Abstract
Non-surgical therapies for human malignancies must negotiate complex cell signaling pathways to impede cancer cell growth, ideally promoting death of cancer cells while sparing healthy tissue. For most of the past half century, medical approaches for treating cancer have relied primarily on cytotoxic chemotherapeutics that interfere with DNA replication and cell division, susceptibilities of rapidly dividing cancer cells. As a consequence, these therapies exert considerable cell stress, promoting the generation of ceramide through de novo synthesis and recycling of complex glycosphingolipids and sphingomyelin into apoptotic ceramide. Radiotherapy of cancer exerts similar geno- and cytotoxic cell stresses, and generation of ceramide following ionizing radiation therapy is a well-described feature of radiation-induced cell death. Emerging evidence now describes sphingolipids as mediators of death in response to newer targeted therapies, cementing ceramide generation as a common mechanism of cell death in response to cancer therapy. Many studies have now shown that dysregulation of ceramide accumulation—whether by reduced generation or accelerated metabolism—is a common mechanism of resistance to standard cancer therapies. The aims of this chapter will be to discuss described mechanisms of cancer resistance to therapy related to dysregulation of sphingolipid metabolism and to explore clinical and preclinical approaches to interdict sphingolipid metabolism to improve outcomes of standard cancer therapies.
1. INTRODUCTION
1.1. Ceramide as a mediator of apoptosis
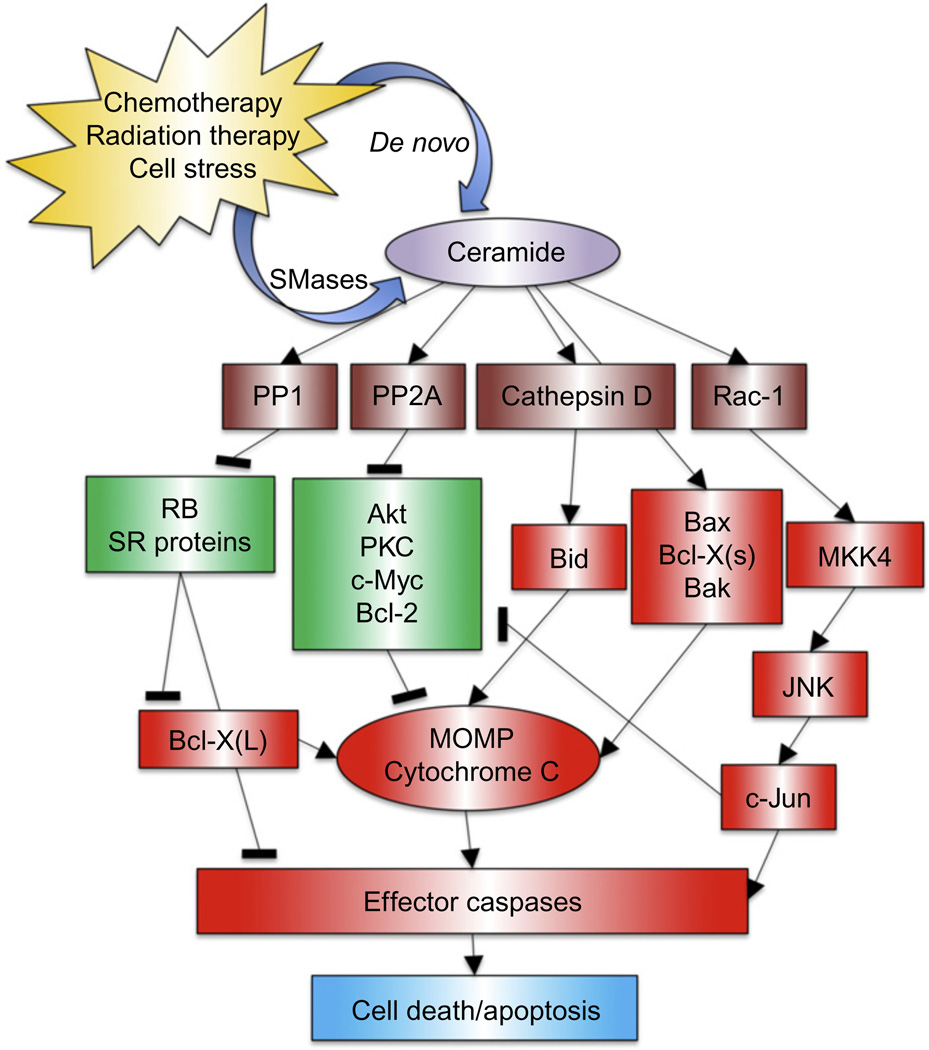
Ceramide is the central molecule in sphingolipid metabolic pathways, and its generation and metabolism are key in understanding advantageous and dysregulated sphingolipid responses to cancer therapy. While many diverse functions have been ascribed to ceramide, for the purposes of this review, ceramide is best characterized to promote apoptosis and cell senescence. Among, the major effectors of ceramide signaling are protein phosphatases PP2A and PP1, which are activated by ceramide (Chalfant et al., 1999; Dobrowsky, Kamibayashi, Mumby, & Hannun, 1993; Galadari, Kishikawa, Kamibayashi, Mumby, & Hannun, 1998). Through activation of PP2A, ceramide promotes numerous signaling alterations including deactivation of Akt (Teruel, Hernandez, & Lorenzo, 2001), PKC (Lee, Hannun, & Obeid, 1996), and c-Jun (Shirakabe et al., 1997); destabilization of c-Myc (Mukhopadhyay et al., 2009); and disruption of the Bax/Bcl-2 interaction (Xin & Deng, 2006). PP1 activation causes dephosphorylation of SR proteins with subsequent alternative splicing of Caspase 9 and Bcl-X (Chalfant et al., 2002) and activation of retinoblastoma (Liu, Wang, & Berndt, 2006). Apart from the myriad functions of ceramide downstream of PP2A and PP1 activation, ceramide generated in the lysosome by acid sphingomyelinase (ASMase) has also been shown to directly bind and induce autoproteolytic cleavage of Cathepsin D (Heinrich et al., 1999), promoting the cleavage-induced activation of proapoptotic Bid. These functions of ceramide, represented in Fig. 1.1, ultimately converge upon causing cell cycle arrest, senescence, and in many cases apoptosis and cell death. While the vast majority of the literature support these anticancer effects of ceramide, it is worthwhile to acknowledge that antiapoptotic roles have been described for some specific ceramide species (Hoye, Davoren, Wipf, Fink, & Kagan, 2008), highlighting the complexities of ceramide signaling that remain to be fully characterized. A more complete review by Ogretmen and Hannun (2004) details these functions of ceramide and more and explains why ceramide accumulation is a hallmark of diverse apoptotic stimuli, including chemo- and radiotherapy and lay the groundwork for why adjuvant therapeutics that promote accumulation of ceramide are promising approaches to improving response to standard therapies for cancer.
Figure 1.1.
