Abstract
Most prior studies of the effects of excessive alcohol intake on the adolescent brain examined alcohol use dependent samples with comorbid psychiatric and substance use disorders. In the Cape Town region, we identified a sizeable cohort of adolescents with alcohol use disorders (AUD) without externalizing or other psychiatric disorders. We examined brain morphology in 64 such adolescents compared to age and gender matched healthy controls. Magnetic resonance imaging data were analyzed using FSL’s FIRST software for subcortical volumes, and cortical gray matter (GM) was analyzed using voxel based morphometry (VBM) and regions of interest (ROI) analysis. AUD boys had smaller thalamic and putamen volumes compared to non-drinking boys, while AUD girls had larger thalamic and putamen volumes compared to non-drinking girls. VBM revealed a large region of decreased GM density in AUDs compared to controls located in the left lateral frontal, temporal, and parietal lobes, extending medially deep into the parietal lobe. Smaller GM volume in this region was also present when examined using ROI analysis. Our lack of findings in other brain regions, particularly hippocampus, suggests that reports of smaller brain volumes in adolescent AUDs in the literature are a consequence of psychiatric and substance abuse comorbidities.
Keywords: magnetic resonance imaging, voxel based morphometry, South Africa
1. Introduction
Alcohol use disorders (AUD, DSM-IV alcohol dependence or abuse) are common amongst adolescents and may pose unique, and serious health consequences. Alcohol is the most widely used intoxicant amongst American adolescents (SAMHSA, 2012a). A quarter of young people aged 12-20, surveyed in 2011, reported drinking during the past month, while 15.8% reported past month binge drinking, and 4.4% reported heavy drinking in the past month (SAMHSA, 2012b). Additionally, a previous report (SAMHSA, 2007) found that individuals who first drank alcohol before the age of 15 were over 5 times more likely to have an adult AUD than those who began alcohol use after the legal age of 21.
A considerable number of studies have investigated the effects of chronic alcohol abuse on the adult brain. The majority of these studies have demonstrated substantial brain atrophy and neuropsychological impairments in adults with chronic AUDs compared to healthy controls (Fein et al., 2008, Fein and McGillivray, 2007, Fein et al., 2009a, Sullivan et al., 2000, Sullivan et al., 1995, Pfefferbaum et al., 1992). These findings suggest chronic alcohol abuse has a deleterious effect on brain structure and function. The adolescent brain is actively developing throughout the teen years and into young adulthood. This development is marked by critical periods of maturation and/or selective pruning in key areas (prefrontal cortex, hippocampus, amygdala) of the brain which may be particularly vulnerable to the effects alcohol and other substances during adolescence (Crews et al., 2007, Lenroot and Giedd, 2006, White et al., 2000). Thus, previous findings from adult studies may not generalize to the developing adolescent brain.
A few studies have looked specifically at adolescents with AUD in order to investigate the unique effects of alcohol consumption on the developing brain. Adolescents with AUD were found to have smaller hippocampal volumes (Nagel et al., 2005, De Bellis et al., 2000, Medina et al., 2007b) and smaller prefrontal cortical volumes (Medina et al., 2008, De Bellis et al., 2005) when compared to healthy non/light-drinking controls. One study by Squeglia and colleagues investigated brain morphology in binge-drinking adolescents, finding thicker frontal cortices in binge drinking girls compared non-drinking girls, and thinner cortices in binge drinking boys compared to age, gender, and family drinking history matched boys (Squeglia et al., 2012). However, the results of these studies must be interpreted with caution. Of the 6 studies mentioned above, the largest experimental sample size was n=29 (14 girls, 15 boys). Additionally, the effect of alcohol consumption is confounded in these studies by comorbid axis I psychiatric disturbances and other substance use. One study in particular, by Nagel and colleagues (2005) attempted to control psychiatric comorbidies, yet over a third of their sample had a conduct disorder diagnosis. Similiarly, in the study by Squeglia and colleagues, exclusion criteria specifically states current or previous DSM-IV Axis I diagnoses as exclusionary with the exception of conduct disorder, oppositional defiant disorder, and simple phobia, but the diagnosis rates of these disorders within their sample is not reported (Squeglia et al., 2012). These examples further underscore the difficulty in disentangling alcohol’s unique effects from the effects of other substance and psychiatric comorbidities.
To isolate the effects of alcohol abuse on the developing brain, it is important to minimize the confounding effects of comorbidities. The Cape Town region of South Africa is historically a wine-growing region, where a range of risk factors creating vulnerability to hazardous alcohol use in adolescence exists. Alcohol is the most popular abused substance among adolescents in this region (Parry et al., 2004, Pluddemann, 2008). Furthermore, surveys in Cape Town schools demonstrate lower incidence of mixed substance abuse than found in the USA (Parry et al., 2004). Although previous studies of adolescents in the Cape Town region show prevalent methamphetamine abuse among school drop-outs (Wechsberg et al., 2010) and associations between substance abuse and psychopathology (Saban et al., 2010), many Cape Town adolescents attend school, exhibit little psychopathology, and are not polysubstance abusers. From this population, we have recruited a community-dwelling (i.e., treatment-naïve) adolescent student cohort that is ideal for studies attempting to isolate the effects of alcohol abuse on the developing brain because of the low rates of substance (other than alcohol) use and our ability to recruit a sample with relatively modest externalizing behavioral problems (i.e., not meeting criteria for a adolescent externalizing diagnosis) among heavy drinkers.
In this report, we present the effects of heavy drinking on cortical and subcortical volumes in South African adolescents with AUDs.
