Abstract
Volatile interactions between unattacked plants can lead to changes in their volatile emissions. Exposure of potato plants to onion plant volatiles results in increased emission of 2 terpenoids, (E)-nerolidol and TMTT. We investigated whether this is detectable by the ladybird Coccinella septempunctata. The odor of onion-exposed potato was significantly more attractive to ladybirds than that of unexposed potato. Further, a synthetic blend mimicking the volatile profile of onion-exposed potato was more attractive than a blend mimicking that of unexposed potato. When presented individually, TMTT was attractive to ladybirds whereas (E)-nerolidol was repellent. Volatile exchange between unattacked plants and consequent increased attractiveness for ladybirds may be a mechanism that contributes to the increased abundance of natural enemies in complex plant habitats.
Keywords: (E)-nerolidol, Coccinella septempunctata, TMTT, aphids, ladybird, natural enemies, onion, plant–plant communication, potato, volatiles
Plants release volatile organic compounds in the course of their normal physiological activities. These volatiles may be received by neighboring plants, which makes plant–plant interaction via volatiles a continuous and dynamic process. Plant volatiles have an important role in mediating multi-trophic interactions; between plants, phytophagous insects, and herbivore natural enemies. The emission of volatiles from plants is significantly increased under stress conditions, caused by abiotic,1,2 or biotic factors.3,4 These volatile chemicals released by plants are available as signals for neighboring plants. For example, volatiles from damaged plants induce responses in neighboring undamaged plants, changing their volatile emission5 and making them less attractive to herbivores6 and more attractive to herbivore natural enemies.7 However, it has been shown that volatile interaction between unattacked plants8 can also occur, reducing attractiveness of the receiving plants to insect herbivores.9-11 Further, volatile interaction between unattacked plants can also lead to attraction of predatory insects, despite the absence of prey feeding on the plants.8,12,13
Increasing diversity of plant species, or even the presence of different genotypes of the same plant species within an environment, has an impact on the abundance of phytophagous insects and their natural enemies.9,13-15 Despite numerous studies on these effects, knowledge of the underlying mechanisms is still limited. Recently we have shown that volatile interaction between unattacked plants can significantly change volatile profile of potato plants after exposure to volatile chemicals from onion, making them less attractive for the aphid Myzus persicae.16 In the field, it was found that migration of M. persicae into an intercrop, where onion plants were grown alongside the potato, was significantly reduced than in potato grown in pure stand. The study found around 4 times greater concentrations of the terpenoids (E)-nerolidol and TMTT in the headspace of potato plants previously exposed to volatiles from onion compared with the headspace of unexposed plants. This showed for the first time that volatile chemical exchange between unattacked plants can cause responding plants to change their volatile profiles. In subsequent laboratory experiments, a synthetic volatile blend mimicking the headspace of potato plants previously exposed to onion was significantly less attractive to M. persicae than a blend based on headspace of unexposed potato, and (E)-nerolidol and TMTT repelled aphids when tested individually, showing similar behavioral responses to the odor of living plants.
