Abstract
Plants often learn from previous infections to mount higher level of resistance during subsequent infections, a phenomenon referred to as systemic acquired resistance (SAR). During primary infection, mobile signals generated at the infection site subsequently move to the rest of plant to activate SAR. SAR activation is associated with alteration in the nucleosomal composition at the promoters of several defense-related genes. However, genetic regulations of such epigenetic modifications are largely obscure. Recently, we have demonstrated that Reduced Systemic immunity1/FLOWERING LOCUS D (RSI1; alias FLD) a homolog of human histone demethylase, is required for SAR development in Arabidopsis. Here, we report that exogenous application of a histone demethylase inhibitor trans-2-phenylcyclopropylamine (2-PCPA) mimics rsi1/fld loss-of-function phenotypes in terms of SAR and associated histone demethylation at the promoters of PR1, WRKY 29, and WRKY6 genes, and as well as flowering phenotypes. Our results suggest histone demethylase activity of FLD is important for controlling SAR activation.
Keywords: Arabidopsis, FLD, flowering, H3K4me2, 2-PCPA, RSI1, SAR, WRKY
Plants, when challenged with pathogens, induce strong resistance at the site of infection to control the spread of the pathogen. In addition, plants often retain the infection memory to activate faster defense responses that provide SAR against subsequent infections.1 The effect of SAR is long lasting and protects against a wide range of pathogens. At the primary infection site, plants synthesize mobile signals, such as salicylic acid and its methylated derivative, azelaic acid, glycerol-3-phosphate, dehydro-abietinalditerpenoid, and pipecolic acid that move to the distal tissues to induce SAR.2-7 The distal tissues upon receiving the SAR inducing signals achieve preparedness for mounting faster defense response during secondary infections, a phenomenon referred to as priming.8 There is substantial evidence that priming is associated with epigenetic modifications at the promoters of defense related genes.9,10 Epigenetic modifications can be long lasting and inherited through meiosis over successive generations.9 Despite knowing the large numbers of mobile signals and their interactions, it is not known how these mobile signals link to alteration chromosomal compositions associated with SAR development.
Earlier we had shown that the FLD gene is required for SAR activation in Arabidopsis.11 Through mutagenesis of wild-type (WT) Arabidopsis and screening for SAR deficient mutants, we identified reduced systemic immunity 1 (rsi1) that is defective in SAR but not in local resistance. Through further map-based positional cloning we identified rsi1 as allelic to FLD. FLD functions at the downstream of mobile signals in the distal tissues to activate SAR. Similar to fld loss-of-function plants, the rsi1 mutant shows delayed flowering phenotype.11,12 FLD codes for a homolog of human lysine specific demethylase 1 (LSD1); In accordance, the fld mutants contain higher levels of H3K4me2.13 Further we reported that rsi1/fld mutation affects H3K4me2 accumulation at the promoters of several WRKY genes.14 However, it was not known whether histone demethylase activity of FLD is required for SAR activation.
In order to investigate the requirement of histone-demethylase activity for SAR, we exogenously applied trans-2-phenylcyclopropylamine (2-PCPA), which is widely used for inhibiting LSD1 activity15,16 and followed SAR activation in Arabidopsis. Five-week-old Arabidopsis plants were SAR induced with Pseudomonas syringae pv tomato DC3000 carrying AvrRpt2 gene (Avr-Pst) at 107 CFU/ml concentration, suspended in 10 mM MgCl2, or mock induced with 10 mM MgCl2 by infiltrating 3 lower leaves.11,14,17 After 3 days of primary inoculation, both SAR and mock induced plants were treated with same dose of Pseudomonas syringae pv maculicola ES4326 (Psm) (5 X 105 CFU/ml) and bacterial numbers were determined after 3 days of secondary inoculation. To study the effect of inhibitor, in parallel sets, 2-PCPA was co-applied along with either primary or secondary inoculations at 10mM final concentrations. We observed that the exogenous 2-PCPA application, either during primary or secondary inoculations, effectively blocks SAR induction in Arabidopsis (Fig. 1A). However, 2-PCPA does not affect growth of bacteria in plants (Fig. 1B), suggesting that the effect of 2-PCPA is SAR specific, similar to fld/rsi1 mutant.
