Abstract
A memristor is a nonlinear element because its current-voltage characteristic is similar to that of a Lissajous pattern for nonlinear systems. We investigated the possible presence of memristors in the electrical circuitry of the Venus flytrap’s upper and lower leaves. The electrostimulation of this plant by bipolar sinusoidal or triangle periodic waves induces electrical responses in the upper and lower leaves of the Venus flytrap with fingerprints of memristors. The analysis was based on cyclic voltammetric characteristics where the memristor, a resistor with memory, should manifest itself. Tetraethylammonium chloride, an inhibitor of voltage gated K+ channels, or NPPB, a blocker of voltage gated Cl- and K+ channels, transform a memristor to a resistor in plant tissue. Uncouplers carbonylcyanide-3-chlorophenylhydrazone (CCCP) and carbonylcyanide-4-trifluoromethoxy-phenyl hydrazone (FCCP) decrease the amplitude of electrical responses at low and high frequencies of bipolar periodic electrostimulating waves. Our results demonstrate that voltage gated K+ channels in the Venus flytrap have properties of memristors of type 1 and type 2. The discovery of memristors in plants creates a new direction in the modeling and understanding of electrical phenomena in plants.
Keywords: electrophysiology, ion channels, memristor, signal transduction, Venus flytrap
Introduction
Electrical processes play important roles in electrophysiology of plants. Electrical form of energy has no entropy content and 100% of this energy can be used to do work or in information transfer and analysis. These signals propagate along sophisticated electrical circuitry of plants consisting of many electrical components developed by nature. The standard electrical circuits comprise 4 basic elements: a resistor, a capacitor, an inductor, and a memristor.1 The fourth basic circuit element is a memristor, or a resistor with memory.1 Memristors are memory circuit elements whose properties depend on the history and state of the system. Here we are going to analyze the memristance in different parts of the Venus flytrap plant.
A memristor is a nano-scale memory device, which has huge potential technical applications.1-14 A memristor is a nonlinear element because its current-voltage characteristic is similar to that of a Lissajous pattern observed from nonlinear systems. No combination of nonlinear resistors, capacitors and inductors can reproduce this Lissajous behavior of the memristor. It is a fundamental 2-terminal electrical circuit element described by a state- dependent Ohm’s Law. A voltage-controlled memristor can be defined by
| (1) |
where G is the memductance of the memristor.8 The “n” state variables (x1, x2,...,xn) depend on the internal state of the memristor and is defined by “n” 1st-order differential equations called the associated state equations.8 A current-controlled memristor is defined by,
| (2) |
where M is the memristance of the memristor. The unit of the memristance is the Ohm. The unit of the memductance is the Siemens.
Mathematically memristance can be described by equation:
| (3) |
where φ and q denote the flux and charge, respectively.
Chua, 8 Chua et. al. 3 , 4 and Sah et al. 5 presented theoretical proofs that the voltage-gated K+ channel is a locally-active memristor. Since plants and animals have similar voltage gated K+ channels, it would be interesting to investigate the possible presence of memristors in plants. Chua 8 and Adhikari et al. 14 found that a memristor has 3 characteristic fingerprints: “When driven by a bipolar periodic signal (such as sine waves with zero average value) the device must exhibit a pinched hysteresis loop in the voltage-current plane, assuming the response is periodic; starting from some critical frequency, the hysteresis lobe area should decrease monotonically as the excitation frequency increases; the pinched hysteresis loop should shrink to a single-valued function when the frequency tends to infinity.” However, in a plant tissue, the pinched hysteresis loop transforms to a non-pinched hysteresis loop instead of a single line I = V/R at high frequencies of the applied voltage 12 because the amplitude of electrical current depends also on the capacitance of the plant tissue and electrodes, the scanning frequency and direction of scanning:
| (4) |
where the capacitance C had been observed to be also a function of scanning frequency.
The pinched hysteresis loop of memory elements, when subject to a periodic stimulus, can be self crossing (type I memristor) or not (type II memristor).8 Recently, we found memristors of both types in different plants.12
Electrical signaling and rapid closure of the carnivorous plant Dionaea muscipula Ellis (Venus flytrap) have attracted the attention of researchers since XIX century. This carnivorous plant is capable of very fast movements to catch insects. Mechanisms of this movement were debated for many years. In Hydroelastic Curvature model15 the upper leaf of the Venus flytrap is visualized as a thin, weakly-curved elastic shell with principal natural curvatures that depend on the hydrostatic state of the 2 surface layers of cell, where different hydrostatic pressures are maintained. Unequal expansion of these layers results in the bending of the leaf, and it had been described in terms of bending elasticity. The external triggers, either mechanical or electrical, result in the opening of pores connecting these layers; water then rushes from the upper layer to the lower layer, and the bilayer couple quickly changes its curvature from convex to concave and the trap closes. Equations describing this movement were derived and verified with experimental data.15
We found that the electrical stimulus between a midrib and a lobe closes the Venus flytrap leaf by activating motor cells without mechanical stimulation of trigger hairs.15-20 The closing time of Venus flytrap by electrical stimulation is about 0.3 s, the same as mechanically induced closing. Ion channel blockers such as Zn2+, Ba2+, TEACl as well as uncouplers FCCP, CCCP, 2,4-dinitrophenol and pentachlorophenol have been observed to dramatically decrease the speed of the trap closing.16-20 Plants have sensory, short-term and long-term memory. We found that sensory and short-term memories in the Venus flytrap are electrical phenomena.21 It was shown by a novel charge injection method.15-22 The Venus flytrap can accumulate small subthreshold charges, and when the threshold value is reached, the trap closes.21 The cumulative character of electrical stimuli points to the existence of short-term electrical memory in the Venus flytrap.21
Results
Experimental setup is shown in Figure 1. Bipolar sinusoidal or triangle periodic waves with amplitude VFG were applied from a function generator. To measure the electrical current we included in the circuit an additional “current sensing” resistor R, so that electrical current was found as I = VR/R. Potential difference, VP, between electrodes in plants is equal to VP = VFG – VR (Fig. 1).
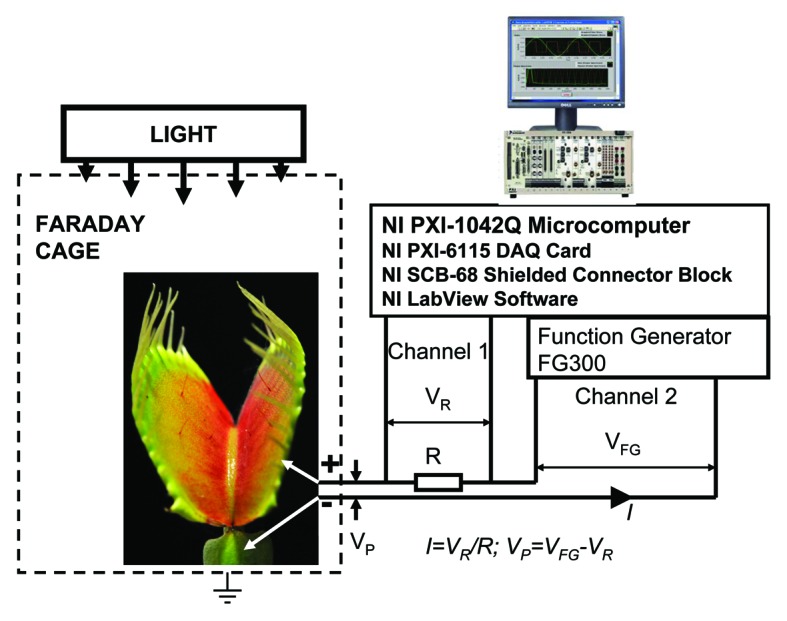
Figure 1. Block diagram of the data acquisition and electrostimulation system.