2. Methods
2.1 Procedures
All study procedures were approved by the Research Ethics Committee of the Stellenbosch University Faculty of Health Sciences. Participants were screened for eligibility after written informed assent/consent was obtained from volunteers and parents or guardians. Screening involved detailed medical history-taking, physical and psychiatric examination, and urine analysis and breathalyzer testing (to confirm that the adolescents were not intoxicated during the testing procedures), all performed by a fully qualified and licensed psychiatrist. The Schedule for Affective Disorders and Schizophrenia for School Aged Children (6–18 years) Lifetime Version (K-SADS-PL) (Kaufman et al., 1996), a semi-structured clinician-rated diagnostic scale, was used to ascertain current and past psychiatric diagnoses, as reported by the participants. The subjects then completed demographic and Childhood Trauma – Short Form Questionnaires (Bernstein et al., 2003) and underwent MRI brain scans. A research assistant was available to assist participants in completing the self-report demographic and early adversity questionnaires. All test materials were available in the participant’s language of preference. Cognitive testing was individually administered. Participants were provided with meals and refreshments, and at the conclusion of the session were compensated for their time with gift vouchers (to the value of ZAR 50 per visit). All study information was kept confidential, except where statutory requirements dictated the reporting of newly identified or ongoing threats to the safety of minor participants. The study protocol and procedures complied with and were conducted in strict adherence to the guidelines contained in the (Association, 2008). Full written approval to conduct the study was obtained from the Western Cape Education Department and the Research Ethics Committee of the Stellenbosch University Faculty of Health Sciences.
2.2 Participants
We recruited English- and Afrikaans-speaking adolescents from 19 schools within the Cape Flats region of the greater Cape Town area. All participants were from moderately low socioeconomic backgrounds, residing in permanent housing with potable water and electricity, but mostly without luxury items such as computers and cars. The median gross annual income level per household was ZAR 62 035. The mean age of the entire sample was 14.82 years (±.78) and subjects had completed 7.82 years (±.81) of education. Girls (n = 70, 54.69%) outnumbered boys (n = 58, 45.31%).
Exclusion criteria for study participation were: mental retardation, lifetime DSM-IV Axis I diagnoses other than AUD (including depressive, anxiety, psychotic, post-traumatic stress, elimination, eating, tic, attention-deficit/hyperactivity, oppositional defiant, and conduct disorders); lifetime dosages exceeding 30 cannabis joints or 3 methamphetamine doses; current use of sedative or psychotropic medication; signs or history of fetal alcohol syndrome or malnutrition; sensory impairment; history of traumatic brain injury with loss of consciousness exceeding 10 minutes; presence of diseases that may affect the CNS (e.g., meningitis, epilepsy, HIV); less than 6 years of formal education; and lack of proficiency in English or Afrikaans. Collateral information verifying the absence of medical, psychiatric, and psychosocial problems was obtained from consenting parents by a social worker at the consent explanation interview.
2.3 Measures
2.3.1 Early adversity
We used the Childhood Trauma Questionnaire-Short Form (CTQ-SF; (Bernstein et al., 2003) to measure early adversity. The CTQ-SF is a 28-item retrospective self-report questionnaire comprising five subscales, each of which is aimed at measuring a distinct dimension of childhood mistreatment: physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect. Each type of maltreatment is represented by five items. An additional three-item minimization/denial subscale is included to detect the underreporting of maltreatment. Item response options are structured to reflect the frequency of maltreatment experiences (i.e., never true, rarely true, sometimes true, often true, very often true), and are scored from 1 to 5 accordingly. Each of the five primary subscales has demonstrated good internal consistency across a range of heterogeneous samples, including individuals with substance use disorders (physical abuse subscale = .83 to .86; sexual abuse = .92 to .95; emotional abuse = .84 to .89; physical neglect = .61 to .78; emotional neglect = .85 to .91).
2.3.2 Neuropsychological test performance
A general-purpose neuropsychological test battery was selected. Due to the unavailability of current, culturally appropriate, unbiased South African tests (Foxcroft, 2004, van Ommen, 2005), age-appropriate international tests with established utility in cross-cultural and multilingual contexts and in substance use disorders studies were selected. Test instructions, stimuli, and response booklets were translated into Afrikaans and back-translated into English by independent translators. In consultation with an Afrikaans linguistics specialist, appropriate cultural and language adaptations were made to the tests. Examples of these adaptations included replacement of items/terminology unfamiliar to South Africans, simplifications of test instructions, and substitution of items to ensure equivalent difficulty levels in both Afrikaans and English. From the battery three composite measures were made: (1) Verbal Story Memory, (2) Self-Monitoring, and (3) Psychomotor Speed and Coordination. The Verbal Story Memory composite included the immediate recall of thematic units of Stories E and F from the Children’s Memory Scale (CMS) (Cohen, 1997), and delayed recognition of thematic units of Stories E and F from the CMS. The Self-Monitoring composite measure was derived from phonemic (letters L, B, and S) and semantic (animal category) fluency error scores (Strauss et al., 2006), the Auditory Verbal Learning Test total error score (Maj et al., 1993), time and rule violation scores from the Tower of London (Culbertson and Zillmer, 2001), error scores from the Children’s Color Trails Test (Llorente et al., 2003), and error scores from the Stroop Color-Word test. The Psychomotor Speed and Coordination composite included non-dominant peg insertion time from the Grooved Pegboard Test (Russell, 1993) and error scores from the Stroop Color-Word test (Golden and Freshwater, 2002). For full description of composite measure computation please see (Ferrett et al., 2010).
2.3.3 Substance use
The Timeline Followback (TLFB) procedure (Sobell and Sobell, 1992), a semi-structured, clinician-administered assessment of lifetime history of alcohol use and drinking patterns, was used in collaboration with the K-SADS-PL to elicit alcohol-use data. A standard drink was defined as one beer or wine cooler, one glass of wine, or one 1.5-oz shot of liquor (alone or in a mixed drink). AUD group membership was defined by a lifetime dosage in excess of 100 units of alcohol plus a DSM-IV diagnosis of alcohol abuse or dependence. The control group were non-drinkers (who had never consumed alcohol) and light drinkers (lifetime dosage not exceeding 76 units of alcohol), with no history of an AUD.