If volatile interaction between unattacked plants leads to reduced numbers of herbivores, could such communication contribute to understanding of the mechanisms underlying the increased abundance of their natural enemies in botanical diverse habitats? A number of studies have examined the effects of HIPVs on the behavior and effectiveness of natural enemies including parasitic wasps,17-19 predatory mites,4,20,21 and ladybirds.22-25 In nature, however, not every plant is attacked by herbivores, particularly not in species rich habitats where the number of phytophagous insects is reduced, and few studies have examined the effects of volatile interaction between undamaged plants on the behavior of herbivore natural enemies.8,12,13
In the present study we investigated whether volatile interaction between unattacked onion and potato had an effect on the 7-spot ladybird Coccinella septempunctata, an important natural enemy of aphids. Plants were grown in a greenhouse maintained at 18–22 °C with a light regime of L16:D8. Ladybirds were fed on different aphid species and pollen under same conditions as the test-plants. Olfactory responses of ladybirds were measured using a 2-way airflow olfactometer.8 When plants were used as an odor source, 5 different treatment arrangements were designed: a potato plant that had been previously exposed to an onion plant compared with an unexposed potato plant; an odor mixture of potato and onion compared with an odor mixture of 2 potato plants; an unexposed potato plant compared with an onion plant; an unexposed potato plant compared with soil without a plant; an onion plant compared with soil without a plant. Exposures were made in a series of “2-chamber cages.”16
To investigate ladybird olfactory response to the synthetic chemical blends which mimicked the volatile profiles of onion-exposed and unexposed potatoes, and also their behavioral activity to the compounds (E)-nerolidol and TMTT, we conducted dose-response olfactometer experiments based on previous odor collections from the plants.16 Ladybird response to the synthetic blend of exposed potato plants was tested against the synthetic blend of unexposed potato plants. Test concentrations were 1/100, 1/10, 1x, 10x, and 100x the concentration of volatiles collected from plants. The chemicals were tested against redistilled n-hexane in 5 different concentrations: 0.01ng, 0.1ng, 1ng, 10ng, and 100ng. Wilcoxon matched pairs test was used for comparisons of the number of ladybird visits in each olfactometer arm.26
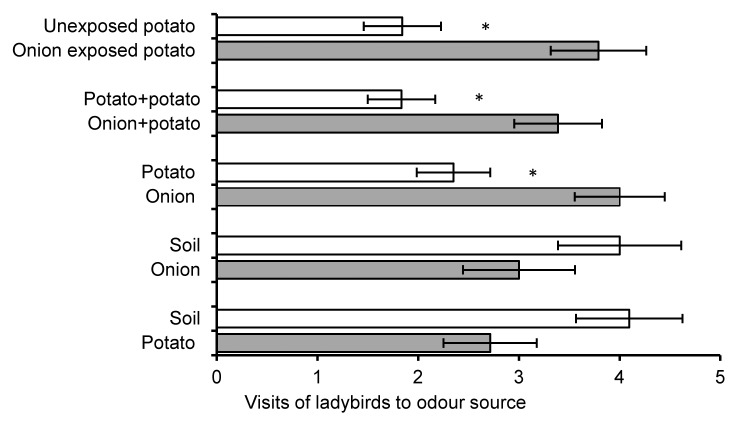
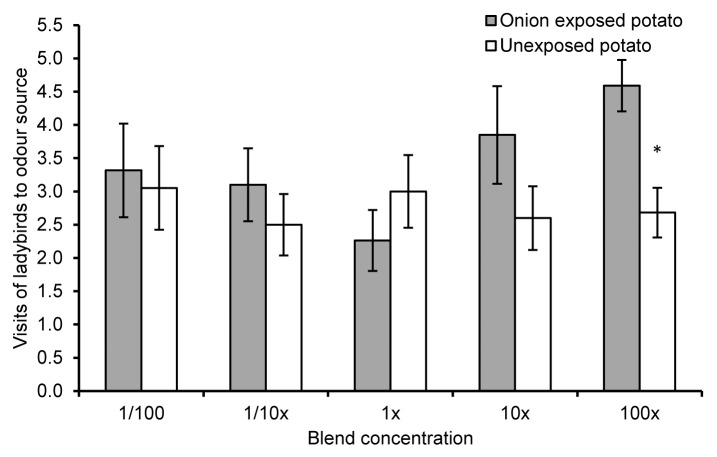
We found that potato exposed to volatiles from onion was more attractive to ladybirds than unexposed potato (Z = 2.19, P = 0.03, n = 16) (Fig. 1). Further, a synthetic chemical blend which mimicked the volatile profile of onion-exposed potato was significantly more attractive for ladybirds than a synthetic blend mimicking unexposed potato (Z = 2.2, P = 0.03, n = 22) at the highest concentration tested (Fig. 2). It has been shown previously that M. persicae behaved in the opposite way; odor of exposed potato was less attractive than unexposed potato, and a synthetic blend based on exposed potato headspace was repellent at the highest concentration tested.16 It is interesting that these relatively minor changes in the volatile profile are detectable by 2 polyphagous insects such as M. persicae and C. septempunctata.
Figure 1. Ladybird olfactory responses to volatiles from plants. Behavioral responses of ladybirds to volatiles from plants. Error bars indicate ± SEM. Asterisks indicate statistical significance levels of * P ≤ 0. 05, and *** P ≤ 0. 001 (Wilcoxon matched pairs test).