Ceramide in apoptosis. Several direct targets of ceramide have been identified, including Cathepsin D and the serine/threonine protein phosphatases PP1 and PP2A. These phosphatases act on several substrates such as the retinoblastoma gene product Rb, Bcl-2, PKC, Akt, and SR proteins. Increasingly, these pathways seem to be compartmentalized. In particular, Cathepsin D is activated by ceramide generated in lysosome membranes leading to activation of the proapoptotic protein Bid. Mitochondrial membrane potential can also be altered by these pathways, probably through disparate signal cascades affecting various Bcl-2 family members. Apoptosis induction through JNK activation is mediated by ceramide-dependent signaling by MKK4. These downstream effects can lead to changes in growth arrest, senescence, and apoptosis.
1.2. Ceramide generation and metabolism
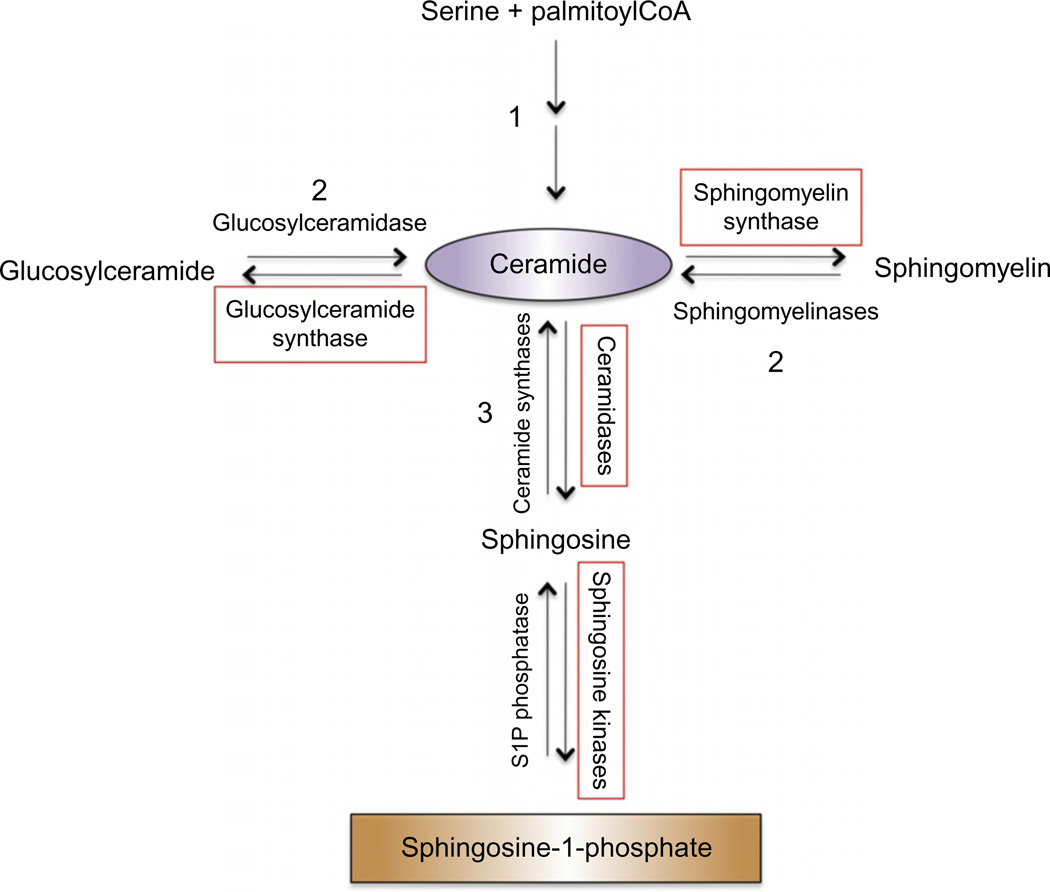
A favorable sphingolipid response to cancer therapy is frequently a net accumulation of ceramide; therefore, it is convenient to view metabolic pathways of ceramide generation and consumption, outlined in Fig. 1.2, in terms of steps that generate ceramide and those that consume it. Ceramide is N-acylated sphingosine with naturally occurring acyl chain lengths from 14 to 26 carbons. It can be generated by de novo synthesis, a multistep process initiated by the condensation of serine and palmitoyl CoA (Xu et al., 1998); by the salvage pathway, which recycles cellular sphingosine (Kitatani, Idkowiak-Baldys, & Hannun, 2008); or by hydrolysis from complex sphingolipids such as sphingomyelin and cerebrosides (galactosyl- and glucosylceramide). De novo synthesis and hydrolysis of sphingomyelin frequently play key roles in generating ceramide in response to cancer therapy, and defects in generation of ceramide by these pathways are implicated in therapy resistance. The following sections will highlight known defects in ceramide generation and how they impact cancer therapy. Conversely, consumption of free ceramide by incorporation into sphingomyelin or cerebrosides or by deacylation of ceramide to form sphingosine is associated with defects in therapy-induced apoptosis. As will be discussed in this chapter, response of cancer cells and tumors to standard therapies like chemotherapy and radiation can be improved by potentiating accumulation of ceramide whether by promoting its generation or by inhibiting its catabolism. Importantly, deacylation of ceramide by ceramidases generates sphingosine, the substrate of sphingosine kinases. Sphingosine 1-phosphate (S1P) is a pleiotropic signaling lipid that frequently opposes apoptosis and promotes angiogenesis and cell migration, justifying prevention of S1P formation in addition to enhancement of ceramide generation as a worthy goal in interdiction of sphingolipid metabolism to improve response to cancer therapy. While this chapter will include discussion of sphingosine kinases and S1P, their roles in cancer promotion and therapy are dealt with more completely in Chapters 5, 6, and 7 of this volume.
Figure 1.2.
Basic sphingolipid metabolism and nodes of interdiction: Ceramide is generated by (1) de novo biosynthesis, a multistep process which is initiated by condensation of serine and palmitoyl CoA; (2) metabolism of existing complex sphingolipids such as glucosylceramide and sphingomyelin; and (3) salvage of free sphingosine by N-acylation by ceramide synthases. Enzymes that catalyze steps that reduce cellular ceramide and have been investigated as potential nodes of interdiction of ceramide metabolism to improve clinical response to standard therapies are boxed in red and will be further discussed. Note that other sphingolipids and sphingolipid metabolizing enzymes, such as ceramide-1-phosphate and ceramide kinase, are not further discussed in this chapter and are not included in this diagram. A complete review of sphingolipid metabolism is referenced (Gault, Obeid, & Hannun, 2010).
1.3. Dysregulation of sphingolipid metabolism in cancer
One of the defining characteristics of cancer cells is the ability to evade apoptosis by development of signaling mechanisms that prevent response to apoptotic stimuli (Hanahan & Weinberg, 2000). Numerous mechanisms are described for death resistance in cancer, including upregulation of pro-survival genes and downregulation of apoptotic genes. Cancer cells also frequently cultivate dysregulation of enzymes involved in sphingolipid metabolism to escape cell death. Dysregulation of sphingolipid metabolic pathways is a common theme in human cancers, perhaps because sphingolipid signaling operates upstream of several apoptotic mediators, suggesting that alteration of sphingolipid metabolism may be an important step in tumorigenesis and, as such, that manipulation of sphingolipid metabolism may improve response to therapeutics. Multiple sphingolipid enzymes are known to be aberrantly expressed in human cancer (Table 1.1).