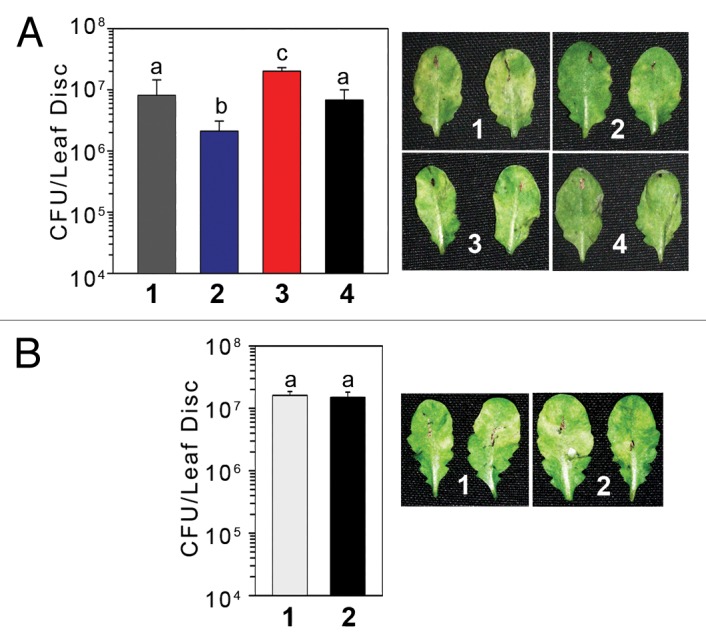
Figure 1. Effect of 2-PCPA on SAR and local resistance. (A) Bacterial numbers and disease symptoms after 3 d of challenge inoculation with Psm (2°). The plants were either pre-treated with 10 mM MgCl2 or Avr-Pst (1°) at 1 X 107cfu/ml with or without 2-PCPA 3 d before the challenge inoculation. Secondary challenge inoculation in all plants were performed with Psm at 5 X 105 CFU/ml (2 o). 1- 1° treatment with 10 mM MgCl2 and 2° treatment with Psm. 2- 1° treatment with Avr-Pst and 2° treatment with Psm, 3 - 1° treatment with Avr-Pst + 2-PCPA and 2° treatment with Psm, 4 - 1° treatment with Avr-Pst, and 2° treatment with Psm+ 2-PCPA. (B) Psm counts from locally inoculated leaves and disease symptom at 3 dpi with or without 2-PCPA.Inoculation was done at 5X105 CFU/ml. Each bar represents the mean ± standard deviation (SD) of 4 samples each carrying 5 leaf discs of 5 mm diameters randomly taken from different plants. Letters above the bars indicate values that are significantly different (P < 0.05) from each other as analyzed by one-way ANOVA (post hoc Holm Sidak method).
Though putative function of FLD is to remove methylations from histones, the fld mutants accumulate reduced levels of H3K4me2 at the promoters of several WRKY genes including WRKY29 and WRKY6.14 It was predicted to be an indirect effect of FLD on defense-related promoters.14 Nevertheless, in order to examine whether 2-PCPA application exerts similar effects on these promoters, we performed Chromatin-immuno-precipitation (ChIP) using anti-H3K4me2 antibody, as described earlier.14 Arabidopsis leaves were infiltrated with 10 mM 2-PCPA and samples were harvested after 48 hours for the ChIP experiment. Chromatins were precipitated using anti-H3K4me2 antibody (Cat#16–157, Millipore, USA). Relative occupancy of H3K4me2 was determined by quantitative real-time PCR (qRT-PCR) and plotted as fold difference with ACTIN2 (At3g18780).14 As shown in Figure 2, 2-PCPA application effectively reduces H3K4me2 accumulation at the promoter of PR1, WRKY29, and WRKY6 genes. This effect of 2-PCPA is also highly similar to fld loss-of-function phenotypes.
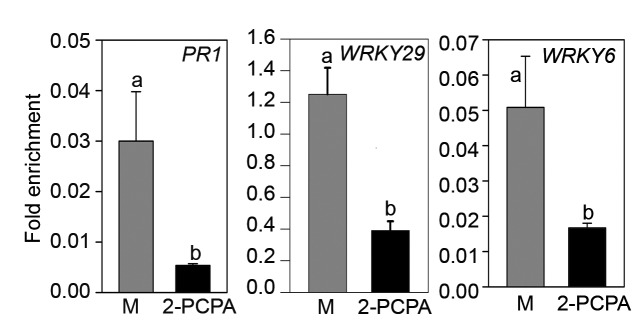
Figure 2. Effect of 2-PCPA on H3K4me2 occupancy in promoters of defense related genes. Five-week-old wild type plants were infiltrated with either 10mM MgCl2 (M) or 10 mM of 2-PCPA. Three days later upper untreated leaves were harvested, and chromatin isolated from these leaves was precipitated with anti-H3K4me2 antibody. Abundance of individual target loci (relative to ACTIN 2) that were associated with H3K4me2 was determined by real-time PCR. Each bar represents the mean ± SD (n = 3). Different letters above the bars indicate values that are significantly different (P < 0.05) from each other as determined by one-way ANOVA (Holm-Sidak method).
FLD negatively regulates floral repressor FLC and thus promotes flowering. Therefore, the fld mutants are delayed in flowering.11,12 However, the flowering phenotype of FLD is not associated with SAR development. The flowering phenotype is rescued in fld flc double mutant but not the SAR phenotype.11 However, since FLD affects both the phenotypes, we anticipated that 2-PCPA would also affect flowering phenotype. We grew Arabidopsis under normal growth conditions for 3 weeks, after which we applied 10 µl of 2-PCPA (10 mM dissolved in water) per leaf, in 3 leaves of each plant. The control plants received only water. The treatment was repeated once a week until plants showed transition. We found significant delay in flowering in the 2-PCPA treated plants, compared with water treated plants. In our growth conditions, at 12 h-12 h light-dark cycle, Arabidopsis plants flower at the end of 5 weeks. The 2-PCPA treated plants took 7 to 9 weeks to flower, and also produced more rosette leaves (Fig. 3).