Figure 2. Ladybird olfactory responses to synthetic blends of volatile organic compounds of onion-exposed and unexposed potato. Behavioral responses of ladybirds to synthetic blends of volatile organic compounds of potato plants that had been previously exposed (treatment) and unexposed (control) to onion plants. Synthetic blends were at 1/100, 1/10, 1, 10, and 100 times the original concentration of volatiles identified in potato headspace. Error bars indicate ± SEM. Asterisks indicate statistical significance levels of * P ≤ 0. 05 (Wilcoxon matched pairs test).
Ladybirds in the present study were strongly attracted by the mixture of potato and onion compared with potato alone (Z = 2.16, P = 0.03, n = 14) (Fig. 1). Responses to odor mixtures have been shown previously in this ladybird. In laboratory and field experiments with different varieties of barley, ladybirds preferred a specific combination of barley varieties over single varieties alone,12,13 and also responded positively to mixtures of barley and weeds, both in the field and with odors in the laboratory,8 suggesting a preference for foraging in species-rich habitats.
In the present study, ladybirds did not prefer odor of potato or onion over soil alone, suggesting that volatiles of the single healthy plants were not attractive for them. However, ladybirds did prefer onion when given a choice between odor of onion and potato (Z = 2.09, P = 0.04, n = 20) (Fig. 1). Plant volatiles that absorbed on neighboring plant surfaces can considerably change the volatile profile of the exposed plant with their re-emission.27 In our previous study, chemical analysis of the plant headspace showed that the 2 terpenoids released in higher amounts by onion-exposed potato were not detectable in the headspace of onion itself,16 suggesting the insect responses were not affected by absorption and re-release of onion volatiles from the surface of potato plants.
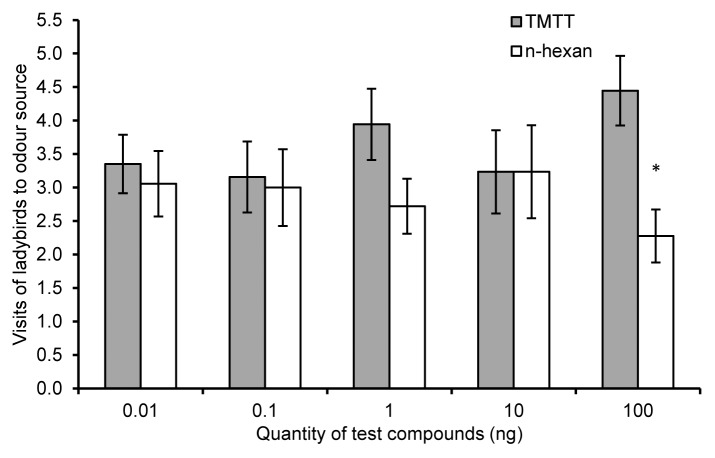
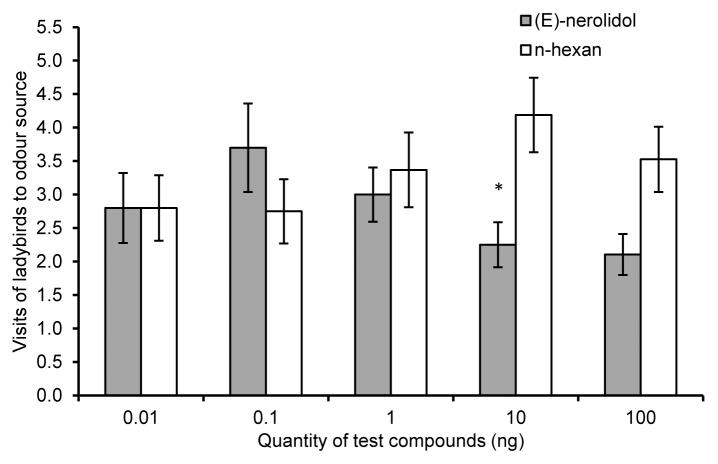
We tested the olfactory responses of ladybirds to (E)-nerolidol and TMTT, terpenoids emitted in greater concentrations by potato after exposure to volatiles from onion.16 A concentration of 10ng/µl TMTT (dosed at volume of 10µl on small piece of filter paper) was significantly more attractive for ladybirds than the control (n-hexane) (Z = 2.37, P = 0.02, n = 18) (Fig. 3). Interestingly, a concentration of 1 ng/µl (E)-nerolidol was repellent to ladybirds (Z = 2.16, P = 0.03, n = 15) (Fig. 4). (E)-nerolidol is known to repel aphids16,28 and to attract predatory mites,29 but had no influence on the behavior of the hover fly Episyrphus balteatus whose larvae are natural enemies of aphids.28 Individual HIPV components can increase predator attraction both independently and when individually enhanced within plant blends.7
Figure 3. Ladybird olfactory responses to (3E, 7E) 4, 8, 12-trimethyl-1, 3, 7, 11-tridecatetraene (TMTT). Behavioral responses of ladybirds toTMTT, a terpenoid released in higher amounts from potato plants after exposure to volatiles from onion plants, vs. n-hexane controls. Error bars indicate ± SEM. Asterisks indicate statistical significance levels of * P ≤ 0. 05 (Wilcoxon matched pairs test).