Table 1.1.
Summary of the literature demonstrating alterations in sphingolipid-metabolizing enzymes in primary human cancer tissues
| Sphiningolipid enzymes |
Expression incancer |
Cancer model | Reference |
|---|---|---|---|
| Glucosylceramide synthase (GCS) | Elevated | Bladder, breast | Liu et al. (2011) and Sun et al. (2010) |
| Acid ceramidase | Elevated | Head and neck, prostate, T-cell large granular lymphocyte (LGL) leukemia | Elojeimy et al. (2007), Norris et al. (2006), and Shah et al. (2008) |
| Sphingomyelinase | Decreased (Alkaline) | Colon, liver | Di Marzio et al. (2005) and Hertervig, Nilsson, Nyberg, and Duan (1997) |
| Sphingosine kinases | Elevated | Breast, astrocytomas, head and neck, prostate, thyroid, gastric | Erez-Roman, Pienik, and Futerman (2010), Guan et al. (2011), Li et al. (2008, 2009), Malavaud et al. (2010), and Shirai et al. (2011) |
| Ceramide synthases | Elevated (Cer2/4/6) | Breast | Erez-Roman et al. (2010) and Schiffmann et al. (2009) |
| Ceramide kinase | Elevated | Breast, non-small cell lung | Kossenkov et al. (2011) and Ruckhäberle et al. (2009) |
| S1P lyase | Decreased | Colon | Oskouian et al. (2006) |
| S1P phosphatase | Decreased | Colon | Oskouian et al. (2006) |
1.4. Susceptible nodes of sphingolipid metabolism for interdiction in cancer therapy
Study of sphingolipid metabolism in cancer has led to the identification of several nodes of sphingolipid metabolism that are susceptible to interference as we seek to improve outcomes of cancer therapy. These nodes can generally be divided into those that promote ceramide generation and those that prevent its consumption. Multiple therapeutic approaches cause generation of ceramide through de novo synthesis and sphingomyelin hydrolysis, and thus ceramide generation is an important part of standard cancer therapy. Increasingly, approaches that rely on inhibition of enzymes that consume ceramide such as glucosylceramide synthase (GCS), ceramidases, or sphingosine kinases are being combined with standard cancer therapies as known dysregulation of these enzymes occurs in cancer and contribute to therapy resistance. Each of the approaches outlined in Fig. 1.2 will be discussed in terms of both known defects in these pathways that contribute to resistance to standard cancer therapies as well as work that demonstrates the principles and feasibility of targeting these enzymes to improve patient response to standard of care therapies including cytotoxic chemotherapy, radiation therapy, and more recently introduced targeted therapies like tyrosine kinase inhibitors (TKIs), deacetylase inhibitors, and monoclonal antibodies (mAbs).
2. SPHINGOLIPIDS AND CYTOTOXIC CHEMOTHERAPIES
The past decade has brought on a new wave of therapeutic approaches to medical management of cancer including TKIs, mAbs, and cancer vaccines; however, mainstay therapy for most human cancer remains, as it has since the mid-century development of mustine (Mechlorethamine Hydrochloride) in the treatment of lymphoma, cytotoxic chemotherapy that targets DNA synthesis and cell division, thus, affecting rapidly proliferating cancer cells more than most normal tissues. Interestingly, several classes of cytotoxic chemotherapeutics cause accumulation of ceramide. These include vinca alkaloids (vincristine and vinblastine), anthracyclines (doxorubicin and daunorubicin), taxanes (paclitaxel), and topoisomerase inhibitors (irinotecan, etoposide), among others. Here, we will discuss how cytotoxic chemotherapeutics elicit ceramide generation, research that demonstrates dysregulation of sphingolipids as a mechanism of resistance to chemotherapy, and how augmentation of a favorable sphingolipid response to therapy could improve outcomes.
2.1. Chemotherapy-induced ceramide generation
Mammalian cells respond to diverse stressors including radiation, nutrient deprivation, and oxidative stress with ceramide generation, largely through hydrolysis of sphingomyelin and de novo synthesis (Hannun, 1996; Ogretmen & Hannun, 2001). Evidence of stress-induced ceramide generation in yeast points to ceramide generation as an evolutionarily ancient response to cell stress (Cowart & Obeid, 2007). While how exactly remains incompletely understood, it seems that cytotoxic chemotherapeutics co-opt this stress response, inducing ceramide generation and thereby the goal of such therapies: apoptosis. Ceramide accumulation in response to chemotherapy occurs both through de novo synthesis and hydrolysis of sphingomyelin. Danuorubicin, etoposide, camptothecin, and gemcitibine appear to cause de novo ceramide biosynthesis either by activation of dihydroceramide synthases or by increasing the activity of serine palmitoyl transferase, the first step in the de novo pathway (Bose et al., 1995; Chalfant et al., 2002; Chauvier, Morjani, & Manfait, 2002; Perry et al., 2000). Interestingly, inhibiting the de novo pathway of ceramide synthesis antagonizes the cytotoxic effects of these drugs, highlighting the role of de novo ceramide generation in chemotherapy-induced cell death. Chemotherapy also promotes ceramide generation through the sphingomyelinase (SMase) pathway, liberating death-inducing ceramide from the vast reservoir of membrane sphingomyelin. Treatment of HL-60 cells with DNA antimetabolite cytosine arabinoside increased ceramide levels following stimulation of neutral sphingomyelinase (NSMase) activity (Strum, Small, Pauig, & Daniel, 1994), demonstrating sphingomyelin hydrolysis in response to a chemotherapy drug. Jaffrezou et al. (1996) described biphasic generation of ceramide in leukemia cells in response to daunorubicin that was unaffected by the ceramide synthase inhibitor Fumonisin B1, again implicating SMase activation as a mechanism of chemotherapy-induced ceramide generation. In another study, etoposide was found to cause ceramide generation by activating NSMase in glioma cells (Sawada et al., 2000). Interestingly, overexpression of Bcl-2 suppressed NSMase activation, preventing ceramide accumulation and cell death. This study is an excellent example of how cancers can suppress ceramide generation following chemotherapy by inhibiting SMase, thereby escaping treatment-induced cell death.
