Abstract
Several metals are essential nutrients for plants. However, they become toxic at high levels and deleteriously affect crop yield and quality. We recently reported the spatial gene expression profiles of iron (Fe)-deficient and cadmium (Cd)-stressed rice using laser microdissection and microarray analysis. The roots of Fe-deficient and Cd-stressed rice were separated into the vascular bundle (VB), cortex (Cor), and epidermis plus exodermis (EP). In addition, vascular bundles from new and old leaves at the lowest node, which are important for metal distribution, were analyzed separately (newDC and oldDC, respectively). Genes expressed in a tissue-specific manner in the VB, Cor, EP, newDC, and oldDC formed large clusters. The genes upregulated in all of the VB, Cor, and EP by Fe deficiency formed a substantial cluster that was smaller than the tissue-specific clusters. Significant numbers of genes expressed in newDC or oldDC were also expressed in VB in roots, suggesting that vascular bundles in the lowest nodes and roots have a partially common function. The expression patterns of transporter families involved in metal homeostasis were investigated, and members of each family were either expressed differentially in each tissue or showed different responses to Fe deficiency. One potassium transporter gene, OsHAK22, was upregulated by Fe deficiency in VB, Cor, and EP, suggesting that OsHAK22 is involved in potassium transport associated with mugineic acids secretion.
Keywords: Iron, cadmium, laser microdissection, transcriptome, rice
The availability of essential metals such as iron (Fe), zinc (Zn), manganese (Mn), and copper severely affects crop yield and quality. Under conditions of low Fe availability, graminaceous plants utilize Fe(III) chelaters known as mugineic acid family phytosiderophores (MAs) to absorb Fe.1 Biosynthesized MAs are secreted into rhizospheres through TOM1,2 where they chelate Fe(III). The resulting Fe(III)–MAs complex is taken up via yellow stripe 1-like (YSL) family transporters.3 The expression of many genes involved in MAs biosynthesis and Fe transport are upregulated coordinately in response to Fe deficiency in rice. Kobayashi et al.4,5 and Ogo et al.6,7 demonstrated that the transcription factors IDEF1, IDEF2, and OsIRO2 regulate crucial steps of gene regulation in response to Fe deficiency.
In contrast, cadmium (Cd) is toxic to living organisms. Cd-polluted soil, which is found across a wide global area and causes Cd accumulation in crops, is a growing threat to agriculture and human health. Cd-induced toxicity in plants disturbs the balance of essential metals in metalloenzymes.8,9 Cd is thought to be absorbed and translocated by the transporters of essential metals, including Fe, Zn, and Mn, which have chemical properties similar to those of Cd. Recently, several metal transporters such as OsNRAMP5 were shown to play a key role in Cd uptake from the soil into rice.10-12
The molecular mechanisms underlying the absorption of Fe and Cd from the soil and their distribution in plants are being explored by analyzing individual genes. However, numerous genes related to metal homeostasis remain uncharacterized. Therefore, we investigated the tissue expression profiles and changes in expression during the response to Fe deficiency and Cd stress using a combination of laser microdissection (LM) and rice microarrays.13 Genes encoding transporters involved in metal homeostasis, proteins associated with heavy metal detoxification, and phytohormone-related proteins were then investigated comprehensively. Rice roots grown under normal, Fe-deficient, or Cd-stressed conditions were separated by LM into 3 distinct tissue types: vascular bundles (central cylinder; VB), cortex (Cor), and exodermis plus epidermis (EP). In graminaceous plants, the lowest node of the shoots, known as the discrimination center (DC), is important for the distribution of metals and metabolites to leaves,14-16 and usage of xylem and/or phloem in Fe transport differs between new and old leaves.17 Therefore, vascular bundles in the DC from new and old leaves (newDC and oldDC, respectively) under normal and Fe-deficient conditions were isolated separately. RNA from each tissue was then extracted from 3 biological replicates, and 44 K rice microarrays were analyzed.
