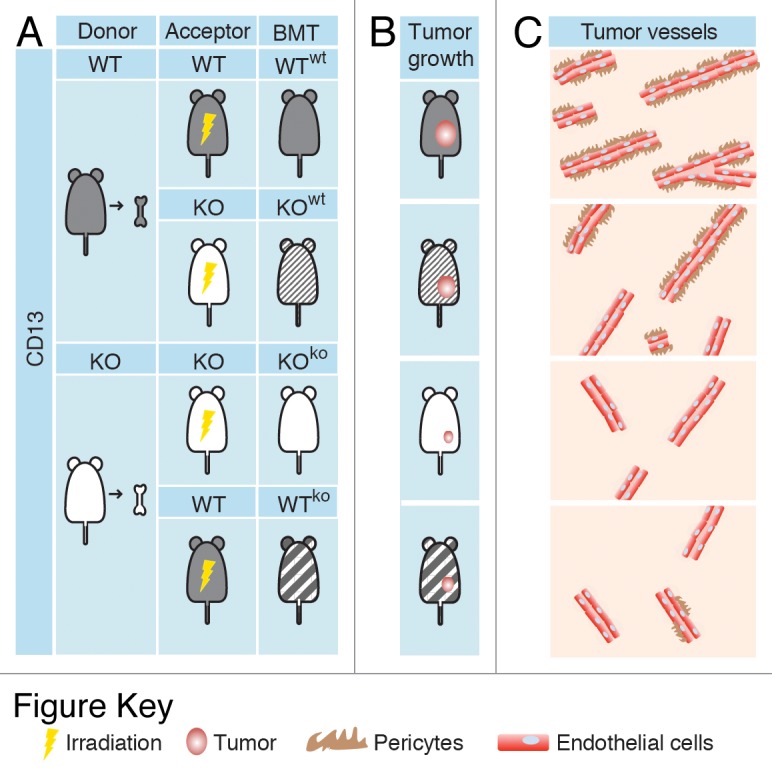
Figure 1. Experimental tumor models used to study the function of CD13+ bone marrow-derived cells. (A) Wild-type (WT) and CD13-null (KO) mice were irradiated and transplanted with wild-type (wt) or CD13-null (ko) bone marrow-derived cells (BMDCs) recovered from WT and KO mice (donors), as indicated. Bone marrow-transplanted (BMT) mice were then subcutaneously implanted with syngeneic cancer (melanoma, mammary adenocarcinoma, or lung carcinoma) cells. (B and C) Tumor growth was severely impaired in mice lacking CD13+ BMDCs (KOko and WTko mice), correlating with a reduction in both vascular density and pericyte coverage within neoplastic lesions.
Our findings establish CD13+ BMDCs as a previously unrecognized non-malignant candidate for the development of new anticancer therapies. CD13 inhibitors and neutralizing antibodies have previously been shown to limit angiogenesis and hence inhibit tumor growth.8-10 Our observation that CD13+ BMDCs are almost exclusively localized within neoplastic lesions makes them an attractive target for ligand-directed delivery of biological response modifiers and/or drugs. For example, CD13 ligands as well as molecules that bind other receptors selectively expressed by CD13+ BMDCs could be exploited for the targeted delivery of cytotoxic compounds. Interestingly, previous studies have shown that CD13 is expressed in different immunoreactive forms by tumor-associated blood vessels, myeloid cells, and epithelial cells, and that the vasculature-associated variant of CD13 is selectively recognized by peptides containing an NGR motif.8-10 This very small domain, which we identified by in vivo phage display technology, has been exploited to selectively deliver chemotherapeutic drugs, cytokines, pro-apoptotic peptides, liposomes, viruses, vascular-targeting, and/or imaging agents to tumor blood vessels, resulting in significant therapeutic responses or improvements in imaging procedure.8-10 Although CD13+ BMDCs are not recognized by NGR-containing ligands, perhaps as they express a form of CD13 that differs from that found on endothelial cells and/or owing to vascular accessibility issues, other ligands targeting the BMDC-associated variant of CD13 or other markers might be developed to target this cell subset, as previously accomplished with endothelial cells. In summary, the discovery that CD13+ BMDCs promote tumor growth and angiogenesis has potential therapeutic implications that merit further investigation. If our finding were confirmed in translational settings, this newly recognized cell subpopulation could serve as a non-malignant target for the development of novel anticancer therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Dondossola E, Corti A, Sidman RL, Arap W, Pasqualini R. Bone marrow-derived CD13+ cells sustain tumor progression: a potential non-malignant target for anticancer therapy. OncoImmunology 2014; 3:e27716; 10.4161/onci.27716
References
- 1.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–66. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176:1564–76. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman-Rojas L, Rangel R, Salameh A, Edwards JK, Dondossola E, Kim YG, Saghatelian A, Giordano RJ, Kolonin MG, Staquicini FI, et al. Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc Natl Acad Sci U S A. 2012;109:1637–42. doi: 10.1073/pnas.1120790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol Med. 2008;14:361–71. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangel R, Sun Y, Guzman-Rojas L, Ozawa MG, Sun J, Giordano RJ, Van Pelt CS, Tinkey PT, Behringer RR, Sidman RL, et al. Impaired angiogenesis in aminopeptidase N-null mice. Proc Natl Acad Sci U S A. 2007;104:4588–93. doi: 10.1073/pnas.0611653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dondossola E, Rangel R, Guzman-Rojas L, Barbu EM, Hosoya H, St John LS, Molldrem JJ, Corti A, Sidman RL, Arap W, et al. CD13-positive bone marrow-derived myeloid cells promote angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:20717–22. doi: 10.1073/pnas.1321139110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev. 2012;32:1078–91. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 9.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–80. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 10.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–7. [PMC free article] [PubMed] [Google Scholar]