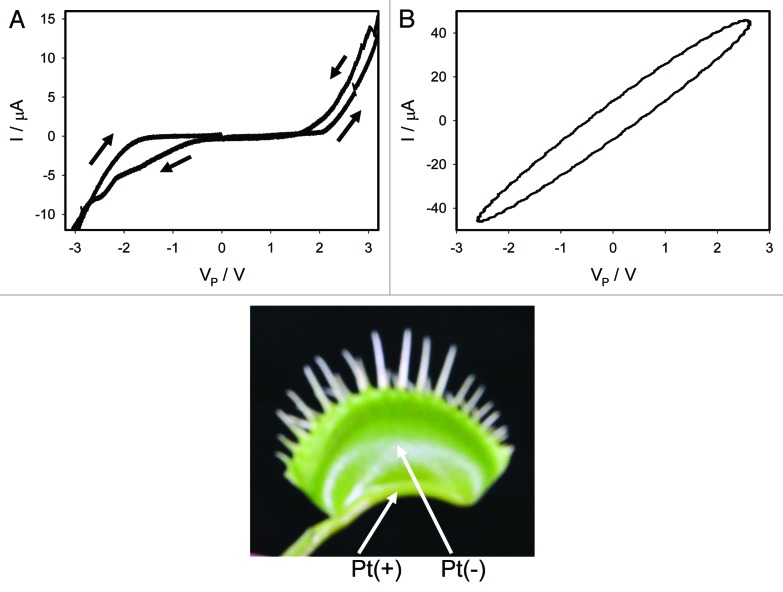
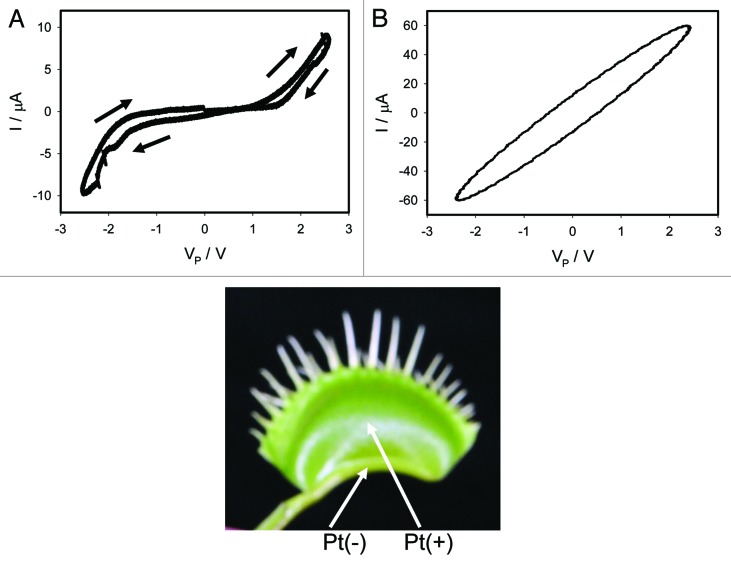
We recorded the current flowing through the plant generated by a bipolar sinusoidal wave with frequency of 0.001 Hz for an open trap (Fig. 2) and for a closed trap (Figs. 3A, 4A). In both cases we obtained a pinched hysteresis loop in the voltage-current plane with one important difference. If the trap is closed with a Pt reference electrode in the midrib, the plot displays a common pinched point with self-crossing between curves (Mean 94%, Std. Dev. 40%, Std. Err. 10%, n = 16) when I = 0 μA (Figs. 3A), which corresponds to properties of a memristor type 1. If the trap is closed with a Pt reference electrode in the lobe, the plot displays a common pinched point without self-crossing between curves (Mean 81%, Std. Dev. 25%, Std. Err. 6%, n = 16) when I = 0 μA (Figs. 4A), which corresponds to properties of a memristor type 2. If the trap is open with a Pt reference electrode in the midrib, the plot also displays a common pinched point but without self-crossing between curves (Mean 100%, n = 16), which corresponds to properties of a memristor type 2 (Fig. 2). At first glance it might be not obvious that the curves in Figure 3A intersect each other (i.e., self-cross type). So, to avoid the confusion we added the arrows in this panel. If one just follows these arrows, the crossing of the curves becomes immediately clear. It is interesting that the same plant tissue of the Venus flytrap has properties of memristor types 1 and 2 in the closed state, and memristor type 2 in the open trap.
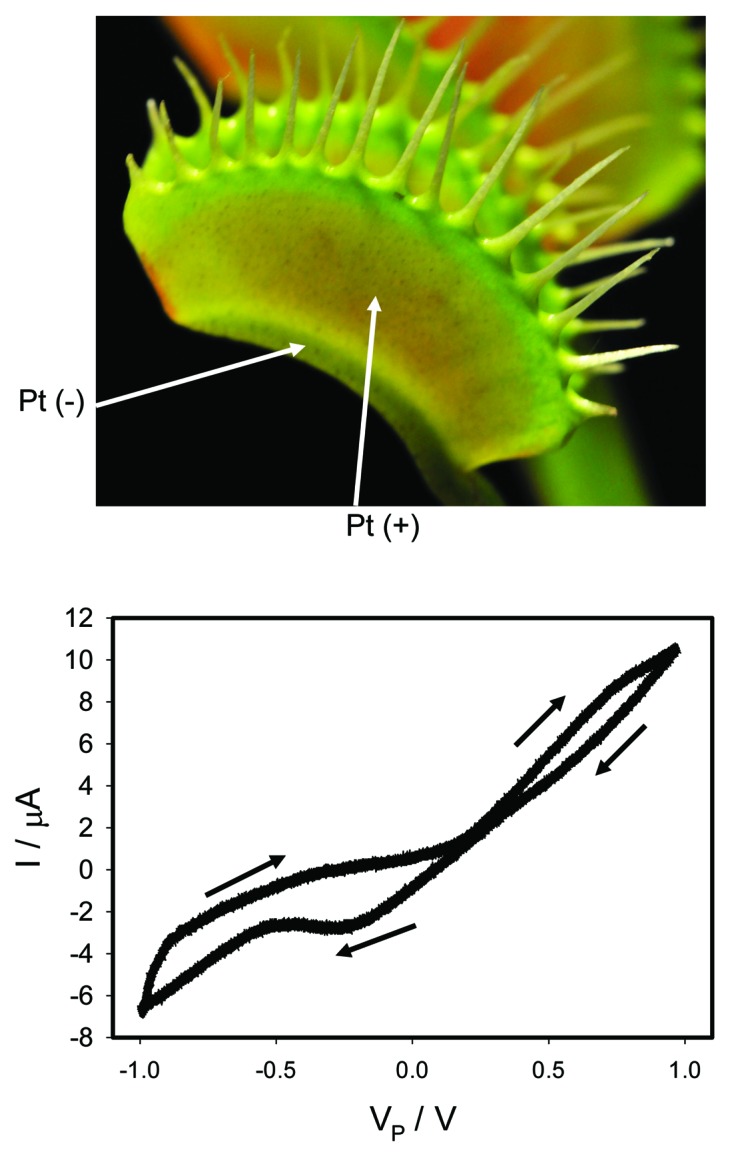
Figure 2. Electrical current I vs. voltage VP, applied between a lobe and a midrib, induced by a bipolar sinusoidal voltage wave VFG from a function generator; Frequency of voltage scanning was 0.001 Hz. Data acquisition: 1,000 scans/s, 1 000 000 scans. Positions of Pt electrodes in the Venus flytrap are shown. The trap was open.