2.4 Image acquisition
All MRIs were collected on a 3T Siemens Magnetom Allegra MR Headscanner using Syngo MR software. The scanner is located in the Cape Universities Brain Imaging Center at the Stellenbosch University Health Sciences Campus in Stellenbosch, South Africa. The first 58 subjects were acquired using a transaxial T1-weighted SPGR acquisition (TR = 2080 ms, TE = 4.88 mm, acquisition matrix = 256 × 192) at 1.0 mm thickness. Review of these images revealed undesirable presence of blood-vessels in the images, resulting from the scanner being a head-only model, not allowing full saturation of the blood to suppress its signal before it enters the head. To reduce this signal from unsaturated blood, a sagittal SPGR protocol was instituted (TR = 2200 ms, TE = 5.16 ms, acquisition matrix 256 × 256) at 1.0 mm thickness. 21 subjects were acquired using both protocols and the remaining 61 subjects were acquired only using the sagittal protocol.
2.5 Image analysis
Of the 140 total particpants, we analyzed all alcohol abusing participants who could be matched with a control participant on age (within 1 year), gender, and structural imaging protocol, resulting in 64 (35 female, 29 male; 31 axial, 33 sagittal acquisition) AUD and 64 non-drinking matched controls.
2.5.1 Subcortical structures
FIRST (FMRIB Image Registration and Segmentation Tool), a fully automated method within the FSL (FMRIB Software Library) suite of tools (Woolrich et al., 2009, Patenaude, 2007, Smith et al., 2004) was used to delineate subcortical structures and measure their volumes. This method has been used successfully in a number of recent investigations (Corthorn et al., 1997, Rinehart et al., 1997, Markov et al., 1997, Figueroa et al., 1997, Angstrom et al., 2004, Sameti et al., 2011). The brainstem was excluded because the shape model used in FIRST extended beyond the inferior boundary of the images; left and right volumes were added to create a total volume for each of 8 subcortical structures. For participants studied with both MRI acquisition protocols, we estimated the subcortical volumes on both images and computed the Pearson’s correlation to assess the comparability of subcortical volumes. All correlation coefficients were significant (P<0.001) and ranged from r=0.673-0.992. Please refer to (Sameti et al., 2011) for full description of subcortical structure analysis method.
2.5.2 Voxel based morphometry
FSL-VBM ((Douaud et al., 2006), http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM) was used to create gray matter density images for voxel-wise analysis. First, structural images were brain-extracted and gray-matter segmented before being registered to the MNI 152 standard space using non-linear registration (Andersson and Smith, 2007). The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific grey matter template. Second, all native gray matter images were non-linearly registered to this study-specific template and “modulated” to correct for local expansion (or contraction) due to the nonlinear component of the spatial transformation. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 10 mm. Voxel-wise statistics were computed using valmap (http://www.nitrc.org/projects/valmap), correcting for multiple comparisons across space using permutation testing of suprathreshold clusters above p=0.01. Maps of GM density were the dependent variables, and factors were group and gender, covaried by head size and acquisition protocol nuisance variables.
2.5.3 Cortical gray matter ROIs
We attempted to corroborate findings from VBM by applying ROI masks, corresponding to the GM density clusters of interest, to whole brain GM segmentations created with FAST (FMRIB’s Automated Segmentation Tool), a fully automated FSL utility that segments 3D brain images in to diferent tissue types (Zhang et al., 2001). For participants studied with both MRI acquisition protocols, we estimated GM volumes in 23 cortical regions on both images and computed the Pearson’s correlation to assess the comparability of GM segmentations. All correlation coefficients were significant (p<0.002) and ranged from r=0.632-0.963. For each significant or nearly significant VBM cluster, voxels comprising the suprathreshold cluster were set to 1 and saved as a separate mask. The masks were then transformed into each subject’s space and applied to the GM segmented images created by FAST, in order to compute GM volumes within the mask, and confirm that the VBM results were not an artifact of misregistration, where tissue displacement can be mistaken for smaller volume.
2.6 Statistical analysis
Demographic and alcohol use variables were investigated with a multivariate analysis with group and sex as the first factors. The General Linear Model (GLM) (SPSS Inc., 2009) was first used to determine whether subcortical volumes were correlated with the cranium size index, calculated by FSL’s SIENAX. The FSL cranium size index has been previously shown to be an excellent surrogate for intracranial vault volume (Fein et al., 2004). Since all 8 subcortical volumes were significantly positively correlated with the cranium size, linear regression was used to adjust each structure volume for the cranium size index. The cortical ROIs were also adjusted for cranium size. Multivariate analyses of variance were used to examine cranium size adjusted subcortical and cortical GM volumes, with group, sex, and acquisition protocol used as fixed factors.
Composite neuropsychological measures were computed as described by (Ferrett et al., 2010), adapted from (Medina et al., 2007a), where measures were grouped based on theoretical assumptions (Lezak et al., 2004). Scores within groupings were standardized and a Cronbach’s alpha was assessed for goodness-of-fit for each composite measure. Bi-variate correlations were used to assess associations of selected cortical regions and subcortical structures, with early childhood adversity, neuropsychological test performance, and measures of alcohol use.
3. Results
3.1 Demographics, substance use, trauma, and cognition
Table 1 presents demographics, substance use, trauma measures, and cognitive scores for adolescents with AUD and non-alcoholic controls (NAC). There were no significant group or gender differences in age, education, language, body-mass index, or handedness. Smoking was more prevalent among the AUD adolescents. Although a higher percentage of AUD adolescents had tried cannabis compared to controls, on average those who had tried cannabis had smoked only 7 joints total. One AUD adolescent had tried methamphetamine a single time, another AUD adolescent had tried meth twice; no NAC adolescent had tried methamphetamine. In our sample, there was no reported use of cocaine, opioids, PCP, hallucinogens, inhalants, stimulants other than methamphetamine, or other drugs (including prescription drugs). AUD boys suffered from more physical abuse (p<0.01) and sexual abuse (p<0.01) than NAC boys, AUD girls, or NAC girls. There were no other differences in trauma exposure between AUD and NAC groups. AUD adolescents showed impaired memory as indexed by lower scores on the Verbal Story Memory composite (p<0.01), and worse self-monitoring as indexed by higher scores on the Self-Monitoring composite (p<0.01).
Table 1.