Clustering analysis was performed on the genes whose expression changed in at least one tissue or under one condition (Fig. 1). The large clusters formed by genes expressed in a tissue-specific manner in the VB, Cor, EP, newDC, or oldDC revealed a specific function of each tissue, such as long-distance transport, radial transport, absorption/secretion, and distribution to new or old leaves, respectively. Many genes expressed in the vascular bundles in DC (newDC and oldDC), particularly the newDC, were also expressed in the VB, although there were also numerous genes that were expressed differentially in the newDC/oldDC and VB (Fig. 1). These suggest that vascular bundles in DC and roots might have a common function, such as the long-distance transport of various kinds of molecules but also have distinct functions. The genes upregulated by Fe deficiency in all of VB, Cor, and EP formed a smaller cluster than the tissue-specific clusters, but it remained substantial. These genes included those of MAs biosynthetic enzymes, the transporters involved in Fe uptake, the Fe-deficiency inducible transcription factor OsIRO2, and the gibberellin-deactivating enzyme EUI (Fig. 1).
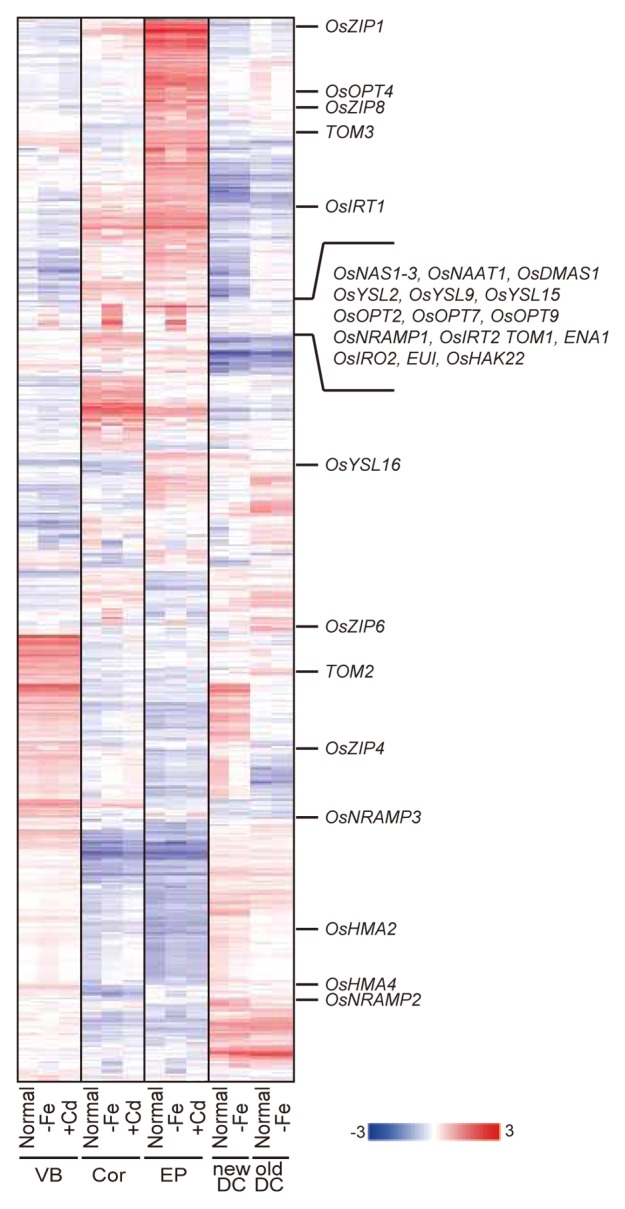
Figure 1. Expression profiles of the genes involved in metal homeostasis. Clustering analysis of genes whose expression changed in at least one tissue or under one condition. The gene-normalized signal intensities are shown in heat maps using a log10 scale. Hierarchical clustering was performed as described previously.13 OsNAS1–3, nicotianamine synthase; OsNAAT1, nicotianamine aminotransferase; OsDMAS1, deoxymugineic acid synthase; OsYSL2, Fe(II)–nicotianamine synthase; OsYSL15, 16, Fe(III)–DMA transporter; OsYSL9, YSL transporter with unknown substrates; OsIRT1, 2, Fe(II) transporter; TOM1, DMA efflux transporter; TOM2, 3, homologous genes of TOM1; ENA1, nicotianamine efflux transporter; OsIRO2, Fe deficiency-inducible transcription factor; OsNRAMP1, Fe transporter with broad substrates; OsNRAMP2 and 3, NRAMP transporters with unknown substrates; OsZIP4 and 8, Zn transporters; OsZIP1 and 6, ZIP transporters with unknown substrates; OsHMA2, Zn/Cd transporter; OsHMA4, HMA transporter with unknown substrates; EUI, gibberellin-deactivating enzyme; OsHAK22, high-affinity potassium (K+) transporter.
