Abstract
Donor lymphocyte infusion (DLI) is an established and potentially curative immune therapy for relapsed leukemia after hematopoietic stem cell transplant (HSCT). Herein, we describe the utility of DLI as a tractable model system to glean fresh insights into understanding and predicting effective anti-leukemia immunity.
Keywords: T cell exhaustion, donor lymphocyte infusion, CML, stem cell transplantation, tumor-infiltrating lymphocytes
Convincingly demonstrating the potency of the graft-vs-leukemia (GvL) effect, DLI has remained a cornerstone of therapy for hematologic relapse after HSCT since its initial discovery in 1990.1,2 Subsequently, its widespread adoption unveiled a varied spectrum of clinical activity, with chronic myelogenous leukemia (CML) exhibiting an exquisite sensitivity.3
However, complications of DLI exist, including graft-vs.-host disease (GvHD) and the development of marrow aplasia. Efforts to divorce the morbidity of GvHD from an active GvL effect led to CD8+ T cell depletion from the donor graft. Infusion of CD8+-depleted (CD4+) DLI mitigated GvHD risk while preserving GvL activity in multiple settings.1,3,4
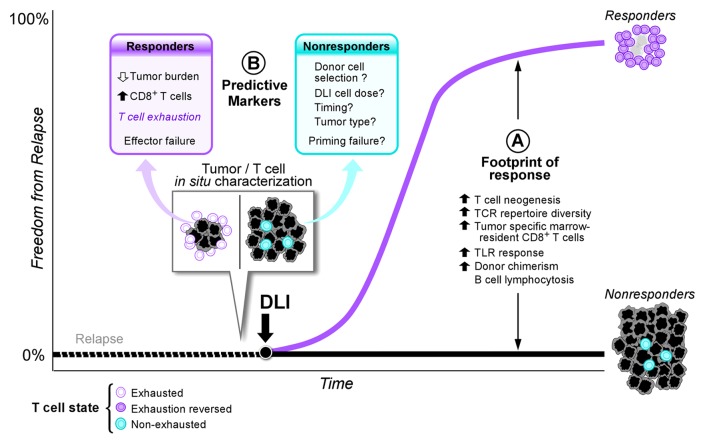
Mechanistic insights into DLI’s GvL effect stem mainly from studies of CD4+ DLI responses. Immune effector analyses uncovered a “footprint of response” occurring both systemically in peripheral blood and locally within bone marrow, the site of disease (Fig. 1A). Systemic effects of DLI on both cellular and humoral immunity include increases both in T cell neogenesis and TCR repertoire diversity coupled with induction of B cell lymphocytosis and development of tumor-specific antibodies.1,5 Moreover, effective DLI responses elicit innate immune involvement via systemic TLR 8/9 activation.6 Finally, local expansion of preexisting tumor-specific marrow-resident CD8+ T cells after DLI further define the footprint of response distinguishing responders from nonresponders in the wake of DLI treatment (Fig. 1A).5
Figure 1. Signatures of DLI therapy. A “footprint of response” exists downstream of DLI therapy that distinguishes responders from nonresponders (A) Comprising immunostimulatory benefits in both adaptive and innate immunity, this footprint elucidates the diverse consequences of DLI; however, studies to detect these signals have not been generally designed to predict the likelihood of benefit. (B) On the other hand, detailed characterization of pre-DLI in situ tumor and T cell infiltrates identifies novel predictive markers of DLI responsiveness. In addition, T cell exhaustion is uncovered as a potential mechanism of efficacy. Outstanding questions include further definition of the mechanisms driving DLI resistance in the nonresponding population.
Yet while downstream events correlating to DLI response have been well characterized, the identification of biologic response predictors and understanding of the precise basis of DLI effectiveness remain elusive. To address this question, we exploited the historically clear response of CML to DLI and retrospectively analyzed marrow and peripheral blood samples from patients with relapsed CML after HSCT pre- and post-treatment with CD4+ DLI, all from the pre-imatinib era. Our study cohort of 22 responders and 7 nonresponders identified predictive markers of DLI response that elucidated a mechanistic basis for DLI efficacy.7 Three key lessons derive from our study (Fig. 1B):
In Situ Characterization of Tumor and T Cells
We learned that in situ (marrow) T cell responses served as a more accurate indicator of T cell immunity than those found in peripheral blood. Whereas no inter-cohort differences in the temporal kinetics of T cells manifested peripherally, we noted significantly different temporal patterns of CD8+ T cells infiltrating the marrow before and after DLI between the 2 cohorts. These results suggest that local anti-tumor immune responses can be modulated systemically.
Identification of Novel Predictive Markers
The robust DLI response rate in CML also enabled us to pinpoint novel predictive markers. Similar to others, we noted an inverse relationship between tumor burden and likelihood of response.1,3,8 Additionally, we found that an increased preexisting CD8+ T cell infiltrate correlated with response, even in patients with high disease burden. In fact, incorporation of pre-DLI “burden” of both tumor and CD8+ T cells in response prediction perfectly distinguished our cohort with 100% sensitivity/specificity. Thus, the pretreatment immunologic state of the marrow, specifically preexisting CD8+ T cell infiltrates, emerged as a strong novel predictor of DLI response.