Figure 3. Electrical current I vs. voltage VP in the Venus flytrap induced by a bipolar sinusoidal voltage wave from a function generator; Frequency of a voltage wave was 0.001 Hz (A) and 1,000 Hz (B). Data acquisition: 1,000 scans/s, 1 000 000 scans (A); 1 000 000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. The trap was closed.
Figure 4. Electrical current I vs. voltage VP in the Venus flytrap induced by a bipolar sinusoidal voltage wave from a function generator; Frequency of a sinusoidal bipolar voltage scanning wave was 0.001 Hz (A) and 1,000 Hz (B). Data acquisition: 1,000 scans/s, 1 000 000 scans (A); 1,000,000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. The trap was closed.
Increasing of a bipolar sinusoidal wave frequency to 1 kHz changes the shape of the line: it is still a loop but without a pinched point for both open and closed traps (Figs. 3B, 4B). The branches of the loop are approximately parallel to each other. So, the electrostimulation of the Venus flytrap by a periodic wave induces electrical responses in the Venus flytrap with fingerprints of a memristor of a type 1 (closed trap, Fig. 3) and type 2 (open trap, Fig. 2).
Voltage gated ionic channels regulate generation and transduction of electrical signals. For their analysis there are very efficient tools such as blockers of ionic channels. It was intriguing to investigate if these blockers would change the characteristics or even the presence of memristors in plants. Tetraethylammonium chloride (TEACl) is known as a blocker of a voltage gated K+ channel. We found that deposition of 10 μL of 10 mM TEACl on the midrib of the Venus flytrap decreased the amplitude of electrical current between electrodes in the trap and the hysteresis shrank (Fig. 5A). This can be caused by the increasing of resistance in the plant tissue. An inhibitor of voltage gated K+ channels tetraethylammonium chloride transforms a memristor to a simple resistor in plant tissue. Increasing of a bipolar sinusoidal wave frequency to 100 Hz changes the shape of the line: it is still a loop but without a pinched point (Fig. 5B). These results demonstrate that a voltage-gated K+ channel in the excitable tissue of the Venus flytrap is an essential component of plant memristor. It was found that 10 mM aqueous solution of TEACl decreased the speed of the trap closure (induced both by mechanical and electrical stimuli).
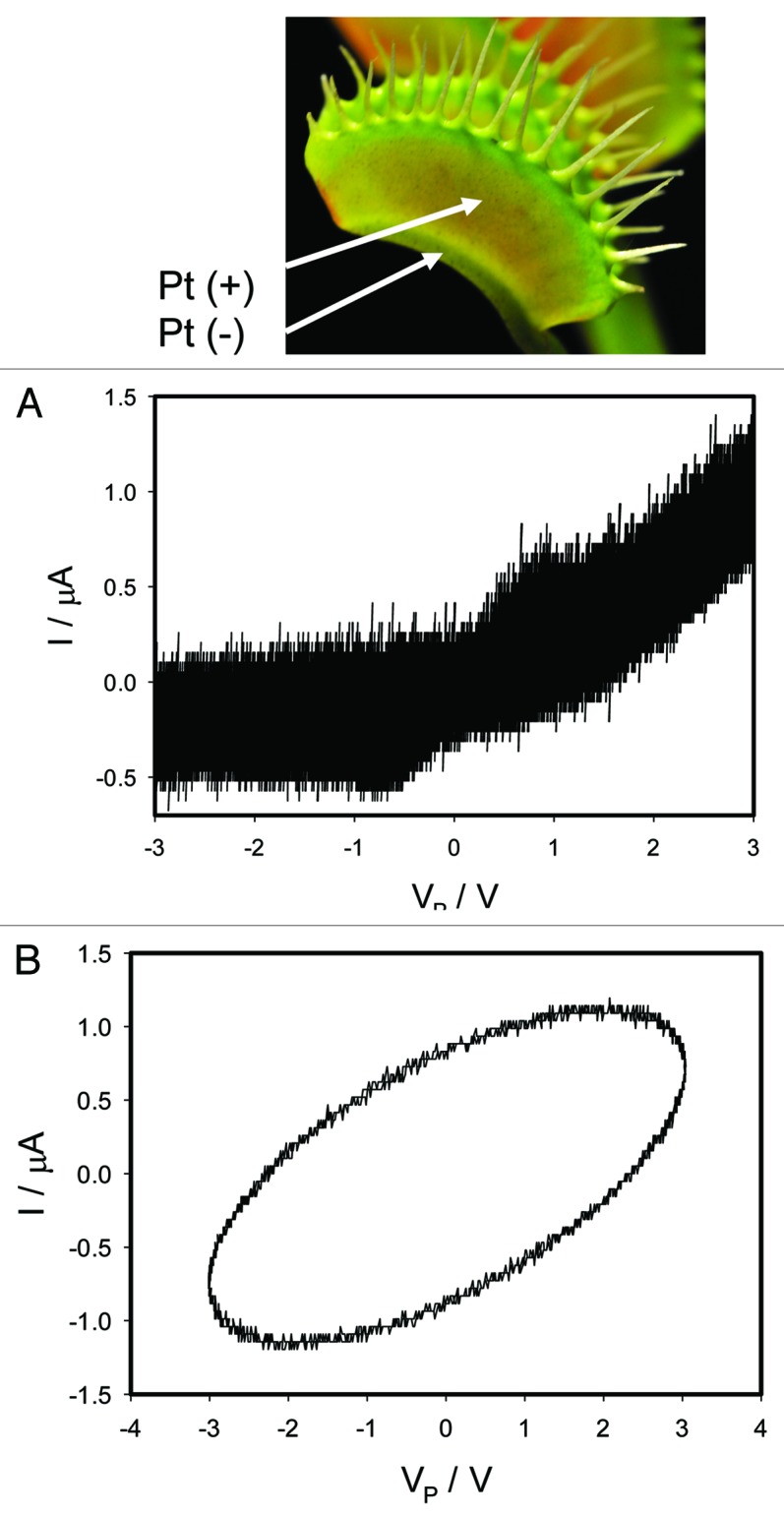
Figure 5. Effects of 10 μL drop of 10 mM TEACl deposited on the midrib of the Venus flytrap without touching the mechanosensitive trigger hairs 5 h before electrical measurements. Frequency of a bipolar sinusoidal voltage wave was 0.001 Hz (A) and 100 Hz (B); R = 47 kΩ. Data acquisition: 1,000 scans/s, 1,000,000 scans (A); 100,000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. The trap was open. These results were reproduced 16 times.