The expression patterns of the transporter families involved in metal homeostasis, YSL transporters, oligopeptide transporters (OPTs), major facilitator superfamily (MFS) antiporters, natural resistance-associated macrophage proteins (NRAMP), zinc-regulated transporter, iron-regulated transporter-like proteins (ZIPs), and heavy metal ATPase (HMA) were investigated. Their expressed tissues and expression changes caused by Fe deficiency are shown in Figure 2. Members of each family were either expressed differently in each tissue, or they showed different responses to Fe deficiency. The transporters expressed in a tissue-specific manner in the VB, Cor, and EP might be involved specifically in long-distance transport (OsNRAMP3, OsZIP6, OsHMA4, and OsHMA5), radial transport, and metal absorption (OsYSL16, TOM3, OsNRAMP5, and OsZIP8), respectively. The transporters expressed in newDC and oldDC might be involved in distributing metals to new leaves (OsYSL2, 7, 15, OsNRAMP1, 2, OsIRT2, OsZIP8, and OsHMA2) and old leaves (OsYSL17, OsOPT5), respectively. The upregulation of certain transporters by Fe deficiency in various tissues (such as OsYSL2, OsIRT2, TOM1, and ENA1) suggests that they are involved both in Fe uptake and translocation.
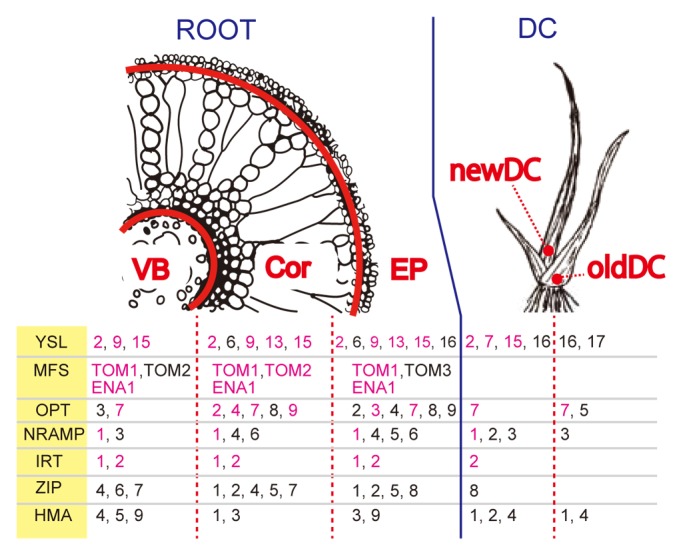
Figure 2. Schematic representation of the tissue expression and expression changes by Fe deficiency of representative transporters involved in metal homeostasis. VB, vascular bundle; Cor, cortex; EP, exodermis plus epidermis; newDC, vascular bundles from new leaves in the DC; oldDC, vascular bundles from old leaves in the DC. The genes listed in the Table are expressed in the indicated tissues. Genes in pink and black letters are upregulated and not upregulated by Fe deficiency, respectively, in the indicated tissue.
Numerous genes were upregulated by Fe deficiency in various tissues; the effect of several of these genes on Fe deficiency has not yet been elucidated. Among these, the potassium (K) transporter OsHAK22 was upregulated by Fe deficiency in the VB, Cor, and EP, whereas the other OsHAKs were not upregulated significantly by Fe deficiency in any tissue (Fig. 3). OsHAK22 is also upregulated during the early stages of Fe deficiency.17 MAs are thought to be secreted as monovalent anion, and equamoler potassium was secreted while MAs secretion.19-21 Fe-deficient barley roots contain more K than Fe-sufficient barley roots, and K in barley roots is dramatically decreased after secretion of MAs,22 suggesting that a large amount of K is transferred into and from root cells during Fe deficiency. However, the precise mechanism of K secretion was unclear. The expression pattern of OsHAK22 was similar to that of the MAs efflux transporter TOM1, which is upregulated by Fe deficiency in the VB, Cor, and EP. OsHAK22 is thought to be involved in the K transport associated with MAs secretion and is expressed in a coordinated manner with TOM1.