2.2. Glucosylceramide synthase dysregulation
While mechanisms employed by cancer cells to prevent generation of ceramide to avoid therapy-induced death are interesting proof of concept that ceramide is a potent mediator of successful chemotherapy, inhibition of enzymes that catabolize ceramide is ultimately more likely to result in treatment protocols with sphingolipid metabolizing enzyme inhibitors as chemosensitizing agents. Perhaps the best-studied mechanism of chemotherapy resistance due to sphingolipid dysregulation is the increased activity of GCS and accumulation of glucosylceramide in chemotherapy resistant cells. Early in the study of sphingolipid dysregulation as a mechanism of chemotherapy resistance, it was discovered that glucosylceramide was markedly elevated in multidrug resistant cells compared to their drug-sensitive counterparts (Lavie et al., 1997), as well as the presence of glucosylceramide in the tissues of patients who did not respond to chemotherapy, but not in the tissues of patients who had chemotherapy response (Lucci et al., 1998). These studies led to the identification of GCS as a mediator of chemotherapy resistance. Inhibition of GCS restored paclitaxel, etoposide, and doxorubicin sensitivity to drug-resistant MCF-7 cells (Liu et al., 2004; Liu, Han, Giuliano, &Cabot, 2001). GCS has since been shown to be overexpressed in metastatic breast cancer and bladder cancer (Liu et al., 2011; Sun et al., 2010) and has been found by multiple groups to regulate multidrug resistance through regulation of p-glycoprotein and MDR1 expression (Gouaze et al., 2005). Interference with glucosylceramide generation by targeting GCS with small molecule inhibition and RNA interference techniques has consistently shown that inhibition of this enzyme can restore chemotherapy sensitivity and promote cell death and apoptosis by reversing upregulation of MDR1 (Sun et al., 2010) and p-glycoprotein (Gouaze et al., 2005) and causing ceramide accumulation (Baran, Bielawski, Gunduz, & Ogretmen, 2011). Thus, at least in preclinical models, GCS is a prototypical ceramide-metabolizing enzyme that is a prime target for overcoming cancer resistance to standard chemotherapy.
2.3. GCS as a target for chemosensitization
Given the strong body of the literature implicating GCS in multidrug resistance in cancer cell lines, it is surprising and perhaps disappointing how few studies have examined the feasibility and efficacy of targeting GCS in vivo. The data that are reported, however, are encouraging. Treatment of ganglioside-rich murine melanoma cells with the GCS inhibitor 1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol drastically reduced their tumor-forming ability and incidence of metastasis when injected into mice (Deng, Li, & Ladisch, 2000). The same group reported similar results using GCS antisense (Deng, Li, Guerrera, Liu, & Ladisch, 2002), confirming that targeting GCS inhibition is efficacious in inhibiting cancer in vivo. Miglustat is an FDA-approved inhibitor of GCS used in some patients with Gaucher’s disease, who lack glucosylceramidase, as substrate reduction therapy (Machaczka et al., 2012). Though this drug has not been evaluated in cancer and is often poorly tolerated, its approval lends proof of concept to treating patients with GCS inhibitors as chemosensitizing agents.
2.4. Ceramidase dysregulation
Another means of preventing the accumulation of ceramide that is necessary for chemotherapy-induced apoptosis is by action of ceramidases. These enzymes, of which there are five in humans, deacylate ceramide to form sphingosine and a fatty acid. This positions them at a critical juncture in the balance between proapoptotic ceramide and antiapoptotic S1P. As such, ceramidases have been intensively studied as mediators of sphingolipid metabolism and cancer. Much of the focus of the roles of ceramidases in cancer has been on lysosomal acid ceramidase. Our group and others have demonstrated that acid ceramidase is frequently dysregulated in human cancer including >60% of human prostate tumors (Saad et al., 2007) and >70%of head and neck squamous cell carcinoma (Elojeimy et al., 2007). Acid ceramidase has been shown to confer aggressive characteristics to cancer cells in vitro and in vivo, promoting cell proliferation and xenograft growth (Saad et al., 2007), and cancer cell invasion (Beckham et al., 2012). Additionally, acid ceramidase has been shown to mediate resistance to a number of treatment modalities including cytotoxic chemotherapy. Our group has shown that acid ceramidase overexpression promoted prostate cancer cell resistance to chemotherapy with etoposide, cisplatin, doxorubicin, and gemcitabine by preventing accumulation of proapoptotic C16-ceramide following administration of these drugs. Importantly, targeting acid ceramidase with siRNA restored sensitivity to chemotherapy, further highlighting the potential of targeting ceramidases to overcome chemotherapy resistance. Interestingly, evidence suggests that acid ceramidase is upregulated in cancer cells as a response to chemotherapy. Morales et al. (2007) showed that treatment of hepatoma cells, but not normal hepatocytes, with daunorubicin posttranscriptionally upregulated acid ceramidase. When acid ceramidase was pharmacologically inhibited or targeted with siRNA in these cells, ceramide accumulated more than when cells were treated with daunorubicin alone, and the hepatoma cells were sensitized to cell death. We have shown that acid ceramidase overexpression induced by radiation therapy (discussed in Section 3) protected prostate cancer cells from subsequent taxol chemotherapy, an effect that was reversed using a small molecule inhibitor of acid ceramidase, LCL385 (Mahdy et al., 2009). Small molecule inhibitors of acid ceramidase based on the structure of the prototypical ceramidase inhibitors (1R, 2R)-N-myristoylamino-4′-nitro-phenylpropandiol-1,3 (B13) and (1S, 2R)-N-myristoylamino-phenylpropanol-1 (D-e-MAPP) have been developed (Bielawska et al., 2008; Szulc et al., 2008) and tested in vitro and in vivo against cancer cell lines and xenografts with promising results (Holman et al., 2008).
Ceramidases generate sphingosine which is phosphorylated by sphingosine kinases to form S1P. Thus, sphingosine kinase inhibition has been evaluated as a mechanism to prevent generation of frequently oncogenic S1P, but also as a means to prevent consumption of cellular ceramide and sphingosine. Indeed, sphingosine kinases have been well studied as mediators of cancer. Sphingosine kinase 1 (SK1) is overexpressed at the message or protein level in lung, breast, stomach, thyroid, brain, colon, and kidney cancer (Pyne & Pyne, 2010). Myriad studies demonstrate a clear oncogenic role for sphingosine kinases and S1P, which are discussed more completely in Chapters 5, 6, and 7 of this volume and in a number of excellent reviews (Pyne & Pyne, 2010; Takabe, Paugh, Milstien, & Spiegel, 2008).
With the well-described oncogenic roles of sphingosine kinases, it is perhaps unsurprising that sphingosine kinase dysregulation has been observed as a mechanism for resistance to conventional chemotherapy. Resistance of prostate cancer cells to the topoisomerase inhibitor camptothecin was found to be due to upregulation of both SK1 and S1P receptors 1 and 3 in PC3 cells in response to camptothecin treatment. Camptothecin-sensitive LNCaP cells did not exhibit upregulation of these proteins. Knockdown of SK1 with siRNA and inhibition of S1P G-protein-coupled receptor signaling with pertussis toxin predictably resensitized PC3 cells to camptothecin (Akao et al., 2006). These results were confirmed by another group, which also showed that LNCaP resistance to docetaxel was similarly due to upregulation of SK1. Again, inhibition of sphingosine kinases, this time with small molecule inhibition, sensitized PC3 and LNCaP cells to chemotherapy with camptothecin and docetaxel, respectively (Pchejetski et al., 2008). Further work by this group demonstrated that docetaxel chemotherapy in hormone-resistant prostate cancer cells could be potentiated by pharmacological and RNAi targeting of SK1, with inhibition resulting in a fourfold improvement in docetaxel IC50 (Sauer et al., 2009). In notoriously difficult to treat pancreatic cancer, manipulation of the ceramide/S1P ratio using ceramide analogs and SK1 inhibition improved sensitivity to gemicitibine therapy, whereas upregulating SK1 caused gemcitibine resistance. In another interesting study, SK1 overexpression was frequent in human non-small cell lung carcinoma tissues, and positively correlated with nodal involvement and metastasis whereas it negatively correlated with patient survival (Song et al., 2011). The same study determined that SK1 expression mediated resistance to docetaxel and doxorubicin, and that targeting SK1 expression with RNAi sensitized cancer cells to these therapies. While much focus of sphingosine kinase in cancer has been on SK1, sphingosine kinase 2 (SK2) is also considered a target as its inhibition has been effective in in vitro models of cancer (Gao & Smith, 2011). In fact, an isozyme-specific SK2 inhibitor, ABC294640, is in a phase I clinical trial for patients with solid tumors making it the most clinically advanced sphingosine kinase inhibitor to date.