Similar inhibition of memristive properties between a lobe and a midrib in the Venus flytrap induces 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), which is known as a blocker of Cl- and K+ voltage-gated ion channels.24 If the a bipolar sinusoidal (Fig. 6A) or triangle (Fig. 7A) wave with frequency of 0.001 Hz is applied between electrodes in a midrib and a lobe, the amplitude of a hysteresis decreases and practically disappears. This is consistent with the DC behavior of non-ideal generalized memristors23 where at DC the pinched hysteresis loop degenerates into a single-valued function, namely, it’s DC V-I curve. At high frequencies of 1 kHz, cyclic voltammetry show I-V dependencies typical for a resistor in parallel with a capacitor (Figs. 6B, 7B).
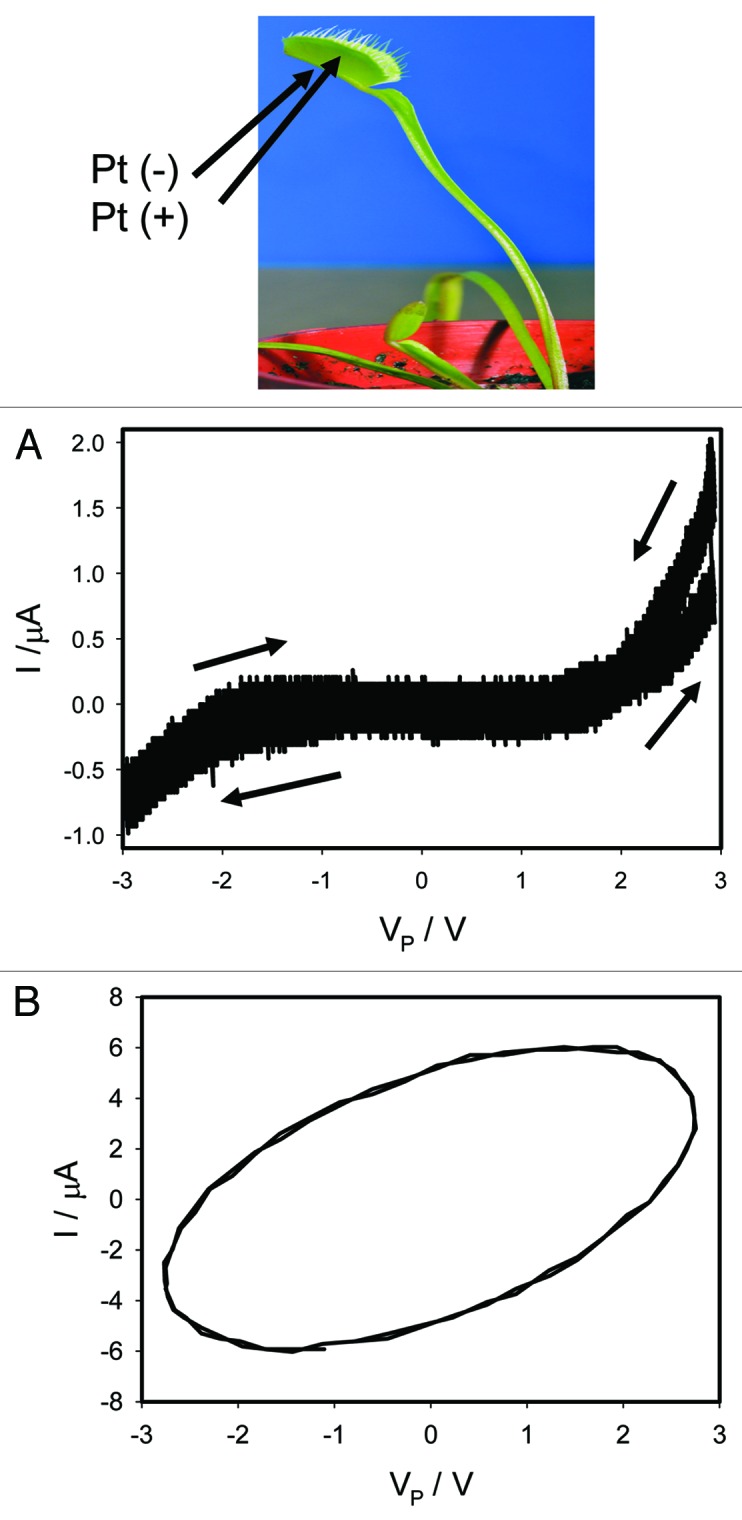
Figure 6. Effects of 50 μL drop of 50 μM NPPB in ethanol deposited on the midrib of the Venus flytrap without touching the mechanosensitive trigger hairs 5 h before electrical measurements. Frequency of a bipolar sinusoidal voltage wave was 0.001 Hz (A) and 1,000 Hz (B). Data acquisition: 1,000 scans/s, 1 000 000 scans (A); 1 000 000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. The trap was open. These results were reproduced 12 times.
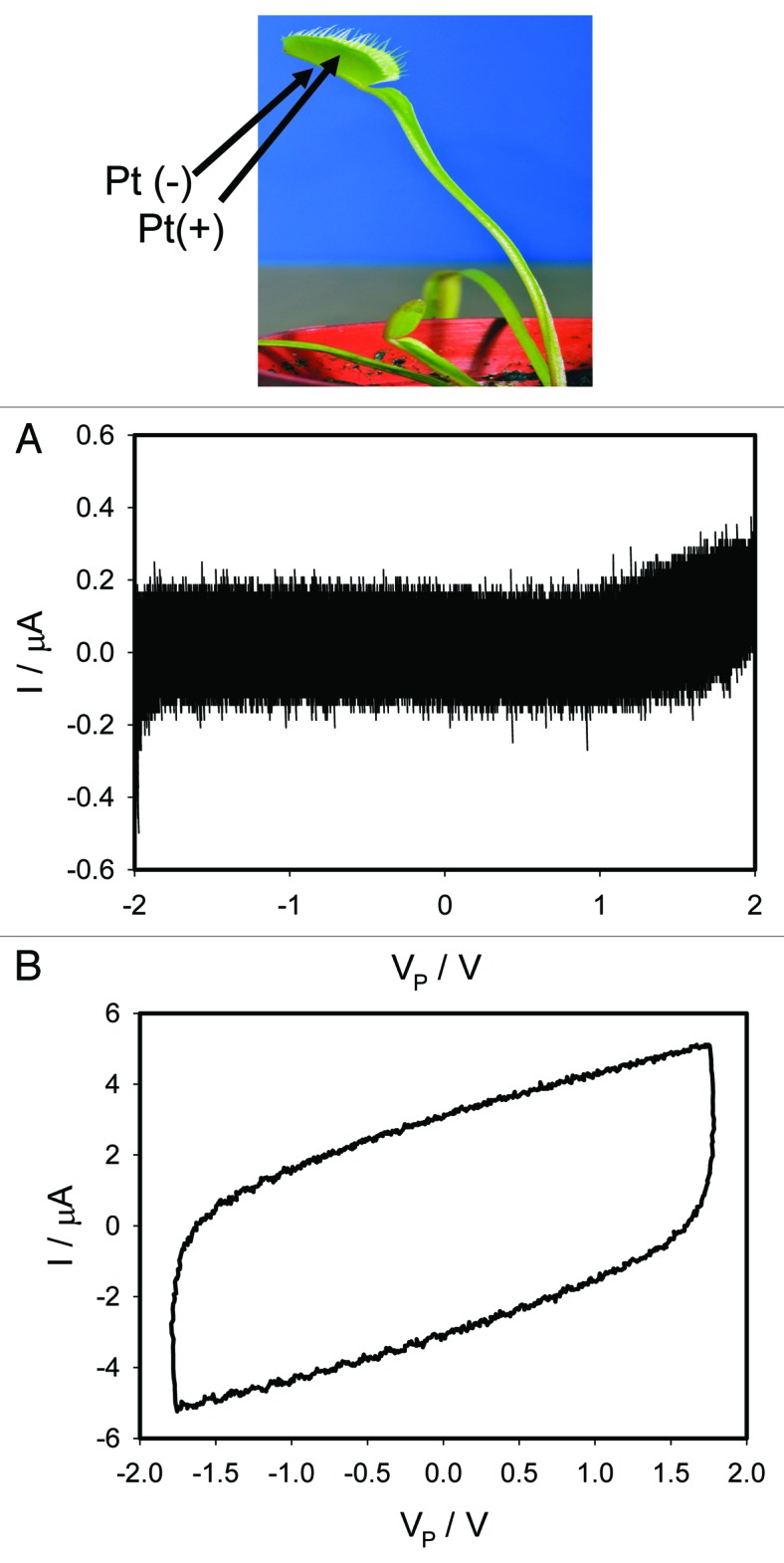
Figure 7. Effects of 50 μL drop of 50 μM NPPB in ethanol deposited on the midrib of the Venus flytrap without touching the mechanosensitive trigger hairs 5 h before electrical measurements. Frequency of a bipolar triangle voltage wave was 0.001 Hz (A) and 1,000 Hz (B). Data acquisition: 1,000 scans/s, 1,000,000 scans (A); 1 000 000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. The trap was open. These results were reproduced 12 times.