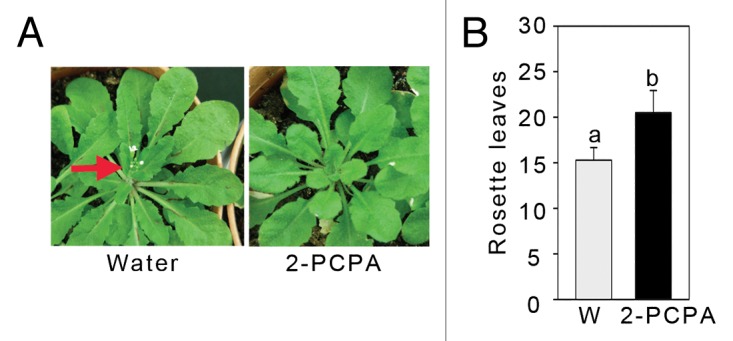
Figure 3. Effect of 2-PCPAon flowering. (A) Flowering phenotypes after treatment with water or 2-PCPA. Arrow indicates flowering in water treated plants. (B) Rosette leaves number at the time of transition. Leaves were counted just before the transition took place. Plants were kept in photoperiod condition 12 h-12 h, light-dark condition. Letters above the bars indicate values that are significantly different (P < 0.05) from each other as analyzed by one-way ANOVA (post hoc Holm Sidak method).
Our results altogether suggest that 2-PCPA application inhibits FLD function in Arabidopsis, and with the established role of histone demethylase inhibition activity of 2-PCPA, we may infer that histone-demethylase activity is required for SAR activation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by Department of Biotechnology (DBT) grant (BT/PR14656/ BRB/10/864/2010) to A.K.N. Z.Z.B. is a recipient of an ICMR fellowship.
Glossary
Abbreviations:
- RSI1
Reduced Systemic Immunity 1
- FLD
Flowering Locus D
- H3K4me2
Dimethylated Histone3 at Lysine4
- ChIP
Chromatin immuno-precipitation
- LSD1
Lysine Specific Demethylase 1
- SAR
Systemic Acquired Resistance
- 2-PCPA
Trans-2-phenylcyclopropylamine
References
- 1.Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–63. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- 2.Návarová H, Bernsdorff F, Döring AC, Zeier J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell. 2012;24:5123–41. doi: 10.1105/tpc.112.103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi R, Venables B, Petros RA, Nalam V, Li M, Wang X, Takemoto LJ, Shah J. An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 2012;71:161–72. doi: 10.1111/j.1365-313X.2012.04981.x. [DOI] [PubMed] [Google Scholar]
- 4.Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–6. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey DA, Klessig DF. SOS - too many signals for systemic acquired resistance? Trends Plant Sci. 2012;17:538–45. doi: 10.1016/j.tplants.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- 7.Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet. 2011;43:421–7. doi: 10.1038/ng.798. [DOI] [PubMed] [Google Scholar]
- 8.Conrath U, Pieterse CM, Mauch-Mani B. Priming in plant-pathogen interactions. Trends Plant Sci. 2002;7:210–6. doi: 10.1016/S1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- 9.Luna E, Bruce TJ, Roberts MR, Flors V, Ton J. Next-generation systemic acquired resistance. Plant Physiol. 2012;158:844–53. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaskiewicz M, Conrath U, Peterhänsel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12:50–5. doi: 10.1038/embor.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh V, Roy S, Giri MK, Chaturvedi R, Chowdhury Z, Shah J, Nandi AK. Arabidopsis thaliana FLOWERING LOCUS D is required for systemic acquired resistance. Mol Plant Microbe Interact. 2013;26:1079–88. doi: 10.1094/MPMI-04-13-0096-R. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Michaels SD, Amasino RM. Regulation of flowering time by histone acetylation in Arabidopsis. Science. 2003;302:1751–4. doi: 10.1126/science.1091109. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Quesada V, Crevillén P, Bäurle I, Swiezewski S, Dean C. The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol Cell. 2007;28:398–407. doi: 10.1016/j.molcel.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Singh V, Roy S, Singh D, Nandi AK. Arabidopsis flowering locus D influences systemic-acquired-resistance- induced expression and histone modifications of WRKY genes. J Biosci. 2014;39:119–26. doi: 10.1007/s12038-013-9407-7. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt DM, McCafferty DG. trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46:4408–16. doi: 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- 16.Neelamegam R, Ricq EL, Malvaez M, Patnaik D, Norton S, Carlin SM, Hill IT, Wood MA, Haggarty SJ, Hooker JM. Brain-penetrant LSD1 inhibitors can block memory consolidation. ACS Chem Neurosci. 2012;3:120–8. doi: 10.1021/cn200104y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandi A, Welti R, Shah J. The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell. 2004;16:465–77. doi: 10.1105/tpc.016907. [DOI] [PMC free article] [PubMed] [Google Scholar]