2.5. Sphingomyelin synthase and ceramide kinase
While somewhat less studied than chemotherapy resistance by metabolism of ceramide by GCS, ceramidases, and sphingosine kinases, activation of sphingomyelin synthase activity is another means by which cancers have been shown to escape death by chemotherapy. Sphingomyelin synthase and GCS activities were found to be elevated in doxorubicin-resistant leukemia cells compared to doxorubicin-sensitive cells, and resistant cells had lower levels of ceramide (Itoh et al., 2003). This study highlights the possibility that sphingomyelin synthesis could be a mechanism of escape from treatment sensitivity in leukemia and perhaps other cancers, and that sphingomyelin synthase inhibition may restore ceramide accumulation and apoptosis in response to chemotherapy. Conversely, a recent study has shown that the efficacy of the novel anticancer drug 2-hydroxyoleic acid is effective in inducing cell cycle arrest and apoptosis glioma cells by virtue of its ability to activate sphingomyelin synthase (Barcelo-Coblijn et al., 2011). The authors point to the critical role of sphingomyelin in organizing lipid raft signaling events that are frequently necessary for apoptosis. These conflicting reports on the impact of sphingomyelin synthase in cancer chemotherapy remind us of the complexity of sphingolipid signaling and the diverse roles that ceramide plays both as a signaling lipid and as a component of more complex structural components of cell membranes. Another ceramide-modifying enzyme, ceramide kinase, and its product ceramide 1-phosphate have been shown to promote cancer in in vitro models (Mitra et al., 2007). While no studies implicating ceramide kinase in resistance to cancer therapy have been published to date, ceramide kinase has been proposed as a potential target for cancer therapy, and could, theoretically, improve outcomes to standard chemotherapies in certain contexts (Lamour & Chalfant, 2008).
The rationale for targeting ceramide-metabolizing enzymes to enhance response to cytotoxic chemotherapies is clear: generation of ceramide is a consequence of cancer chemotherapy, and mechanisms that prevent ceramide accumulation in response to chemotherapy cause resistance. Several enzymes have been identified as worth targets of interdiction of ceramide metabolism in chemotherapy including GCS, acid ceramidase, and sphingosine kinases. With a wealth of data justifying targeting these enzymes to improve standard cancer chemotherapy, it is our hope that clinically viable agents will be developed in the near future.
3. SPHINGOLIPIDS AND RADIATION THERAPY
3.1. Radiation therapy in the standard of care
Radiation therapy aims at deteriorating the malignant cells and tumor bed via delivery of physical lesions resultant of high energy liberation of electrons from vital cellular structures, which in turn activate pathways of programmed cell death. Therefore, unlike the systemic administration of cytotoxic chemotherapy, the efficacy of most radiation modalities is based on targeted delivery to discernible sites of tumor growth, and is limited by toxic exposure to neighboring, noncancerous organs and tissues. A classical understanding of radiation-induced cellular injury centers on DNA damage as a primary target of programmed cell death pathway activation manifesting cellular lethality from double-strand breaks or reproductive/mitosis-associated cell death from unrepaired and misrepaired breaks (Radford, 1986; Yu, Long, Fu, Zhang, & Ni, 2003). Yet, while the p53-mediated pathway is the most widely recognized mechanism of radiation-induced apoptosis (Ali, Schriml, & Dean, 1999), the past two decades have yielded a burgeoning crop of the literature, much of which grows from the work of Richard Kolesnick and colleagues at the Memorial-Sloan Kettering Cancer Center, that continues to elucidate the p53-independent contributions of sphingolipid metabolism and signaling to cellular fate upon irradiation.
3.2. Induction of p53-independent apoptosis
The first foray into defining the DNA damage-independent context of sphingolipids in γ-radiation-induced apoptosis described the rapid, SMase-mediated hydrolysis of sphingomyelin into ceramide (Haimovitz-Friedman et al., 1994). This process was sensitive to phorbol ester and coincided with the induction of apoptosis. Moreover, preparations of nuclei-free cell membrane isolate generated ceramide upon stimulation with ionizing radiation, thereby demonstrating a functional apoptotic response without the involvement of DNA damage. While this phenomenon occurs generally, the literature suggests that the specific SMase isoenzymes driving sphingomyelin metabolism may be cell type specific. For instance, rapid, radiation-induced NSMase activation is preferred in bovine aortic endothelial (BAEC; Haimovitz-Friedman et al., 1994), WEHI-231 murine lymphoid (Chmura, Nodzenski, et al., 1997), and TF-1 human leukemia cells (Bruno et al., 1998), whereas ASMase activation plays a predominant ceramide-generating role in irradiated Rat-1 MycER fibroblasts (Zundel & Giaccia, 1998), human B lymphoblastoid cells, and lung epithelium (Santana et al., 1996). SQ-20B human head and neck squamous carcinoma cells demonstrated involvement of both NSMase and ASMase in the response to radiation (Chmura, Nodzenski, et al., 1997).
Experimental support of the SMase-mediated mechanisms came from the genetic ablation of ASMase in human tissues, immortalized from type I Niemann–Pick Disease patient B lymphoblastoid cells (Santana et al., 1996), or murine models (Lozano et al., 2001; Santana et al., 1996). Here, defects in radiation-stimulated ceramide generation and apoptosis were shown in each model, which was rescued by ectopic restoration of ASMase expression and function. Importantly, radiosensitization of cells was also restored by the exogenous administration of natural ceramides in combination with irradiation, indicating the defect of apoptosis in these cells was caused not by the dysfunction of a particular SMase isoenzyme, but results from the general failure of radiation-induced ceramide generation, thereby fingering ceramide as the obligate molecule necessary for radiation-induced apoptosis.
3.3. DNA damage incites ceramide generation by ceramide synthases
Apart from the ceramide generated via SMase activation, ceramide synthases (CerS) were implicated in radiation-induced apoptosis via cellular responses to DNA damage. Studies by the Kolesnick group demonstrated irradiation of BAEC and HeLa cells induced fumonisin-sensitive ceramide generation and apoptosis (Liao et al., 1999). In the same report, it was shown that the ataxia telangiectasia-mutated (ATM) gene product regulates the apoptotic pathway activated by radiation-induced DNA damage (Liao et al., 1999). Epstein Barr Virus (EBV)-immortalized B-cell lines from six ataxia telangiectasia (AT) patients with different mutations of the ATM gene exhibited radiation-induced CerS activation, ceramide generation, and apoptosis, whereas irradiation of three cell lines from normal patients did not produce these same responses. Ectopic expression of wild-type ATM reversed the response of the AT patient-derived cells, while antisense targeting of ATM in normal B cells produced the AT phenotype. These data provided evidence that signals from DNA damage and ATM can reciprocally regulate the CerS activity and the proclivity to apoptosis upon radiation exposure.