Figure 4. Ladybird olfactory responses to (E)-nerolidol. Behavioral responses of ladybirds to (E)-nerolidol, a terpenoid released in higher amounts from potato plants after exposure to volatiles from onion plants, vs. n-hexane controls. Error bars indicate ± SEM. Asterisks indicate statistical significance levels of * P ≤ 0. 05, and *** P ≤ 0. 001 (Wilcoxon matched pairs test).
The effects of TMTT and (E)-nerolidol as individual components or in blends have been studied mainly in predatory mites.20,29 Coccinella septempunctata is an important predator of aphids, strongly attracted by volatiles of attacked plants, but knowledge of the role of these HIPV components on their behavior, alone or in blends, is lacking. It has been previously shown that ladybirds are attracted by changes in volatile profiles resulting from chemical interaction between unattacked barley plants8,12,13 and the present study supports these findings and gives a potential explanation of the phenomenon. Our results show that C. septempunctata is attracted by the synthetic blend of onion-exposed potato, and by TMTT, one of the components of the blend.16 Although (E)-nerolidol is repellent for ladybirds when presented alone, it was present in higher amounts in the blend released by onion-exposed potato, which was attractive. This supports the idea that some volatiles have different effects on insect behavior when encountered alone or together with other compounds in blends.30
Ladybirds were attracted to potato plants exposed to onion neighbor volatiles, whereas aphids were repelled.16 It may seem non-adaptive for ladybirds to be attracted to plant that had reduced attraction to aphids. However, ladybird immigration into crop fields is not always correlated with aphid abundance.31 The ladybird is polyphagous and may benefit from locating habitats with increased plant diversity, and thus increased prey diversity. Further, a previous study has shown that while plant–plant volatile exchange can reduce aphid numbers on a plant, ladybirds consume more aphids on exposed than on unexposed plants.12 Thus the adaptive significance of predator responses to chemical interaction between undamaged plants is likely depend on several factors and this type of plant interaction might be an underlying mechanism in plant diversity systems, effecting not only phytophagous herbivore insects but also their natural enemies.
A number of studies have shown that complex plant habitats can decrease the occurrence of phytophagous insects and increase that of their natural enemies.14 Studies of tri-trophic interactions usually address the importance of plant chemical compounds in regulating insect herbivore species richness and the abundance of natural enemies. The present study provides additional evidence that even communication between healthy plants has a strong impact on predatory insects, and suggests that volatile interaction between undamaged plants leading to changes in volatile profiles may be a mechanism contributing to these observations. These findings may have practical importance for the development of habitat manipulation strategies, e.g., intercropping, that reduce insect pests and increase the abundance of their natural enemies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Erika Qvarfordt (Swedish University of Agricultural Sciences, Department of Ecology) for her help in the laboratory. Financial support was received from the Swedish Foundation for Strategic Environmental Research (MISTRA) through the PlantComMistra program, Ministry of Education, Science, and Technological Development of the Republic of Serbia (Project No. III 46008), and the Carl Trygger Foundation for Scientific Research.