Demographics, substance use, trauma measures, and cognitive scores for adolescents with alcohol use disorders (AUD) and non-alcoholic controls (NAC).
| Variable | NAC (n=64) | AUD (n=64) | ||||||
|---|---|---|---|---|---|---|---|---|
| Girls | Boys | Total | Girls | Boys | Total | |||
| (n=35) M (SD) |
(n=29) M (SD) |
M (SD) | Range | (n=35) M (SD) |
(n=29) M (SD) |
M (SD) | Range | |
| Age (yrs) | 14.64 (0.75) | 14.79 (0.78) | 14.71 (0.76) | 12.21-15.99 | 14.83 (0.90) | 15.07 (0.61) | 14.94 (0.79) | 12.77-16.00 |
| Education (yrs) | 7.83 (0.86) | 7.62 (0.82) | 7.73 (0.84) | 6-10 | 7.91 (0.78) | 7.90 (0.77) | 7.91 (0.77) | 6-9 |
| % Afrikaans-speaking | 71 | 76 | 73 | NA | 63 | 76 | 69 | NA |
| Body mass index (kg/m) | 22.06 (4.86) | 21.66 (5.02) | 21.87 (4.90) | 15-38 | 23.18 (5.54) | 20.63 (5.02) | 22.05 (5.42) | 15-41 |
| % right-handed | 91 | 79 | 86 | NA | 97 | 93 | 95 | NA |
| % never drank alcohol | 42 | 45 | 44 | NA | NA | NA | NA | NA |
| Age first drink (yrs) | 12.15 (1.57) | 12.00 (1.75) | 12.08 (1.63) | 8-15 | 11.89 (1.70) | 12.07 (1.91) | 11.97 (1.78) | 5-14 |
| # drinks/lifetime | 8.34 (16.94) | 3.07 (5.15) | 5.95 (13.18) | 0-76 | 1435.29 (1466.53) | 1371.66 (1245.29) | 1406.45 (1360.49) | 114-6384 |
| Age first intoxication (yrs) | NA | NA | NA | NA | 12.69 (1.23) | 13.03 (1.27) | 12.84 (1.25) | 9-15 |
| Regular drinking onset age (yrs) | NA | NA | NA | NA | 12.74 (1.17) | 13.17 (1.26) | 12.94 (1.22) | 9-15 |
| Drinking duration (mo) | NA | NA | NA | NA | 57.75 (44.57) | 65.99 (50.05) | 61.48 (46.93) | 5-196 |
| #drinks/mo | NA | NA | NA | NA | 57.75 (44.57) | 65.99 (50.05) | 61.48 (46.93) | 5-196 |
| %non-smoker | 60.0 | 62.1 | 60.9 | NA | 14.3 | 17.2 | 15.6 | NA |
| % <100 cigarettes | 34.3 | 31.0 | 32.8 | NA | 20.0 | 37.9 | 28.1 | NA |
| % >100 cigarettes | 5.7 | 6.9 | 6.3 | NA | 65.7 | 44.8 | 56.3 | NA |
| % tried methamphetamine | 0.0 | 0.0 | 0.0 | NA | 5.7 | 0.0 | 3.1 | NA |
| # methamphetam ine doses | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 (1.4) | 0.0 | 2.0 (1.4) | 1-3 |
| % tried cannabis | 8.6 | 13.8 | 10.9 | NA | 48.6 | 51.7 | 50.0 | NA |
| # joints smoked | 1.0 (0.0) | 1.3 (0.5) | 1.1 (0.4) | 1-2 | 6.5 (8.8) | 7.2 (6.8) | 7.0 (1.4) | 1-30 |
| CTQ-physical abuse | 6.86 (4.06) | 5.90 (1.86) | 6.42 (3.27) | 5-25 | 5.86 (1.26) | 8.24 (1.26) | 6.94 (3.19) | 0-16 |
| CTQ-sexual abuse | 6.37 (3.00) | 5.48 (1.24) | 5.97 (2.40) | 5-15 | 5.86 (2.19) | 7.66 (4.36) | 6.67 (3.44) | 0-16 |
| CTQ-emotional abuse | 8.29 (6.90) | 6.90 (2.58) | 7.66 (3.95) | 5-20 | 8.71 (3.41) | 8.59 (4.09) | 8.66 (3.70) | 0-20 |
| CTQ-physical neglect | 7.66 (3.48) | 8.59 (3.80) | 8.80 (3.63) | 5-19 | 8.60 (2.70) | 8.93 (4.17) | 8.75 (3.42) | 0-15 |
| CTQ-emotional neglect | 11.34 (5.57) | 13.83 (6.67) | 12.47 (6.17) | 5-25 | 12.80 (5.68) | 12.97 (6.67) | 12.88 (6.10) | 0-25 |
| CTQ-total score | 40.51 (14.80) | 40.69 (11.04) | 40.59 (13.13) | 25-83 | 41.83 (9.00) | 46.38 (19.73) | 43.89 (14.90) | 0-73 |
| Verbal story memory | 0.205 (0.921) | 0.481 (0.893) | 0.330 (0.912) | -1.32 – 2.61 | -0.344 (0.804) | -0.314 (0.795) | -0.330 (0.794) | -1.58 – 1.89 |
| Self-monitoring | -0.198 (0.316) | -0.119 (0.405) | -0.162 (0.358) | -0.69 – 0.78 | 0.027 (0.429) | 0.326 (0.524) | 0.162 (0.494) | -0.52 – 1.80 |
| Psychomotor speed and coordination | -0.165 (0.574) | 0.137 (0.878) | -0.028 (0.737) | -1.59 – 2.20 | -0.081 (0.607) | 0.160 (0.742) | 0.028 (0.677) | -1.56 – 1.51 |
3.2 Subcortical structures
Table 2 presents the results obtained through FIRST. The majority of the difference in cranial vault size between subjects was accounted for by gender differences. A multivariate analyses of subcortical GM volumes with group and sex as fixed factors failed to show an effect of group or sex for any of the 8 subcortical structures (P > 0.05). The lack of group findings did not change when scanning protocol was included as a fixed factor. There were two significant group by sex interactions, the thalamus (F=4.334, df=1, P=.039, eta2=3.4%) where boys with AUD had smaller volumes than NAC boys while girls with AUD had greater volume than NAC girls, and the putamen (F=10.63, df=1, P=.001, eta2=7.9) where boys with AUD had smaller volumes than NAC boys while girls with AUD had greater volume than NAC girls. The thalamus and putamen volumes for AUD and NAC boys and girls are shown in the left scatterplot of Figure 1.