Vit and Rosselli (2003) integrated the previous evidence into a model of biphasic ceramide accumulation within the radiation-exposed human lymphoblast cell lines, AHH-1 and HSC-93, consisting of transitory, DNA damage-independent SMase activity followed by a lagging, CerS-dependent generation of ceramide. The late phase ceramide accumulation was found to be dependent upon the early phase ceramide-generating process, but independent of caspase activity. Ultimately, the late phase generation of ceramide depends on the DNA damage machinery, supporting earlier results involving the ATM gene, and CerS-mediated ceramide represents the rate-limiting apoptotic step of radiation-induced cell death. Similar biphasic patterns of ceramide generation were demonstrated in Jurkat leukemia cells (Ardail et al., 2009). The dependence of late phase ceramide generation on the earlier phase was supported by this study which found sphingomyelin-sourced ceramide in plasma membrane lipid rafts only 15 min postirradiation; a second peak in ceramide 4 h later was evidenced at the mitochondria via ASMase targeting of this organelle.
3.4. Downstream effectors of radiation-induced ceramide generation
The downstream signaling of ceramide generation upon radiation exposure harkens to well-characterized responses to other forms of cell stress. The stress-activated protein kinase/c-jun kinase (JNK) cascade and apoptosis are activated in irradiated BAEC and human monocytic U937 cells (Verheij et al., 1996). Dominant negative kinases of the JNK cascade abrogated the apoptotic response to either ionizing radiation or exogenous ceramide administration. Basic fibroblast growth factor (bFGF) inhibited radiation-induced apoptosis through JNK activity (Verheij et al., 1996) by activating the diacylglycerol (DAG)-PKC pathway (Cairns et al., 1997; Haimovitz-Friedman et al., 1994). Zundel and Giaccia (1998) demonstrated in Rat-1 Myc-ER cells ASMase-dependent PI3K inhibition via stress- or ceramide-induced coupling to caveolin which resulted in PI3K inactivation, Akt inhibition, and diminution of death effector phospho-Bad (Zundel & Giaccia, 1998). In addition, cells from Niemann–Pick disease patients were deficient in radiation-induced ceramide generation, PI3K inhibition, and apoptosis.
A definitive study demonstrating the necessity of ceramide biogenesis in radiation-induced apoptosis was reported in the Caenorhabditis elegans germline, which required mitochondrial ceramide generation for ultimate execution (Turner et al., 2011). Investigations of the mitochondrial effectors of cell survival and death on an evolutionary higher order were first plumbed by a study that, on the one hand, generated CD95 (FAS/APO-1) resistance in Jurkat T cells, which resulted in cross-resistance to either ionizing radiation or etoposide, and was resensitized by administration of ceramide analogs (Tepper, de Vries, van Blitterswijk, & Borst, 1999). On the other hand, the report showed that forced Bcl-2 overexpression in wild-type Jurkat cells manifested lower levels of ceramide accumulation upon exposure to etoposide or ionizing radiation. Although other reports demonstrate that Bcl-2 does not block ceramide generation upon stress (Martin et al., 1995; Pesche et al., 1998; Susin et al., 1997), this study established ceramide downstream of radiation and upstream of mitochondrial control of apoptosis execution. In HeLa and BAEC cells, the engagement of Bax downstream of ceramide was first suggested (Bold, Chandra, & McConkey, 1999) and later reported (Lee et al., 2011) by the Kolesnick group. This study showed that radiation-induced fumonisin-sensitive ceramide generation at the mitochondrial outer membrane forms a platform—termed a mitochondrial ceramide-rich macrodomain—into which Bax inserts, oligomerizes, and functionalizes mitochondrial outer membrane permeabilization leading to cytochrome c release.
3.5. Ceramide in γ-irradiation damage in vivo
Ionizing radiation was shown to activate ceramide-mediated endothelial cell apoptosis in several organs in vivo, including respiratory alveolar septi (Fuks, Alfieri, Haimovitz-Friedman, Seddon, & Cordon-Cardo, 1995; Santana et al., 1996), intestinal mucosa (Paris et al., 2001), and central nervous system (Pena, Fuks, & Kolesnick, 2000). Histopathological analyses seemed to indicate the endothelial damage was focused in the microvasculature, while larger vessels remained intact. Endothelial apoptosis developed as an early, dose-dependent event after exposure, peaking at 4–10 h after irradiation. Maximal apoptotic effects were observed in the intestinal mucosa at 15 Gy, in the lung at 25 Gy, and in the CNS at 40 Gy (Fuks, Haimovitz-Friedman, & Kolesnick, 1995; Paris et al., 2001; Pena et al., 2000; Santana et al., 1996). Ceramide kinetics in tissue extracts from the lung demonstrated that the apoptosis induction was preceded by ceramide elevation which peaked at twofold of control by 15 min after 10 Gy (Santana et al., 1996). ASMase was indicated as the obligatory mediator of this response based on the reports of the asmase−/− mice demonstrating abrogated ceramide generation and apoptosis in the endothelium of the lung and intestines and brain after whole body radiation (Paris et al., 2001; Pena et al., 2000; Santana et al., 1996). Interestingly, these animals showed normal, p53-mediated apoptosis in the thymus, whereas p53−/− mice manifested not only normal ceramide generation and apoptosis in the lung and intestines, but also abolition of thymic apoptosis, cementing the independence of the p53 and sphingomyelin pathways of radiation-induced apoptosis. Consistent with the in vitro studies above, intravenous administration of bFGF inhibited both a ceramide generation and an endothelial apoptotic response in the intestines, brain, and lung of wild-type mice (Haimovitz-Friedman et al., 1997; Paris et al., 2001; Pena et al., 2000; Santana et al., 1996).
The microvasculature became the focus of radiation-related organ injury because of evidence from the genetic and pharmacologic studies in intestinal and lung models. These studies posited the microvascular endothelium, rather than tissue stem cells, as the primary target for radiation. Whole-body irradiated C57BL/6 mice receiving 10–13 Gy died between 10 and 13 days with an intact gastrointestinal tract and depleted bone marrow, while mice irradiated with 15 Gy died at 61 days with denuded GI mucosa and moderately damaged marrow (Paris et al., 2001). TUNEL staining demonstrated apoptosis in the crypt/villus microvasculature as early as 4-h postirradiation and the risk of death from the GI syndrome correlated with the severity of endothelial apoptosis. Intravenous bFGF administration abrogated endothelial apoptosis and prevented radiation-induced crypt damage, organ failure, and GI death. Inhibition of endothelial apoptosis and protection from GI death were also found in asmase−/− mice treated with 15 Gy radiation. In situ hybridization showed that the endothelium, and not epithelial crypt cells, expressed high affinity bFGF receptors, thereby stratifying the endothelial lesion upstream of crypt stem cell damage in the manifestation of the radiation-induced GI syndrome. Consistent with this notion, bFGF administration also protected C3H/HeJ mice from lethal radiation pneumonitis (Fuks, Alfieri, et al., 1995).