The next group of active substances studied in this work included uncouplers of oxidative phosphorylation. They are protonophores soluble in both water and lipid phases, permeate the lipid phase of a membrane by diffusion and transfer protons across the membrane, thus eliminating the proton electrochemical gradient and/or a membrane potential. Hodick and Sievers25 reported an excitability inhibition of Dionaea leaf mesophyll cells using uncoupler 2,4-dinitrophenol. Uncoupler CCCP caused the delay of the trap closing in addition to a significant decrease of the speed of closing as a result of membrane depolarization or dissipation of a proton gradient. CCCP and FCCP inhibit both mechanically and electrically induced the trap closure.20
Uncouplers are generally weak acids, and are often used to inhibit photosynthetic water oxidation. This phenomenon is due to their ability to be oxidized by the manganese cluster of the O2-evolving complex of photosystem II (PSII) and chloroplast. The membrane pool of plastoquinone can reduce these oxidized uncouplers, leading to formation of an artificial cyclic electron transfer chain around PSII involving uncouplers as redox carriers. Protonophores also uncouple electron transport, accelerate the deactivation of the S-2 and S-3 states on the donor side, and facilitate the oxidation of cytochrome b559 on the acceptor side of PSII. Uncouplers CCCP (Fig. 8) and FCCP inhibit the memristive properties of the trap.
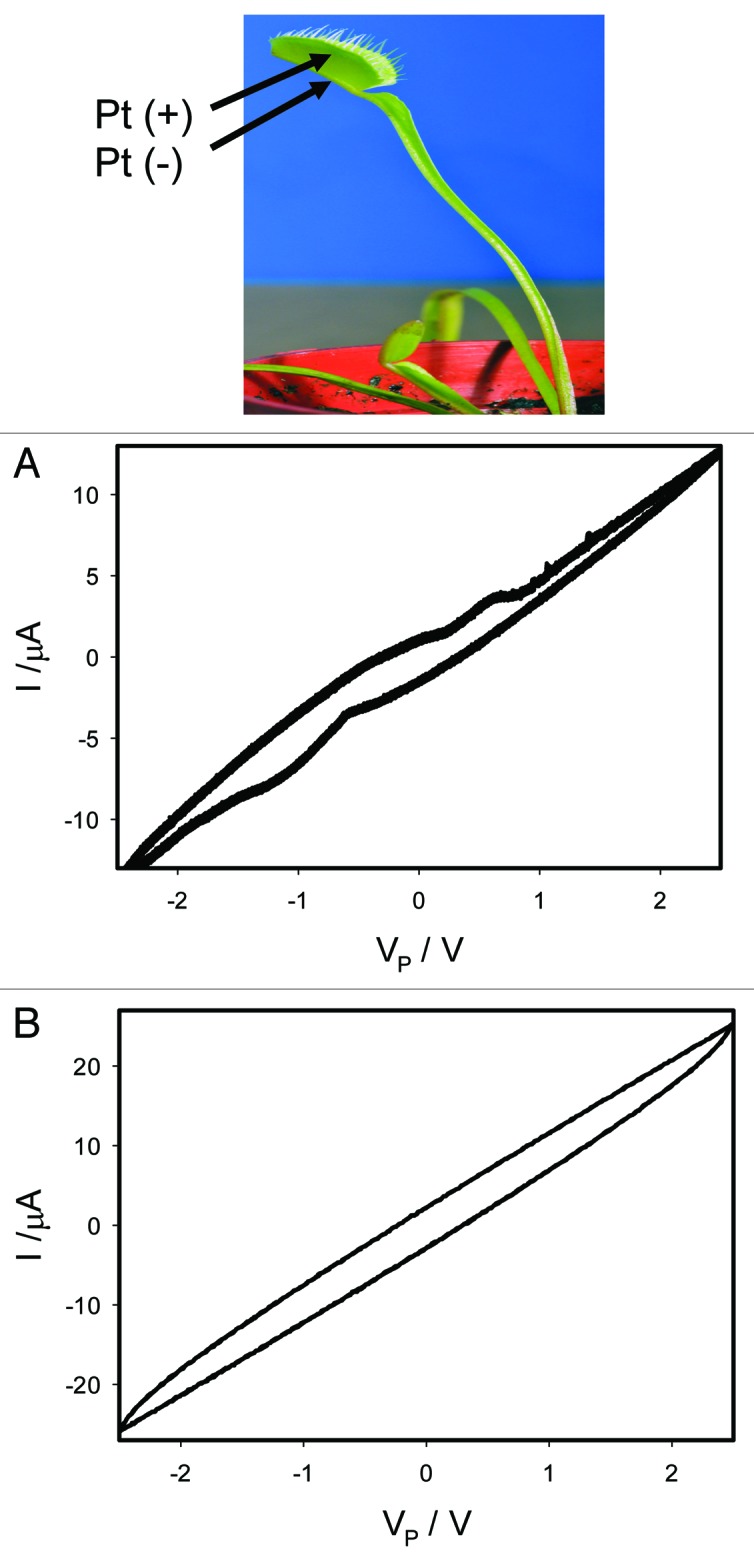
Figure 8. Effects of 10 μL drop of 10 μM CCCP deposited on the midrib of the Venus flytrap without touching the mechanosensitive trigger hairs 5 h before electrical measurements. Frequency of bipolar triangle voltage wave was 0.001 Hz (A) and 1,000 Hz (B); R = 47 kΩ. Data acquisition: 1,000 scans/s, 1 000 000 scans (A); 1,000,000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. The trap was open. These results were reproduced 16 times.