T Cell Exhaustion and Outstanding Questions
Cancers employ a barrage of immunoevasive strategies including T cell exhaustion—a dysfunctional state transcriptionally distinct from anergy or senescence that is induced by chronic antigen exposure and marked by loss of effector and proliferative functions.9 Transcriptional profiling of infiltrating CD3+ T cells revealed enrichment of exhaustion gene sets in responders before DLI and reversal of discrete exhaustion modules after therapy. These data strongly implicate this key pathway as a potential marker and mechanism of DLI responsiveness in relapsed CML after HSCT. Intriguingly, the clinical debut of anti-PD1/PDL1 antibodies that may reverse T cell exhaustion suggests their use in lieu of DLI to promote GvL responses after allogeneic HSCT.
The involvement of T cell exhaustion in predicting DLI response suggests effector failure of an anti-tumor immune response wherein DLI responders harbor a reservoir of infiltrating anti-tumor CD8+ T cells that have presumably already encountered CML tumor antigens (thus are exhausted). Given that these T cells have already achieved tumor specificity, immunological “help” in the form of CD4+ T cells may be sufficient to reinvigorate a dormant anti-tumor response.
Of course, many questions persist. Our small, though well-defined, cohort should be expanded to determine applicability to other hematologic malignancies. More importantly, what occurs in the nonresponder’s tumor milieu (Fig. 1B)? Our data argue that the tumor microevironment of nonresponders harbors very few preexisting CD8+ T cells that lack phenotypic evidence of prior strong antigenic activation. Hence, these T cells are perhaps incapable of mounting a specific and potent anti-tumor response. Infusions of alternate donor grafts, perhaps containing activated CD8+ T cells, could be considered for these patients.3 Increasing the graft cell dose is another possibility, and risk-adapted strategies targeting minimal residual disease states may improve DLI efficacy for this population by taking advantage of a lower tumor burden.3 Finally, given the failure of effector immunity in responders, it is tempting to speculate an upstream failure of priming consistent with reduced T cell infiltrates in those without response. Such a scenario may be particularly amenable to multi-epitope tumor vaccination strategies.10
Nevertheless, future delineation of immunoevasive maneuvers deployed by leukemias resistant to DLI may prove feasible with the use of next-generation sequencing and T cell profiling technologies. Concordantly, the mechanisms driving DLI efficacy may prove quite relevant to other adoptive cell transfer therapies that promise anti-leukemia potential. DLI, then, has become a familiar face in the treatment arsenal against leukemic relapse whose study remains informative today.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- DLI
donor lymphocyte infusion
- HSCT
hematopoietic stem cell transplant
- GvL
graft-vs-leukemia
- GvHD
graft-vs-host-disease
- CML
chronic myelogenous leukemia
Citation: Bachireddy P, Wu CJ. Understanding anti-leukemia responses to donor lymphocyte infusion. OncoImmunology 2014; 3:e28187; 10.4161/onci.28187
References
- 1.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–73. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HJ, Mittermüller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. [PubMed] [Google Scholar]
- 3.Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473–87. doi: 10.1517/14712598.2011.554811. [DOI] [PubMed] [Google Scholar]
- 4.Soiffer RJ, Alyea EP, Hochberg E, Wu C, Canning C, Parikh B, Zahrieh D, Webb I, Antin J, Ritz J. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol Blood Marrow Transplant. 2002;8:625–32. doi: 10.1053/bbmt.2002.v8.abbmt080625. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Choi J, Zeng W, Rogers SA, Alyea EP, Rheinwald JG, Canning CM, Brusic V, Sasada T, Reinherz EL, et al. Graft-versus-leukemia antigen CML66 elicits coordinated B-cell and T-cell immunity after donor lymphocyte infusion. Clin Cancer Res. 2010;16:2729–39. doi: 10.1158/1078-0432.CCR-10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Y, Zhang L, Cai AX, Lee M, Zhang W, Neuberg D, Canning CM, Soiffer RJ, Alyea EP, Ritz J, et al. Effective posttransplant antitumor immunity is associated with TLR-stimulating nucleic acid-immunoglobulin complexes in humans. J Clin Invest. 2011;121:1574–84. doi: 10.1172/JCI44581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachireddy P, Hainz U, Rooney M, Pozdnyakova O, Aldridge J, Zhang W, Liao X, Hodi FS, O’Connell K, Haining WN, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123:1412–21. doi: 10.1182/blood-2013-08-523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, Volin L, Gürman G, Maertens J, Bordigoni P, et al. EBMT Acute Leukemia Working Party Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–45. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 10.Burkhardt UE, Hainz U, Stevenson K, Goldstein NR, Pasek M, Naito M, Wu D, Ho VT, Alonso A, Hammond NN, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest. 2013;123:3756–65. doi: 10.1172/JCI69098. [DOI] [PMC free article] [PubMed] [Google Scholar]