Exquisite sensitivity to ASMase-mediated apoptosis upon irradiation was demonstrated in the ovary of fertile female mice. Tilly et al. demonstrate a defect in asmase−/− mice of normal apoptotic deletion of fetal oocytes during embryogenesis, leading to neonatal ovarian hyperplasia (Morita et al., 2000). The cell autonomy of this defect was confirmed ex vivo in oocytes from asmase−/− mice, or wild-type oocytes treated with S1P-resisted daunorubicin-induced apoptosis. Of note, Bax was found to function downstream of ceramide in this pathway. Irradiation at only 0.1 Gy induced 90% loss of oocytes and infertility in wild-type female mice, which was abrogated in vivo by administration of S1P. Furthermore, oocytes superovulated from S1P-pretreated and irradiated animals generated normal blastocysts after in vitro fertilization, while oocytes from vehicle-treated animals yielded defective blastocysts, indicating preservation of organ function by S1P.
3.6. Restoration and perturbation of sphingolipid balance in radiotherapeutic enhancement
Armed with an understanding of the ceramide response to ionizing radiation, we have witnessed a number of pharmacologic interventions in sphingolipid metabolism that produce predictable functional outcomes to alter the balance between ceramide generation and its removal, thereby influencing radiation-induced apoptotic signaling and its pathologic consequences. Chmura, Nodzenski, et al. (1997) selected a population of WEHI-231 cells after long-term selection with the acid ceramidase inhibitor N-oleoylethanolamine, which purportedly selects for ceramide-intolerant cells. The resultant WEHI-231 subline exhibited resistance to SMase-mediated apoptosis in response to radiation.
Much attention has been paid to prostate cancer radiotherapy, because it is a leading indication for local solid organ radiation modalities (Nguyen et al., 2011). Kimura, Bowen, Spiegel, and Gelmann (1999) found that androgen-sensitive human LNCaP prostate cancer cells produced neither a ceramide nor an apoptotic response to ionizing radiation, but were apoptotic under the influence of TNF-α (Kimura et al., 1999). However, the combination of radiation and TNF-α affected synergistically ceramide elevation and apoptosis. Moreover, substitution of TNF-α with ceramide analogs, at doses that did not induce apoptosis under singular treatment, yielded synergistic cell killing in combination with radiation. An alternative approach to target radiation resistance in LNCaP cells was reported by Garzotto et al. (Facher & Law, 1998; Garzotto et al., 1999). Although most cell types treated with phorbol ester signal antiapoptotic signals via PKC activation (Cairns et al., 1997; Haimovitz-Friedman et al., 1994), in LNCaP cells, TPA treatment demonstrated a CerS-mediated apoptotic response in combination with radiation (Garzotto et al., 1999). Pretreatment of LNCaP cells with TPA significantly enhanced ceramide generation by CerS activation, and resulted in a synergistic, fumonisin-sensitive apoptotic response. Orthotopic transplantation into the prostates of nude mice, recapitulated the responses of LNCaP cell-derived tumors to combination treatment within 24 h of irradiation. An additional benefit was noted, in that neighboring rectal tissues were protected by TPA administration, thereby improving the therapeutic window of radiation therapy in this small animal model.
Norris and colleagues have targeted prostate cancer cells undergoing radiation therapy with rationally designed ceramide analog inhibitors of acid ceramidase to effect radiation sensitization and enhanced tumor killing (Mahdy et al., 2009). The strategy is based on the observation that ionizing radiation stimulates overexpression of the acid ceramidase and alleviates this choke point of ceramide accumulation by conversion to sphingosine and rapid phosphorylation to S1P. Rational design of ceramide analogs based on the structures of d-e-MAPP and B13 has led to development of several lead compounds (Bai et al., 2009; Bielawska et al., 2008; Szulc et al., 2008) that demonstrate efficacious inhibition of acid ceramidase activity and enhancement of cancer cell killing either with monotherapeutic administration (Holman et al., 2008; Samsel et al., 2004; Selzner et al., 1999) of the inhibitor or in combination with other modalities (Elojeimy et al., 2007; Liu, Elojeimy, et al., 2006; Norris et al., 2006), including radiation therapy (Mahdy et al., 2009).
Targeting the sphingosine kinases in order to elevate ceramide and sphingosine levels, as well as shut down S1P production and signaling, is another mechanistic node for investigation. Cuvillier and colleagues utilized the sphingosine analog, FTY720 (fingolimod), in combination with ionizing radiation on prostate cancer cell lines in vitro and in vivo (Pchejetski et al., 2010). In prostate cancer cell lines, FTY720 inhibited SK1 and induced prostate cancer cell apoptosis in a manner independent of S1P receptors. Ionizing radiation did not affect SK1 activity in prostate cancer cells; yet, synergized with FTY720 to inhibit SK1. Combination therapy in orthotopic and subcutaneous mouse models demonstrated marked radiosensitization of the tumors. N,N-dimethyl-d-erythro-sphingosine also has demonstrable applications as a sphingosine kinase inhibitor that, when combined with radiation, produced increased sphingosine accumulation and apoptosis in lNCaP prostate adenocarcinoma cells (Nava et al., 2000) and Lewis lung carcinoma cells (Park et al., 2004).
Several studies have shown that PKC modulation affects the apoptotic response to radiation (Cairns et al., 1997; Haimovitz-Friedman et al., 1994), likely through the blockade of radiation-induced DAG generation (Bruno et al., 1998; Chmura, Mauceri, et al., 1997; Okahara, Ikawa, Kanaho, & Maehama, 2004). As mentioned above, PKC activation with phorbol ester blocked ceramide generation and apoptosis in most cell types tested with radiation exposure (Bruno et al., 1998; Cairns et al., 1997; Haimovitz-Friedman et al., 1994; Nakamura et al., 2000; Okahara et al., 2004; Vazquez, Ramaswamy, Nakamura, & Sellers, 2000). Conversely, pharmacologic inhibition of PKC augmented radiation-induced cell killing (Hallahan et al., 1992). The ceramide-related mechanism by which phorbol ester and DAG affect apoptosis apparently likely occurs through SMase activation (Nakamura et al., 2000). As mentioned above, bFGF similarly protected endothelial cells against radiation-induced apoptosis, in part through activation of PKC and MAPK cascades (Cairns et al., 1997; Verheij et al., 1996).