Glossary
Abbreviations:
- HIPVs
herbivore-induced plant volatiles
- TMTT
(3E, 7E)-4, 8, 12-trimethyl-1, 3, 7, 11-tridecatetraene
References
- 1.Gouinguené SP, Turlings TCJ. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002;129:1296–307. doi: 10.1104/pp.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piesik D, Łyszczarz A, Tabaka P, Lamparski R, Bocianowski J, Delaney KJ. Volatile induction of three cereals: influence of mechanical injury and insect herbivory on injured plants and neighbouring uninjured plants. Ann Appl Biol. 2010;157:425–34. doi: 10.1111/j.1744-7348.2010.00432.x. [DOI] [Google Scholar]
- 3.Rajabaskar D, Wu Y, Bosque-Pérez NA, Eigenbrode SD. Dynamics of Myzus persicae arrestment by volatiles from Potato leafroll virus-infected potato plants during disease progression. Entomol Exp Appl. 2013;148:172–81. doi: 10.1111/eea.12087. [DOI] [Google Scholar]
- 4.Arimura G, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 2009;50:911–23. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- 5.Le Guigo P, Rolier A, Le Corff J. Plant neighborhood influences colonization of Brassicaceae by specialist and generalist aphids. Oecologia. 2012;169:753–61. doi: 10.1007/s00442-011-2241-4. [DOI] [PubMed] [Google Scholar]
- 6.Tscharntke T, Thiessen S, Dolch R, Boland W. Herbivory induced resistance and interplant signal transfer in Alnus glutinosa. Biochem Syst Ecol. 2001;29:1025–47. doi: 10.1016/S0305-1978(01)00048-5. [DOI] [Google Scholar]
- 7.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the “cry for help.”. Trends Plant Sci. 2010;15:167–75. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Ninkovic V, Pettersson J. Searching behaviour of the sevenspotted ladybird - Coccinella septempunctata – effects of plant – plant odor interaction. Oikos. 2003;100:65–70. doi: 10.1034/j.1600-0706.2003.11994.x. [DOI] [Google Scholar]
- 9.Ninkovic V, Olsson U, Pettersson J. Mixed barley cultivars affects aphid host plant acceptance in field experiments. Entomol Exp Appl. 2002;102:177–82. doi: 10.1046/j.1570-7458.2002.00937.x. [DOI] [Google Scholar]
- 10.Ninkovic V, Glinwood R, Dahlin I. Weed-barley interactions affect plant acceptance by aphids in laboratory and field experiments. Entomol Exp Appl. 2009;133:38–45. doi: 10.1111/j.1570-7458.2009.00900.x. [DOI] [Google Scholar]
- 11.Glinwood R, Ninkovic V, Pettersson J. Chemical interaction between undamaged plants--effects on herbivores and natural enemies. Phytochemistry. 2011;72:1683–9. doi: 10.1016/j.phytochem.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V, Pettersson J. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod-Plant Interact. 2009;3:215–24. doi: 10.1007/s11829-009-9072-9. [DOI] [Google Scholar]
- 13.Ninkovic V, Al Abassi S, Ahmed E, Glinwood R, Pettersson J. Effect of within-species plant genotype mixing on habitat preference of a polyphagous insect predator. Oecologia. 2011;166:391–400. doi: 10.1007/s00442-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andow DA. Vegetational diversity and arthropod population response. Annu Rev Entomol. 1991;36:561–86. doi: 10.1146/annurev.en.36.010191.003021. [DOI] [Google Scholar]
- 15.Dahlin I, Ninkovic V. Aphid performance and population development on their host plants is affected by weed – crop interactions. J Appl Ecol. 2013;50:1281–8. [Google Scholar]
- 16.Ninkovic V, Dahlin I, Vucetic A, Petrovic-Obradovic O, Glinwood R, Webster B. Volatile exchange between undamaged plants - a new mechanism affecting insect orientation in intercropping. PLoS One. 2013;8:e69431. doi: 10.1371/journal.pone.0069431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agbogba BC, Powell W. Effect of the presence of a nonhost herbivore on the response of the aphid parasitoid Diaeretiella rapae to host-infested cabbage plants. J Chem Ecol. 2007;33:2229–35. doi: 10.1007/s10886-007-9379-x. [DOI] [PubMed] [Google Scholar]