Cyclic voltammetry along the vascular bundles of the lower leaf of the Venus flytrap is shown in Figure 9. There is a pinched hysteresis in I-V plane without self crossing at low frequency of scanning (Fig. 9A), which is typical for memristors of type 2. The pinched hysteresis disappears at high frequencies (Fig. 9B).
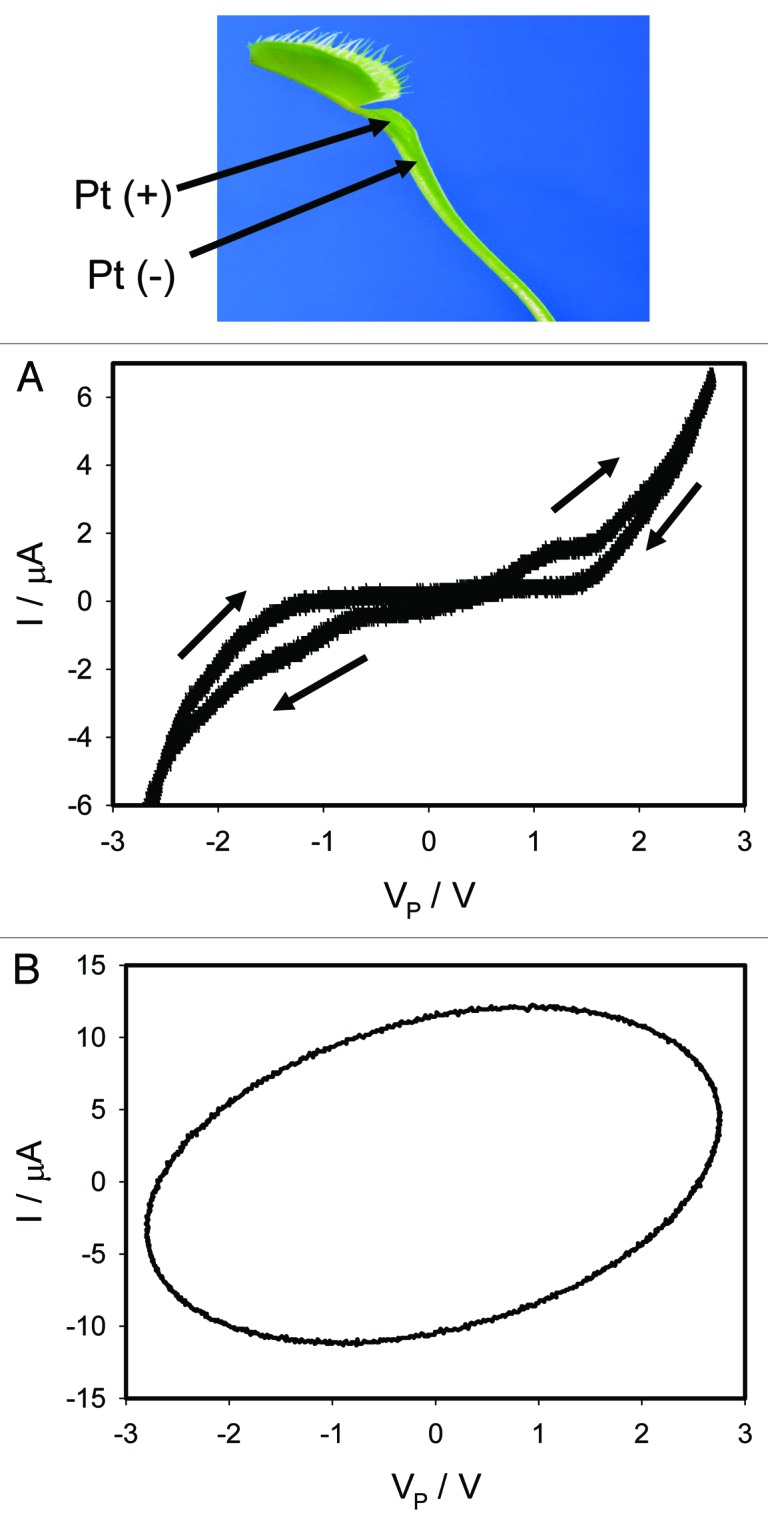
Figure 9. Electrical current I vs. voltage VP along vascular bundles in the lower leaf of the Venus flytrap induced by a bipolar sinusoidal voltage wave from a function generator. Frequency of bipolar sinusoidal voltage wave was 0.001 Hz (A) and 1,000 Hz (B); R = 47 kΩ. Data acquisition: 1,000 scans/s, 1 000 000 scans (A); 1 000 000 scans/s, 1,000 scans (B). Positions of Pt electrodes in the Venus flytrap are shown. Distance between electodes was 7 mm. The trap was open. These results were reproduced 12 times.
Figure 10 presents voltammetry between electrodes located perpendicular to the vascular bundles in the lower leaf of the Venus flytrap. There are no a pinched hysteresis loop in the voltage-current plane at low and high frequencies.
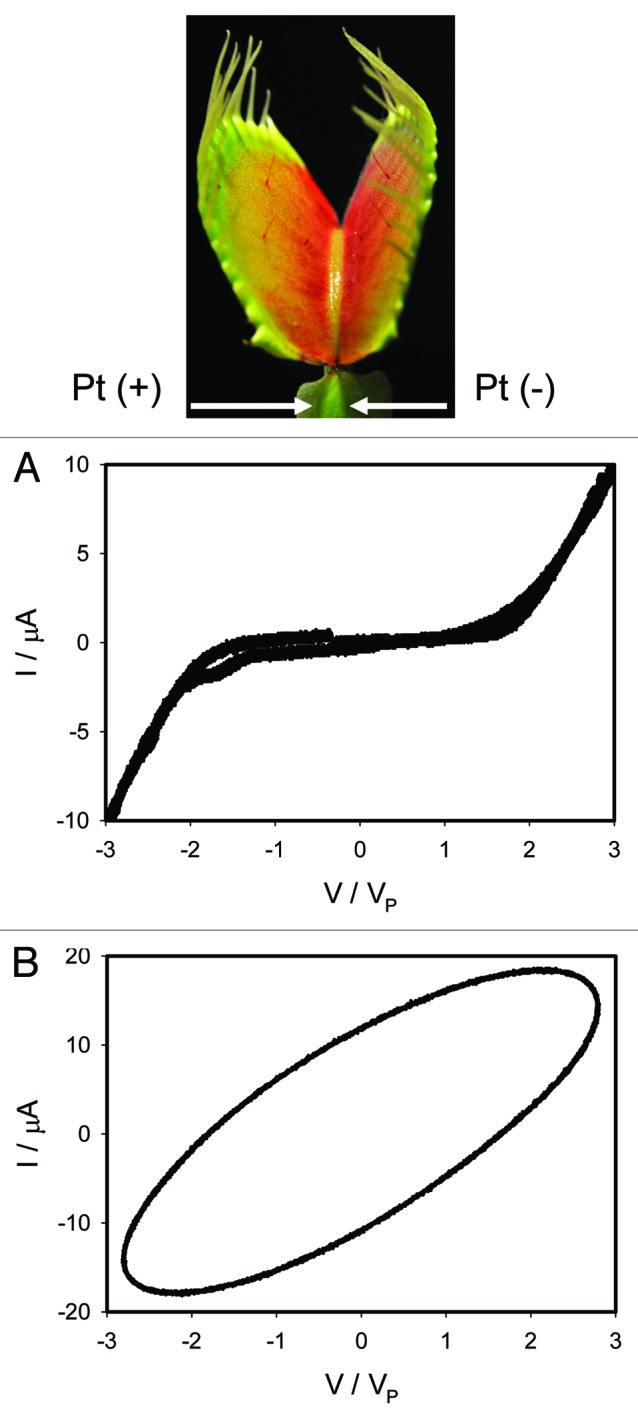
Figure 10. Electrical current I vs. voltage VP in the lower leaf of the Venus flytrap induced by bipolar triangular voltage wave from a function generator. Frequency of triangle voltage scanning was 0.001 Hz (A) and 1,000 Hz (B). R = 47 kΩ. Data acquisition: 1,000 scans/s, 1 000 000 scans (A); 1 000 000 scans/s, 1,000 scans (B). Position of Pt electrodes in the Venus flytrap is shown. The trap was open. These results were reproduced 14 times.