Table 2.
Mean volumes (mm3) of subcortical structures and cortical regions of interest.
| Region | NAC | AUD | ||
|---|---|---|---|---|
| Girls (N=35) Mean (SD) |
Boys (N=29) Mean (SD) |
Girls (N=35) Mean (SD) |
Boys (N=29) Mean (SD) |
|
| Lateral ventricles | 13377 (4495) | 10601 (3872) | 12513 (5124) | 12382 (5408) |
| Thalamus* | 15920 (867) | 16502 (819) | 16355 (934) | 16277 (939) |
| Caudate | 7347 (894) | 7330 (786) | 7660 (858) | 7461 (868) |
| Putamen* | 9237 (878) | 10088 (710) | 9623 (1092) | 9395 (975) |
| Globus pallidus | 3439 (298) | 3579 (256) | 3496 (281) | 3497 (216) |
| Hippocampus | 6491 (540) | 6474 (678) | 6486 (610) | 6429 (893) |
| Amygdala | 2208 (732) | 2098 (398) | 2108 (386) | 2116 (350) |
| Nucleus accumbens | 816 (144) | 780 (185) | 824 (188) | 814 (187) |
| Left frontal/temporal/parietal** | 8463 (1566) | 9267 (1165) | 7575 (1689) | 8699 (1078) |
| Left dorsolateral prefrontal | 4507 (568) | 4500 (662) | 4307 (777) | 4516 (741) |
| Right dorsolateral prefrontal | 3882 (420) | 4036 (564) | 3775 (505) | 4031 (448) |
AUD Boys < NAC Boys, AUD Girls > NAC Girls, P < 0.05
AUD < NAC, P < 0.005
Figure 1.
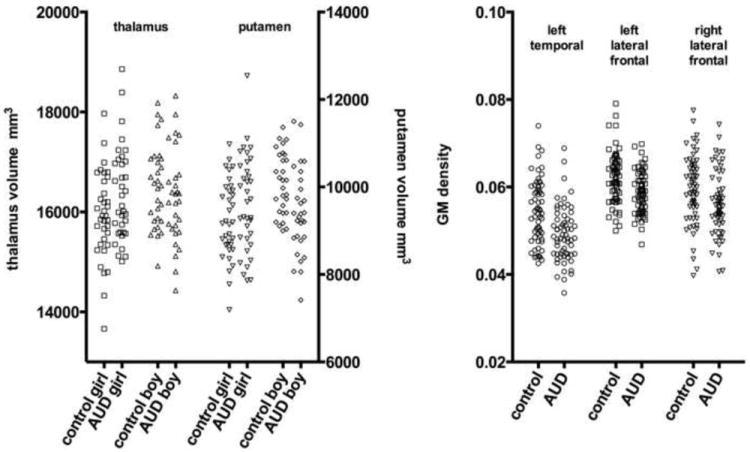
The subcortical volumes for the thalamus and putamen for each subject is shown on the left, grouped by alcohol status and sex, showing that AUD girls had larger volumes and AUD boys had smaller volumes. The average GM density within the left temporal/parietal/frontal, left lateral frontal, and right lateral frontal clusters is shown for each participant on the right, grouped by alcohol status, illustrating the generally smaller GM density in the AUD subject of each pair matched on age, sex, and acquisition protocol.
3.3 Voxel based morphometry
Table 3 summarizes significant and nearly significant clusters from the VBM analysis. A significant cluster of lower GM density was in the AUDs compared to the controls (P<0.025). As shown in Figure 2, this region includes the left temporal cortex, extending into the left frontal and parietal cortex. This region comprises 2865 voxels (22920 mm3) with an average 12.5% smaller GM density due to alcohol. There were also two smaller clusters showing a trend of lower GM density in the AUDs compared to the controls (P<0.1). These clusters were located in the left and right dorso- and ventro-lateral prefrontal cortex (the left cluster can be seen in Figure 1). Scatterplots of the average GM density within these three clusters for all subjects are shown in Figure 1.
Table 3.
Cluster characteristics from VBM analysis.
| Independent variable | # voxels | Cluster volume (mm3) | Corrected cluster P-value | estimated effect | Location |
|---|---|---|---|---|---|
| Group | 2865 | 22920 | P<0.025 | -12.5% | Left temporal extending into frontal and parietal |
| 1372 | 10976 | P<0.1 | -11.8% | Left dorso- and ventro-lateral | |
| 1267 | 10136 | P<0.1 | -12.5% | Right dorso- and ventro-lateral | |
| Gender | 2347 | 18776 | P<0.05 | -12.8% | Left lateral frontal |
| 2081 | 16648 | P<0.05 | -11.1% | Right anterior | |
| 1896 | 15168 | P<0.05 | -8.7% | Left temporal and parietal | |
| 1319 | 10552 | P<0.1 | -11.7% | Left orbitofrontal cortex |
A negative group effect denotes smaller GM density in AUD vs. controls. A negative gender effect denotes smaller GM density in females vs. males.
Figure 2.
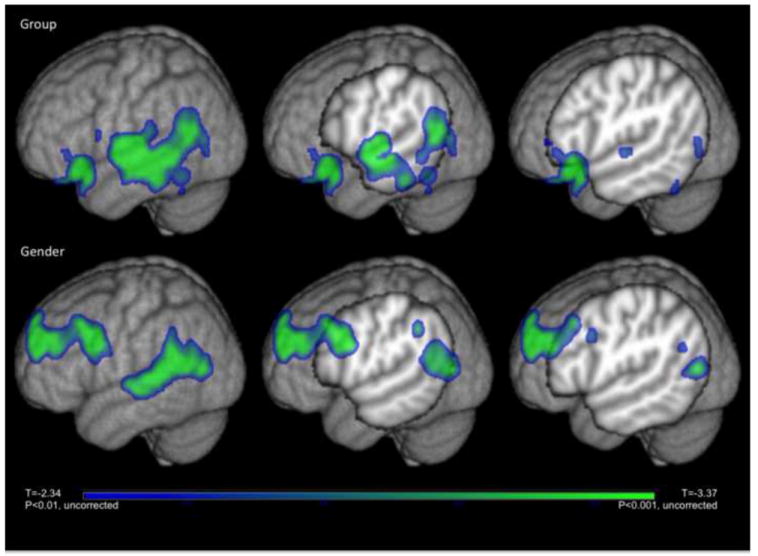
T-statistic maps showing voxels within significant clusters (corrected p<0.1) are overlaid on the MNI-152 T1-weighted image. The top row shows voxels with smaller GM density in AUD compared to controls and the bottom row shows voxels with smaller GM in females compared to males.