- 18.Blande JD, Pickett JA, Poppy GM. A comparison of semiochemically mediated interactions involving specialist and generalist brassica-feeding aphids and the Braconid parasitoid Diaeretiella rapae. J Chem Ecol. 2007;33:767–79. doi: 10.1007/s10886-007-9264-7. [DOI] [PubMed] [Google Scholar]
- 19.Kugimiya S, Shimoda T, Tabata J, Takabayashi J. Present or past herbivory: a screening of volatiles released from Brassica rapa under caterpillar attacks as attractants for the solitary parasitoid, Cotesia vestalis. J Chem Ecol. 2010;36:620–8. doi: 10.1007/s10886-010-9802-6. [DOI] [PubMed] [Google Scholar]
- 20.Brillada C, Nishihara M, Shimoda T, Garms S, Boland W, Maffei ME, Arimura G. Metabolic engineering of the C16 homoterpene TMTT in Lotus japonicus through overexpression of (E,E)-geranyllinalool synthase attracts generalist and specialist predators in different manners.(Erratum) New Phytol. 2013;200:1200–11. doi: 10.1111/nph.12442. [DOI] [PubMed] [Google Scholar]
- 21.van Wijk M, de Bruijn PJA, Sabelis MW. Complex odor from plants under attack: herbivore’s enemies react to the whole, not its parts. PLoS One. 2011;6:e21742. doi: 10.1371/journal.pone.0021742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninkovic V, Al Abassi S, Pettersson J. The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol Control. 2001;21:191–5. doi: 10.1006/bcon.2001.0935. [DOI] [Google Scholar]
- 23.Han B, Chen Z. Composition of the volatiles from intact and tea aphid-damaged tea shoots and their allurement to several natural enemies of the tea aphid. J. Appl. Ent. 2002;126:497–500. doi: 10.1046/j.1439-0418.2002.00692.x. [DOI] [Google Scholar]
- 24.Gencer NS, Kumral NA, Sivritepe HO, Seidi M, Susurluk H, Senturk B. Olfactory response of the ladybird beetle Stethorus gilvifrons to two preys and herbivore-induced plant volatiles. Phytoparasitica. 2009;37:217–24. doi: 10.1007/s12600-009-0032-9. [DOI] [Google Scholar]
- 25.Girling RD, Hassall M. Behavioural responses of the seven-spot ladybird Coccinella septempunctata to plant headspace chemicals collected from four crop Brassicas and Arabidopsis thaliana, infested with Myzus persicae. Agric For Entomol. 2008;10:297–06. doi: 10.1111/j.1461-9563.2008.00379.x. [DOI] [Google Scholar]
- 26.StatSoft Inc. STATISTICA, version 10, 2011. www.statsoft.com
- 27.Himanen SJ, Blande JD, Holopainen JK. Plant-emitted semi-volatiles shape the infochemical environment and herbivore resistance of heterospecific neighbors. Plant Signal Behav. 2010;5:1234–6. doi: 10.4161/psb.5.10.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kos M, Houshyani B, Overeem AJ, Bouwmeester HJ, Weldegergis BT, van Loon JJA, Dicke M, Vet LE. Genetic engineering of plant volatile terpenoids: effects on a herbivore, a predator and a parasitoid. Pest Manag Sci. 2013;69:302–11. doi: 10.1002/ps.3391. [DOI] [PubMed] [Google Scholar]
- 29.Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science. 2005;309:2070–2. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- 30.Webster B, Bruce T, Pickett J, Hardie J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim Behav. 2010;79:451–7. doi: 10.1016/j.anbehav.2009.11.028. [DOI] [Google Scholar]
- 31.Cardinale BJ, Weis JJ, Forbes AE, Tilmon KJ, Ives AR. Biodiversity as both a cause and consequence of resource availability: a study of reciprocal causality in a predator-prey system. J Anim Ecol. 2006;75:497–505. doi: 10.1111/j.1365-2656.2006.01070.x. [DOI] [PubMed] [Google Scholar]