Discussion
Recently, we found that the bipolar triangle electrical wave induced pinched hysteresis in cyclic voltammetric measurements between 2 reversible Ag/AgCl electrodes in plant tissue.12 Application of large voltages can create exchange Cl- ions between Ag/AgCl electrodes in a plant tissue and mask the actual electrochemical mechanism:
| AgCl + e- ⇔ Ag + Cl- | (5) |
To avoid this, we use polarizable platinum microelectrodes in the present work. Platinum electrodes are universal electrodes for polarization in cathodic and anodic processes. They are stable over a wide range of potentials, both in alkaline and acidic solutions, and in the presence of redox components. Results obtained with platinum electrodes in the present work are similar to results with Ag/AgCl electrodes in our previous work.12
In the Venus flytrap we found the presence of resistors with memory. When driven by a bipolar periodic sinusoidal or triangle signal, plants exhibit a pinched hysteresis loop in the voltage-current plane (Figs. 2, 3A, 4A, 9A). Starting from some critical frequency, the hysteresis loop changes shape and a pinched hysteresis loop transforms to a non-pinched hysteresis (Figs. 3B, 4B, 9B) as the excitation frequency increases. Adhikari et al.14 have shown that there are 2 types of pinched hysteresis loops in memristors: a transversal type where 2 branches of the hysteresis loop are self-crossing and a non-transversal or tangential type where 2 branches of the hysteresis loop are tangent at the pinched point. In the open trap the electrical circuit has properties of a memristor type 1 and in the closed trap has properties of a memristor type 2.
Voltage gated channels can exhibit more than one self-intersection points. The third fingerprint of a memristor discovered by Chua and Adhikari et al.: “the pinched hysteresis loop should shrink to a single-valued function when the frequency tends to infinity” is correct for ideal memristors. For a plant tissue, the pinched hysteresis loop transforms to a non-pinched hysteresis loop instead of a single line I = V/R at high frequencies of the applied voltage because the amplitude of electrical current hysteresis depends also on capacitance of a plant tissue and electrodes, frequency and direction of scanning (Equation 4).
Figure 11 shows simple equivalent electrical circuits for low frequency measurements with a memristor (Figs. 2, 3A, 4A, 9A) and for high frequency measurements with a resistor (Figs. 3B, 4B, 9B) in parallel with a capacitor. The goal of this work is not to offer an exact memristor model of the plants, which would be subject of future research, but to point out that the Venus flytrap exhibits memristive characteristics.
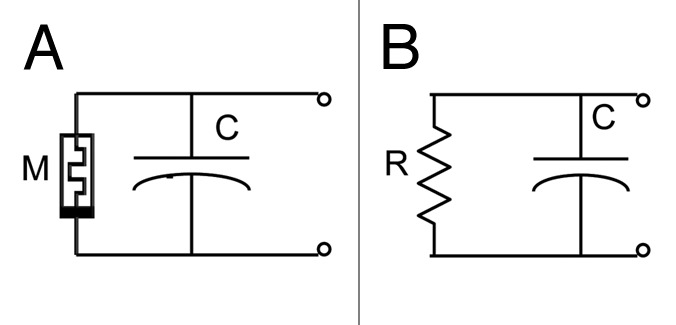
Figure 11. Equivalent electrical circuits for low frequency measurements with a memristor and a capacitor (A) and for high frequency measurements with a resistor and a capacitor (B).
The plant physiology must include memristors as essential model building blocks in electrical networks in plants. However, the memristor is an “ideal” circuit element and no real-world biodevices can be exactly mimicked by an “ideal” circuit model.26 Currently, there are different models of increasing accuracy that can be developed at the cost of increasing complexity.
Materials and Methods
Plants
The Dionaea muscipula Ellis (Venus flytrap) plants were purchased from Fly-Trap Farm Supply (North Carolina, USA) and grown in well drained peat moss in plastic pots at 22 °C with 12:12h light: dark photoperiod. We used plants grown from seeds only. All these plants can be closed by a mechanical stimulation of trigger hairs less than 1 s. We did not use plants grown using “cell culture” methods, which usually have properties different from native plants in North and South Carolina. The soil was treated with distilled water. Irradiance was 700–800 μmol photons m−2s−1. The humidity averaged 40–50%. All experiments were performed on healthy adult specimens. The Venus flytrap is a carnivorous plant. The trap can be closed by mechanical stimulation of trigger hairs or by an electrical pulse between the midrib and a lobe of the upper leaf. The Venus flytrap is considered to be an anticancer drug with oncolytic, anti-proliferative and immunomodulatory effects.27 Due to the high demand in medicine, there are many methods to grow plants in vitro using “cell culture” methods.28 There are problems with properties of the Venus flytrap produced by plant tissue culture technique: some traps do not close or close too slowly after mechanical stimulation of the trigger hairs. We used in our experiments plants grown from seeds only. All these plants can be closed by a mechanical stimulation of trigger hairs less than 1 s.
Chemicals
Tetraethylammonium chloride (TEACl), carbonylcyanide-4-trifluoromethoxyphenyl hydrazone (FCCP), and carbonylcyanide-3-chlorophenylhydrazone (CCCP) were obtained from Fluka (New York), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) was obtained from Tocris Bioscience.
Electrodes for extracellular measurements
Platinum electrodes were prepared from Teflon coated platinum wires (A-M Systems, Inc.) with a diameter of 76 μm. In all experiments we used identical electrodes as a measuring and as reference electrodes.
Data acquisition
All measurements were conducted in the laboratory at constant room temperature of 22 oС inside a Faraday cage which was mounted on a vibration-stabilized table (Fig. 1). High speed data acquisition of low-pass filtered signals was performed using microcomputer NI-PXI-1042Q (National Instruments) with simultaneous multifunction I/O plug-in data acquisition board NI-PXI-6115 (National Instruments) interfaced through a NI SCB-68 shielded connector block to electrodes.