There was a significant effect of gender, with females showing 9-13% lower GM density than the males in several regions of the brain, including the left lateral frontal cortex, the right anterior temporal cortex, the left anterior orbitofrontal cortex, and the left temporal/parietal cortex. These clusters are described in detail in Table 3, and Figure 2 shows the left temporal/parietal cluster. Of note, the left temporal/parietal cortex cluster overlaps the alcohol cluster, so that the left temporal cortex in females with AUD have lower GM density than males with AUD. The group by gender interaction was not significant.
3.4 Cortical gray matter ROIs
Cortical gray matter volumes of the left frontal/temporal/parietal region, left dorso- and ventro-lateral prefrontal region, and right dorso- and ventro-lateral prefrontal region, as defined by the significant VBM clusters, were examined with a multivariate analysis of variance using group and sex as fixed factors. The mean volumes of each ROI are shown in Table 2. The cortical GM volumes corresponding to the left frontal/temporal/parietal region showed smaller volumes in AUD participants compared to controls (F=10.11, df=1, P = 0.002, eta2 = 7.5%). There was also a significant effect of sex in this region where boys had greater volumes than girls (F=8.45, df=1, P = 0.004, eta2 = 6.4%), even after correcting for cranium size. Neither of the other two regions had significant group or sex effects. The findings were not affected when scanning protocol was included in the analysis as a fixed factor. There were no significant group by sex interactions.
3.5 Subcortical structures revisisted
The VBM and cortical ROI analyses showed regions of decreased volume in AUDs compared to controls, primarily localized in the left hemisphere. Taking this into consideration, we reexamined the subcortical data looking specifically at the left hemisphere for each subcortical structure for group differences. There were no significant effects of alcohol revealed for any of the 8 left hemispheric subcortical structures (P > 0.1) when compared across gender with group, sex, and protocol used as fixed factors.
3.6 Effects of smoking
We performed multivariate analyses to separate the effects of smoking from those of alcohol using VBM and region of interest statistics in the AUD group only, but found that there were no significant associations between gray matter volumes and smoking status using either of these methods.
3.7 Morphology associations with childhood adversity, neuropsychological performance, and alcohol use variables
Within the AUD participants, Pearson’s correlations were used to investigate the association of average GM density within the significant clusters with early childhood adversity, neuropsychological test perfomance, and measures of alcohol use. Higher GM density in the left temporal/frontal/parietal region was associated with worse psychomotor performance (r=0.29, P=0.02) and more self-monitoring behaviors (r=0.33, P=0.01). Lower GM density in the left dorso- and ventrolateral frontal region was associated with a higher average number of monthly drinks (r=-0.35, P=0.005), with a similar but less significant association in the right lateral frontal region (r=-0.21, P=0.09). GM density in these regions was not associated with any trauma measure.
4. Discussion
The main findings of this study were 1) significantly smaller thalamus and putamen volume in AUD boys compared to NAC boys 2) significantly larger thalamus and putamen volume in AUD girls compared to NAC girls, 3) no significant differences between groups (or between groups separately within girls or boys) in any other subcortical volume measure, regardless of whether the left and right sides were aggregated or analysed separately; 3) a significant VBM cluster of decreased GM density (average of 12.5%) in AUD compared to controls including parts of the left lateral frontal, parietal and temporal cortex, this finding is also present when examined in an ROI based GLM analysis.
Animal, neuroimaging, and neuropsychological studies have shown the hippocampus to be particularly sensitive to drug and alcohol neurotoxicity. Previous studies have shown smaller hippocampal volumes in AUD adolescents (De Bellis et al., 2005, Medina et al., 2007b, De Bellis et al., 2000, Nagel et al., 2005). However, the AUD samples studied had psychiatric (especially externalizing) comorbidity. We did not find any evidence of smaller hippocampal volumes in our AUD sample. We note that: 1) our AUD sample was larger than those of these other studies (n=14 for Nagel et al, n=12 for deBellis, n=16 AUD for Medina, with n=26 AUD+Marijuana also in Medina et al.); 2) alcohol use in our AUD sample was greater than that in the other studies; and 3) our subjects showed a binge-drinking pattern at least as severe as the other studies. The major factor separating our study from the others is the sample – we studied excessive alcohol use in adolescence in the absence of predisposing psychiatric (including externalizing) disorder and in the absence of comorbid drug abuse. Our study raises the issue of whether smaller hippocampal volumes in adolescents with AUD is a consequence of these predisposing and comorbid factors rather than alcohol abuse per se. Consistent with this, our failure to find amygdala volume differences between AUD and control samples is consistent with a model wherein smaller amygdala volumes represent a vulnerability factor for addiction (including alcoholism) (Hill, 2004, Koob, 1999).
We observed significantly smaller GM density in the left frontal/temporal/parietal region in a VBM analysis, and confirmed this result in a region of interest analysis in the same area. The GM density in this region was not related to any alcohol variable, and therefore probably does not reflect damage due to alcohol per se. Within AUD, larger frontal/temporal/parietal GM density was associated with higher psychomotor and self-monitoring scores, which was not intuitive as higher scores on these measures imply worse performance (e.g., slower reaction times, more self-monitoring behaviors), and one might reasonably expect higher GM to improve cognition. In a study of binge drinking adolescents, Squeglia and colleagues (Squeglia et al., 2012) found that thicker frontal cortex was associated with poor performance in several cognitive domains, another counter-intuitive example. It may be that in these adolescent samples, regions of higher GM density or a thicker cortex index an alcohol-related delay in selective pruning, with consequent poor cognitive performance.