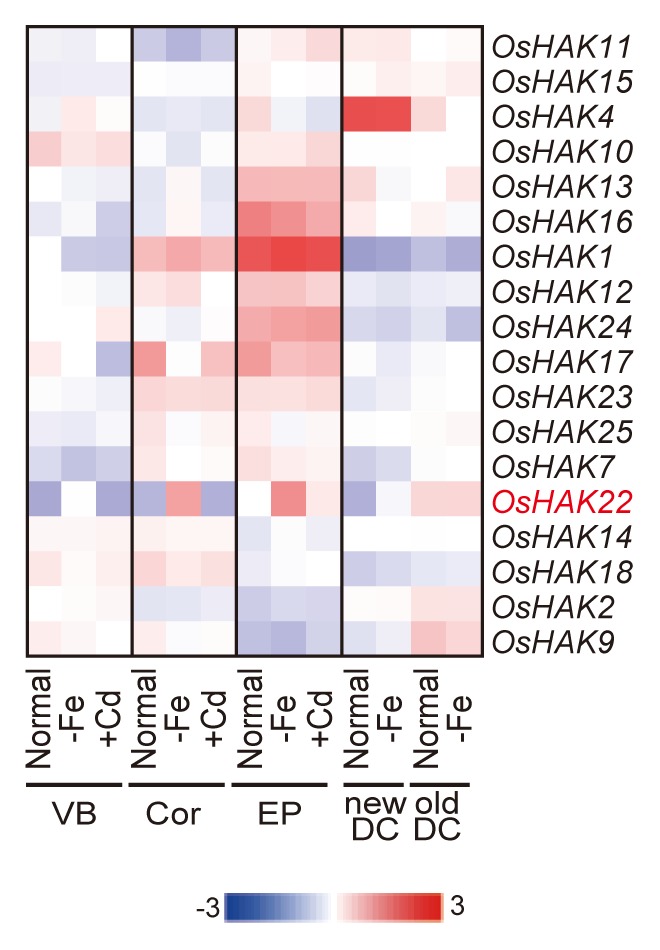
Figure 3. Expression profiles of the KT-HAK-KUP potassium transporter family in rice. The rice KT-HAK-KUP family genes (OsHAK1–25) were described by Amrutha et al.18
In conclusion, this study provides useful information for understanding the molecular mechanisms involved in metal absorption, transport, and distribution, and makes a significant contribution to identifying the tissue-specific responses to metal stress.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- Fe
iron
- MA
mugineic acid
- YSL
yellow stripe 1-like
- DC
discrimination center
- Cd
cadmium
- LM
laser microdissection
- OPT
oligopeptide transporter
- MFS
major facilitator superfamily
- NRAMP
natural resistance-associated macrophage protein
- ZIP
zinc-regulated transporter, iron-regulated transporter-like proteins
- IRT1
Iron-Regulated Transporter 1
- HMA
Heavy Metal ATPase
References
- 1.Takagi S. Naturally occuring iron-chelating compounds in oat- and rice-root washing I Activity measurement and preliminary characterization. Soil Sci Plant Nutr. 1976;22:423–33. doi: 10.1080/00380768.1976.10433004. [DOI] [Google Scholar]
- 2.Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem. 2011;286:5446–54. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem. 2009;284:3470–9. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Ogo Y, Itai RN, Nakanishi H, Takahashi M, Mori S, Nishizawa NK. The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc Natl Acad Sci U S A. 2007;104:19150–5. doi: 10.1073/pnas.0707010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi T, Itai RN, Ogo Y, Kakei Y, Nakanishi H, Takahashi M, Nishizawa NK. The rice transcription factor IDEF1 is essential for the early response to iron deficiency, and induces vegetative expression of late embryogenesis abundant genes. Plant J. 2009;60:948–61. doi: 10.1111/j.1365-313X.2009.04015.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 2007;51:366–77. doi: 10.1111/j.1365-313X.2007.03149.x. [DOI] [PubMed] [Google Scholar]
- 7.Ogo Y, Kobayashi T, Nakanishi Itai R, Nakanishi H, Kakei Y, Takahashi M, Toki S, Mori S, Nishizawa NK. A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. J Biol Chem. 2008;283:13407–17. doi: 10.1074/jbc.M708732200. [DOI] [PubMed] [Google Scholar]
- 8.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–36. doi: 10.1016/0891-5849(94)00159-H. [DOI] [PubMed] [Google Scholar]