Plant electrostimulation
The function generator FG300 (Yokagawa, Japan) was interfaced to NI-PXI-1042Q microcomputer and used for electrostimulation of plants. Resistance between electrodes in plants in all our experiments was between 1.0 MΩ and 1.2 MΩ. We selected a resistor R = 47 kΩ for measuring of voltage, VR, for estimation of electrical current I.
Statistics
All experimental results were reproduced on different plants. Software SigmaPlot 12 (Systat Software, Inc) was used for statistical analysis of experimental data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This article is based upon work supported in part by the National Science Foundation under grant number CBET-1064160 and in part by the US Army Research Office under contract/grant number W911NF-11–1-0132 to A.G.V. L.C.'s research is supported in part by AFOSR grant number FA9550–13–1-0136 and an EC Marie-Curie Fellowship.
Glossary
Abbreviations:
- C
capacitance
- CCCP
carbonylcyanide-3-chlorophenylhydrazone
- DAQ
data acquisition
- FCCP
carbonylcyanide-4-trifluoromethoxyphenyl hydrazone
- I
electrical current
- NPPB
(5-nitro-2-(3-phenylpropylamino)benzoic acid
- PXI
PCI eXtensions for Instrumentation
- TEACl
tetraethylammonium chloride
- VFG
voltage of an function generator
- VP
voltage between electrodes in plants
- VR
voltage on resistor R
References
- 1.Chua L. Memristor – The missing circuit element. IEE Transactions Circuit Theory. 1971;18:507–19. doi: 10.1109/TCT.1971.1083337. [DOI] [Google Scholar]
- 2.Strukov DB, Snider GS, Stewart DR, Williams RS. The missing memristor found. Nature. 2008;453:80–3. doi: 10.1038/nature06932. [DOI] [PubMed] [Google Scholar]
- 3.Chua L, Sbitnev V, Kim H. Hodgkin-Huxlew axon is made of memristors. Internat J Bifurcation Chaos 2012; 22:1230011-1-48.
- 4.Chua L, Sbitnev V, Kim H. Neurons are poised near the edge of chaos. Internat J Bifurcation Chaos 2012; 22:1250098-1-49.
- 5.Sah M, Kim H, Chua L. Brains are made of memristors. IEEE Circuits Systems IEEE Circuits Systems. 2014;14:12–36. doi: 10.1109/MCAS.2013.2296414. [DOI] [Google Scholar]
- 6.MacVittie K, Katz E. Electrochemical systems with memimpedance properties. J Phys Chem C 2013; 117:24943-24947.7.
- 7.Pershin YV, La Fontaine S, Di Ventra M. Memristive model of amoeba learning. Phys Rev E 2009; 80:021926.0. [DOI] [PubMed]
- 8.Chua L. Memristor, Hodgkin-Huxley, and edge of chaos. Nanotechnology. 2013;24:383001. doi: 10.1088/0957-4484/24/38/383001. [DOI] [PubMed] [Google Scholar]
- 9.Macvittie K, Katz E. Self-powered electrochemical memristor based on a biofuel cell - towards memristors integrated with biocomputing systems. Chem Commun (Camb) 2014;50:4816–9. doi: 10.1039/c4cc01540a. [DOI] [PubMed] [Google Scholar]
- 10.Smerieri A, Berzina T, Erokhin V, Fontana MP. Polymeric electrochemical elements for adaptive networks: Pulse mode. J Appl Phys. 2008;104:114513–8. doi: 10.1063/1.3033399. [DOI] [Google Scholar]
- 11.Jo SH, Chang T, Ebong I, Bhadviya BB, Mazumder P, Lu W. Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett. 2010;10:1297–301. doi: 10.1021/nl904092h. [DOI] [PubMed] [Google Scholar]
- 12.Volkov AG, Tucket C, Reedus J, Volkova MI, Markin VS, Chua L. Memristors in plants. Plant Signal Behav. 2014;9 doi: 10.4161/psb.28152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua L. Resistance switching memories are memristors. Appl Phys, A Mater Sci Process. 2011;102:765–83. doi: 10.1007/s00339-011-6264-9. [DOI] [Google Scholar]
- 14.Adhikaru AP, Sah MPd, Kim H, Chua L. The fingerprints of memristor. IEEE Trans Circuits Systems 2013; DOI: 10.1109/TCSI.2013.325671 [DOI]
- 15.Markin VS, Volkov AG, Jovanov E. Active movements in plants: Mechanism of trap closure by Dionaea muscipula Ellis. Plant Signal Behav. 2008;3:778–83. doi: 10.4161/psb.3.10.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markin VS, Volkov AG. Morphing structures in the Venus flytrap. In: Volkov AG, editor. Plant Electrophysiology. Signaling and Responses. Berlin: Springer; 2012. p. 1-31. [Google Scholar]
- 17.Volkov AG, Adesina T, Jovanov E. Closing of venus flytrap by electrical stimulation of motor cells. Plant Signal Behav. 2007;2:139–45. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkov AG, Pinnock MR, Lowe DC, Gay MS, Markin VS. Complete hunting cycle of Dionaea muscipula: consecutive steps and their electrical properties. J Plant Physiol. 2011;168:109–20. doi: 10.1016/j.jplph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Volkov AG, Coopwood KJ, Markin VS. Inhibition of the Dionaea muscipula Ellis trap closure by ion and water channels blockers and uncouplers. Plant Sci. 2008;175:642–9. doi: 10.1016/j.plantsci.2008.06.016. [DOI] [Google Scholar]
- 21.Volkov AG, Carrell H, Baldwin A, Markin VS. Electrical memory in Venus flytrap. Bioelectrochemistry. 2009;75:142–7. doi: 10.1016/j.bioelechem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Volkov AG, Carrell H, Markin VS. Biologically closed electrical circuits in venus flytrap. Plant Physiol. 2009;149:1661–7. doi: 10.1104/pp.108.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua LO, Kang SM. Memristive devices and systems. Proceedings of the IEE. 1976;64:209–23. doi: 10.1109/PROC.1976.10092. [DOI] [Google Scholar]
- 24.Illek B, Fischer H, Kreusel KM, Hegel U, Clauss W. Volume-sensitive basolateral K+ channels in HT-29/B6 cells: block by lidocaine, quinidine, NPPB, and Ba2+ Am J Physiol. 1992;263:C674–83. doi: 10.1152/ajpcell.1992.263.3.C674. [DOI] [PubMed] [Google Scholar]
- 25.Hodick D, Sievers A. The action potential of Dionaea muscipula Ellis. Planta. 1988;174:8–18. doi: 10.1007/BF00394867. [DOI] [PubMed] [Google Scholar]
- 26.Chua LO. Device modelling via basic nonlinear circuit elements. IEEE Trans Circ Syst. 1980;CAS-27:1014–44. doi: 10.1109/TCS.1980.1084742. [DOI] [Google Scholar]
- 27.Gaascht F, Dicato M, Diederich M. Venus flytrap (Dionaea muscipula Solander ex Ellis) contain powerful compounds that prevent and cure cancer. Frontiers Oncology 2013; 3:Article 202, 1-18. DOI: 10.3389/fonc.2013.00202 [DOI] [PMC free article] [PubMed]
- 28.Jang GW, Kim KS, Park RD. Micropropagation of Venus fly trap by shoot culture. Plant Cell Tissue Organ Cult. 2003;72:95–8. doi: 10.1023/A:1021203811457. [DOI] [Google Scholar]