A trend to smaller GM density in the right and left lateral frontal regions was also observed, but smaller volumes were not confirmed by the region of interest analysis. However, within AUD, smaller volumes of the lateral frontal regions were associated with higher alcohol consumption, so this trend to smaller volumes is more likely to be a direct consequence of alcohol use.
In the current study, comorbid DSM axis I disorders and substance dependence were exclusionary critierion whereas in the United States studies of AUD adolescents, comorbid psychiatric (e.g., major depressive disorder, post-traumatic stress disorder; attention deficit hyperactivity disorder, conduct disorder, oppositional defiant disorder, generlized anxiety disorder) and substance use disorders were the norm. It is easy to attribute the different results in the current study to this lack of psychiatric and substance use disorder comorbidity. It would be easier to support this conclusion were we to find effects similar to the U.S. studies in South Africa adolescent AUD samples with such comorbidities. Moreover, both AUD and control adolescents in this study had experienced substantial trauma, had low socioeconomic status (SES), and a substantial number were cigarette smokers. It is possible that exposure to trauma, low SES, and smoking may have resulted in smaller brain volumes within the Cape Town samples (both AUD and controls), such that the additional effect of alcohol on brain volume in the AUD adolescents was small, perhaps reaching a floor. Further study is necessary to assess these possible contributing factors
Several limitations should be noted. First, the analysis of the effects of smoking had relatively low power. Only 15% of the AUD participants were non-smokers, so smoking status and AUD status were highly collinear, compromising the ability to detect any additional effect on brain volumes due to smoking. So while our findings suggest that the level of smoking did not impact the group findings, we cannot rule out that smoking contributes to smaller brain volumes.
Second, there are possible problems of cross-cultural adaptations/translations of the neuropsychological battery used in this study, that may have affected our ability to assess the cognition of our participants. Few tests are developed for the South African population and language groups. Although one may argue that translating the instructions and materials may create compatibility issues, the materials were carefully translated and then back-translated by local linguists who have a good sense of local language usage and nuances. All things considered, we believe that adapting the tests to suit our population allows a closer approximation of ability versus using tests specifically developed for South Africa. Importantly, we did not use United States norms in scoring the tests. A strength of our study, then, is that the neuropsych results are valid in a South African population, although we cannot make a direct comparison to US populations. Despite this, previous work using this battery was able to detect poor performance of AUD adolescents compared to controls, supporting the validity of the approach undertaken (Ferrett et al., 2010).
Third, the images in this study were acquired using two different MRI acquisition protocols. The MRI data collected at the beginning of our study, acquired using a transaxial acquisition without complete blood saturation, had good GM/WM contrast and were easily segmented by the FSL segmentation programs. Despite the adequacy of these images, when we recognized that we could acquire better images, we decided to switch acquisition protocols if we could determine that the segmentation outputs were comparable. We acquired both protocols on 22 subjects and examined the correlation between protocols for multiple subcortical and cortical volumes, all of which were highly correlated. Out of an abundance of caution, however, each AUD participant was matched to a control participant that had been studied using the same MRI protocol. In addition, we included a protocol factor in our statistical analyses to account for systematic volume differences due to acquisition. In this way, any effect of protocol on the brain volumes would be both balanced between groups and accounted for statistically. Although it is still possible that the two acquisition protocols contributed to the absence of alcohol effects on most subcortical and cortical regions, we believe that any acquisition effect is minimal and has been accounted for.
Lastly, this was a cross-sectional study, which cannot address the long-term neural effects of alcohol exposure during adolescence, and which does not allow causal inferences about the associations noted here to be drawn. At the same time, this study is strengthened by the focus on subjects with relatively minimal comorbid substance use and externalizing psychopathology. During adolescence, there is significant neuroplasticity, and it is possible that this provides some resilience against alcohol-induced neural damage. At the same time, findings in individuals with comorbid conditions may reflect the combined effects of the comorbidity and the effects of the comorbidity on individual’s vulnerability to the neurotoxic effects of excessive alcohol use.
Acknowledgments
This work was supported by a NIAAA grant (AA016303).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson MJ, Smith S. Technical Report TRO7JA2. Oxford, U.K.: Oxford University, Oxford Center for Functional Resonsance Imaging of the Brain; 2007. Non-linear registration, a.K.A. Spatial normalization. [Google Scholar]
- Angstrom J, Larsson T, Hansson GC, Karlsson KA, Henry S. Default biosynthesis pathway for blood group-related glycolipids in human small intestine as defined by structural identification of linear and branched glycosylceramides in a group o le(a-b-) nonsecretor. Glycobiology. 2004;14:1–12. doi: 10.1093/glycob/cwh003. [DOI] [PubMed] [Google Scholar]
- Association WM. Declaration of helsinki. Ethical principles for medical research involving human subjects. 59th wma general assembly; seoul. 2008. [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Cohen M. Chuldren’s memory scale: Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Corthorn J, Figueroa C, Valdes G. Estrogen and luminal stimulation of rat uterine kallikrein. Biol Reprod. 1997;56:1432–8. doi: 10.1095/biolreprod56.6.1432. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. Tower of london: Technical manual. New York, NY: Multi-Health Systems Inc; 2001. [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Alcoholism Clinical and Experimental Research. 2000;157:737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism Clinical and Experimental Research. 2005;29:1590–600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R, Verny C, Krystkowiak P, Damier P, Bachoud-Levi AC, Hantraye P, Remy P. Distribution of grey matter atrophy in huntington’s disease patients: A combined roi-based and voxel-based morphometric study. Neuroimage. 2006;32:1562–75. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Flinn P, Shumway R. Psychiatric comorbidity in older long-term abstinent alcoholics. Alcoholism Clinical and Experimental Research. 2008;33:1564–71. doi: 10.1016/j.addbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Taylor C, Moon K, Barakos J, Tran H, Landman B, Shumway R. Controlling for premorbid brain size in imaging studies: T1-derived cranium scaling factor vs. T2-derived intracranial vault volume. Psychiatry Research. 2004;131:169–76. doi: 10.1016/j.pscychresns.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fein G, Mcgillivray S. Cognitive performance in long-term abstinent elderly alcoholics. Alcohol Clin Exp Res. 2007;31:1788–1799. doi: 10.1111/j.1530-0277.2007.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naive actively drinking alcohol-dependent sample. Alcohol Clin Exp Res. 2009a doi: 10.1111/j.1530-0277.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcoholism Clinical and Experimental Research. 2009b;33:1806–14. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrett HL, Carey PD, Thomas KG, Tapert SF, Fein G. Neuropsychological performance of south african treatment-naive adolescents with alcohol dependence. Drug and Alcohol Dependence. 2010;110:8–14. doi: 10.1016/j.drugalcdep.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa K, Feuerstein D, Principe AL. A comparison of three-dimensional spiral computed tomoangiography with renal angiography in the live-donor work-up. J Transpl Coord. 1997;7:195–8. doi: 10.7182/prtr.1.7.4.2p24p5vg11132186. [DOI] [PubMed] [Google Scholar]
- Foxcroft C, Paterson H, Le Roux N, Herbst D. Research Output. Pretoria: Human Sciences Research Council; 2004. Psychological assessment in south africa: A needs analysis: The test use patterns and needs of psychological assessment practitioners. Final report. [Google Scholar]
- Golden CJ, Freshwater SM. Stroop color and word test: A manual for clinical and experimental uses. Los Angeles, CA: Stoelting Co; 2002. [Google Scholar]
- Hill SY. Trajectories of alcohol use and electrophysiological and morphological indices of brain development: Distinguishing causes from consequences. Annals of the New York Academy of Sciences. 2004;1021:245–259. doi: 10.1196/annals.1308.029. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. [March 25, 2010];The schedule for affective disorders and schizophrenia for school aged children (6–18 years) lifetime version. 1996 doi: 10.1097/00004583-199707000-00021. [Online]. Available: http:\\www.wpic.pitt.edu\ksads. [DOI] [PubMed]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Alcoholism Clinical and Experimental Research. 1999;877:445–60. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Llorente AM, Williams J, Satz P, D’elia LF. Children’s color trails test: Professional manual. Odessa: Psychological Assessment Resources; 2003. [Google Scholar]
- Maj M, D’elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, Starace F, Galderisi S, Chervinsky A. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of hiv-1 seropositive persons: A who study. Alcoholism Clinical and Experimental Research. 1993;8:123–35. [PubMed] [Google Scholar]
- Markov AK, Brumley MA, Figueroa A, Skelton TN, Lehan PH. Hemodynamic effects of fructose 1,6-diphosphate in patients with normal and impaired left ventricular function. Alcoholism Clinical and Experimental Research. 1997;133:541–9. doi: 10.1016/s0002-8703(97)70149-2. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007a;13:807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Mcqueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism Clinical and Experimental Research. 2008;32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007b;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CD, Myers B, Morojele NK, Flisher AJ, Bhana A, Donson H, Pluddemann A. Trends in adolescent alcohol and other drug use: Findings from three sentinel sites in south africa (1997-2001) J Adolesc. 2004;27:429–40. doi: 10.1016/j.adolescence.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Patenaude BM. Bayesian statistical models of shape and appearence for subcortical brain segmentation, in department of clinical neurology. Vol. 236 University of Oxford; Oxford: 2007. [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative mri study. Alcoholism Clinical and Experimental Research. 1992;16:1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pluddemann A, Parry C, Cerff P, Bhana A, Pereira T, Potgieter H, Gerber W, Nqini T, Petersen P. Monitoring alcohol and drug abuse trends in south africa. Drug and Alcohol Review. 2008;2:185–189. [Google Scholar]
- Rinehart J, Keville L, Clayton S, Figueroa JA. Corticosteroids alter hematopoiesis in vitro by enhancing human monocyte secretion of granulocyte colony-stimulating factor. Experimental Hematology. 1997;25:405–12. [PubMed] [Google Scholar]
- Russell EW, Starkey RI. Halstead russell neuropshchological evaluation system (hrnes) Alcoholism Clinical and Experimental Research 1993 [Google Scholar]
- Saban A, Flisher AJ, Distiller G. Association between psychopathology and substance use among school-going adolescents in cape town, south africa. J Psychoactive Drugs. 2010;42:467–76. doi: 10.1080/02791072.2010.10400709. [DOI] [PubMed] [Google Scholar]
- Sameti M, Smith S, Patenaude B, Fein G. Subcortical volumes in long term abstinent alcoholics: Associations with psychiatric comorbidity. Alcoholism Clinical and Experimental Research. 2011;35:1067–1080. doi: 10.1111/j.1530-0277.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural mr image analysis and implementation as fsl. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, N.J.: Humana Press; 1992. [Google Scholar]
- Spss Inc. Alcoholism Clinical and Experimental Research. Chicago: SPSS Inc; 2009. Spss statistics 18.0, rel.18.0.0. [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220:529–39. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests : Administration, norms, and commentary. Oxford ; New York: Oxford University Press; 2006. [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 2000;14:341–52. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism Clinical and Experimental Research. 1995;19:110–22. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system : An approach to cerebral imaging. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Van Ommen CH. Putting the pc in iq: Images in the wechsler adult intelligence scale -third edition (wais-iii) South African Journal of Psychology. 2005;35:532–554. [Google Scholar]
- Wechsberg WM, Jones HE, Zule WA, Myers BJ, Browne FA, Kaufman MR, Luseno W, Flisher AJ, Parry CD. Methamphetamine (“Tik”) use and its association with condom use among out-of-school females in cape town, south africa. Am J Drug Alcohol Abuse. 2010;36:208–13. doi: 10.3109/00952990.2010.493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Matthews D, Best P. Ethanol, memory, and hippocampal function: A review of recent findings. HIPPOCAMPUS. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in fsl. Neuroimage. 2009;45:S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Smith S, Brady M. Segmentation of brain mr images through a hidden markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]