- 9.Schützendübel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot. 2002;53:1351–65. doi: 10.1093/jexbot/53.372.1351. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci U S A. 2012;109:19166–71. doi: 10.1073/pnas.1211132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, Ono K, Yano M, Ishikawa S, Arao T, et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep. 2012;2:286. doi: 10.1038/srep00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki A, Yamaji N, Yokosho K, Ma JF. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell. 2012;24:2155–67. doi: 10.1105/tpc.112.096925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogo Y, Kakei Y, Itai RN, Kobayashi T, Nakanishi H, Takahashi H, et al. Spatial transcriptomes of iron-deficient and cadmium-stressed rice. New Phytol. 2013 doi: 10.1111/nph.12577. [DOI] [PubMed] [Google Scholar]
- 14.Kiyomiya S, Nakanishi H, Uchida H, Nishiyama S, Tsukada H, Ishioka NS, Watanabe S, Osa A, Mizuniwa C, Ito T, et al. Light activates H2 15O flow in rice: Detailed monitoring using a positron-emitting tracer imaging system (PETIS) Physiol Plant. 2001;113:359–67. doi: 10.1034/j.1399-3054.2001.1130309.x. a. [DOI] [PubMed] [Google Scholar]
- 15.Kiyomiya S, Nakanishi H, Uchida H, Tsuji A, Nishiyama S, Futatsubashi M, Tsukada H, Ishioka NS, Watanabe S, Ito T, et al. Real time visualization of 13N-translocation in rice under different environmental conditions using positron emitting Ttacer imaging system. Plant Physiol. 2001;125:1743–53. doi: 10.1104/pp.125.4.1743. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukamoto T, Nakanishi H, Uchida H, Watanabe S, Matsuhashi S, Mori S, Nishizawa NK. (52)Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol. 2009;50:48–57. doi: 10.1093/pcp/pcn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itai RN, Ogo Y, Kobayashi T, Nakanishi H, Nishizawa NK. Rice genes involved in phytosiderophore biosynthesis are synchronously regulated during the early stages of iron deficiency in roots. Rice (N Y) 2013;6:16. doi: 10.1186/1939-8433-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amrutha RN, Sekhar PN, Varshney RK, Kishor PBK. Genome-wide analysis and identification of genes related to potassium transporter families in rice (Oryza sativa L.) Plant Sci. 2007;172:708–21. doi: 10.1016/j.plantsci.2006.11.019. [DOI] [Google Scholar]
- 19.Takagi S, Kamei S, Kawai S. Preliminary characterization of mugineic acid secretory transport system in barley roots. In: Abstracts of 5th International Symposium on Iron Nutrition and Interactions in Plants. Israel: 1989, 100. [Google Scholar]
- 20.Takagi S. Mugineic acid. In: Nutrition and Physiology of Metal Relating Compounds. Chino M, ed. Tokyo: Hakuyusya Press, 1991; 5-21. [Google Scholar]
- 21.Mori S. Mechanisms of Iron Acquisition by Graminaceous (Strategy II) Plants. In: Biochemistry of Metal Micronutrients in the Rhizosphere. Manthey JA, Crowley DE, Luster DG. Boca Raton: CRC Press Inc, 1994; 225-249. [Google Scholar]
- 22.Sakaguchi T, Nishizawa NK, Nakanishi H, Yoshimura E, Mori S. The role of potassium in the secretion of mugineic acids family phytosiderophores from iron-deficient barley roots. Plant Soil. 1999;215:221–7. doi: 10.1023/A:1004546112140. [DOI] [Google Scholar]