Studies by Rodriguez-Lafrasse and colleagues have explored mechanisms of achieving high levels of ceramide accumulation in order to sensitize a range of cancer cell lines. In a study of the radioresistant head and neck squamous cell carcinoma line SQ20B versus a radiosensitive cell line of the same histological type, SCC61, the group found that the resistant cell line lacked the typical ceramide response to ionizing radiation and was demonstrably insensitive to SMase-generated ceramide (Alphonse et al., 2002, 2004). The SQ20B cell line was found to lack ASMase activity and translocation to produce ceramide-associated membrane rafts (Bionda et al., 2007). Poly-drug pretreatment of SQ20B cells to bolster the endogenous ceramide levels consisted of: (1) an inhibitor of GCS, DL-PDMP; (2) an inhibitor of ceramidase, D-MAPP; and (3) an amphiphillic amine that disturbs lipid turnover in biological membranes, imipramine (Alphonse et al., 2004; Rodriguez-Lafrasse et al., 2002). This potent cocktail radiosensitized SQ20B to produce clonogenic and apoptotic cell death upon exposure to 10 Gy ionizing radiation, and demonstrated a threshold of intracellular ceramide that was necessary for cell death. Polydrug treatment was similarly effective in Jurkat leukemia cells (Rodriguez-Lafrasse et al., 2002). In addition, attacking the antioxidant defenses of SQ20B cells with either peroxide or GSH depletion also triggered ASMase activation and translocation, lipid raft coalescence, and apoptosis induction (Bionda et al., 2007).
Additionally, untested hypotheses have been posited to drive cancer toward radiosensitization through interdiction of ceramide-related pathways. Pharmacological and biochemical studies suggest that investigating compounds capable of interfering with SMase activity is of potential benefit (Claus, Dorer, Bunck, & Deigner, 2009). Exploitation of a positive feedback loop between PLA2-mediated arachidonic acid generation and ceramide and/or ceramide 1-phosphate has also been hypothesized to provide a viable strategy to mediate ceramide-induced radiosensitivity (Eng, 2003; Huwiler, Johansen, Skarstad, & Pfeilschifter, 2001; Nakamura, Hirabayashi, Shimizu, & Murayama, 2006; Shimizu et al., 2009).
3.7. Swords to ploughshares
As research continues into increasing tumor radiosensitization and improving radiotherapeutic response, radioprotective strategies have also been developed from our understanding of sphingolipid metabolism. Interest in protecting normal tissues from collateral injury during radiation therapy, or, from general radiation contamination, highlighted by recent interest ignited by radiation concerns after the 2011 Japanese earthquake (Bhattacharjee, 2011), has led to investigation of sphingolipid interdiction as well. Richard Kolesnick is leading development of a humanized monoclonal anticeramide antibody, 2A2, for efficacious protection against radiation injury, particularly GI syndrome (Bhattacharjee, 2011). Interference of the acute response to IR via sphingomyelin was investigated with the administration of sphingomyelin synthase inhibitor tricyclodecan-9-yl-xanthogenate (D609), which caused diminution of ionizing radiation (IR)-induced (1) production of reactive oxygen species, (2) decrease in intracellular reduced glutathione, (3) oxidative damage to proteins and lipids, and (4) activation of nuclear factor-kappaB (Davies, Jans, & Wagstaff, 2010). S1P analogs, such as FTY720, (S)-FTY720-phosphonate (fTyS), and SEW-2871, have recently shown efficacy in attenuating radiation-induced lung injury in a murine models (Mathew et al., 2011). At the step of S1P metabolism, oral administration of an S1P Lyase (SPL) inhibitor to mice prolonged their survival after exposure to a lethal dose of total body IR (Kumar et al., 2011). Successful induction of sphingolipid-modulated radioprotection combined with precise delivery to noncancerous tissues present a feasible, alternative strategy to improving the therapeutic window for radiation therapy of solid tumors.
4. SPHINGOLIPIDS AND TARGETED ANTICANCER AGENTS
The discovery of the first human oncogene, Ras, in 1982 by Weinberg and colleagues brought on boundless energy and enthusiasm for the concept of cancer therapies that target cancer-driving signaling pathways specifically rather than relying on cytotoxic chemotherapies, whose tumor specificity was centered around rapidity of cell division (Hanahan & Weinberg, 2000). Since then, myriad oncogenes have been discovered and, in turn, inhibited with high hopes for transforming cancer therapy. Major classes of targeted therapies now approved for cancer include TKIs, histone deacetylase inhibitors (HDACIs), and mAbs. These therapies certainly have improved therapeutic outcome for many cancer patients. Disappointingly, however, targeted therapies infrequently cure patients, as development of resistance is nearly universal. Interestingly, as with cytotoxic chemotherapy and radiotherapy, study of the mechanisms of cell death and resistance to targeted therapies has again implicated sphingolipid metabolism as integral to both favorable outcomes and development of resistance. In this section, we will review the literature on sphingolipid metabolism in response to newer targeted therapies, both in terms of eliciting favorable treatment responses and the development of resistance.
4.1. Tyrosine kinase inhibitors
TKIs are a class of anticancer drugs that function by inhibiting many signal transducing protein kinases. The classic TKI is imatinib, which targets the bcr–abl fusion kinase that drives chronic myelogenous leukemia (CML). Constitutive bcr–abl signaling activates well-characterized oncogenic pathways including Ras/ERK, Jak Stat, PI3K, and c-MYC. The development of imatinib to target the bcr–abl tyrosine kinase by competitive interference at the ATP-binding pocket has been one of the great advancements of cancer therapy. Interestingly, sphingolipid metabolism has been found to be a key element in imatinib effectiveness and resistance against CML. Treatment of CML cells with imatinib caused an accumulation of ceramide in imatinib-sensitive cells, but not in cells that had been cultured to become imatinib resistant (Baran et al., 2007). Ceramide generation was found to occur through CerS1, increasing primarily C18 ceramide and inducing apoptosis. Analysis of gene expression in imatinib-resistant cells led to the discovery that resistant cells had elevated SK1 and S1P. Targeting SK1 restored imatinib sensitivity, whereas SK1 overexpression conferred resistance, demonstrating a clear role for SK1 in imatinib resistance in CML. The same group later showed that imatinib-resistant cells also have elevated GCS activity, and that ceramide accumulation and therapeutic response could be augmented by treatment with the GCS inhibitor PDMP (Baran et al., 2011). Multiple groups have reported similarly that imatinib resistance is mediated by SK1 (Bonhoure et al., 2008; Marfe et al., 2011) and GCS (Huang et al., 2011). Second generation TKIs used in imatinib-resistant CML have also been investigated with regards to sphingolipid signaling. Gencer, Ural, Avcu, and Baran (2011) showed that dasatinib cell killing was due to increased ceramide generation through CerS1 and downregulation of SK1. Similar results were observed by the same group with nilotinib (Camgoz, Gencer, Ural, Avcu, & Baran, 2011), suggesting that ceramide is indeed a critical mediator of TKI-induced treatment of CML.
Sphingolipid manipulation may be further important in combination with TKIs during CML blast crisis. It has been found that during blast crisis, PP2A, which performs many of the apoptotic signaling functions induced by ceramide accumulation, is functionally inactive due to CML-driven expression of the PP2A inhibitor SET (Neviani et al., 2005). FTY720, an S1P analog that induces S1P receptor downregulation and is an approved therapy for the treatment of relapsing multiple sclerosis and with preclinical data supporting its potential use as an antineoplastic agent (Wallington-Beddoe, Hewson, Bradstock, & Bendall, 2011), can lead to the reactivation of PP2A (Perrotti & Neviani, 2008). Additional work has shown that the mechanism of SK1 and S1P in conferring TKI resistance in CML is through inhibition of PP2A (Salas et al., 2011). These studies implicate that targeting PP2A stability and activation, perhaps with the clinical agent FTY720 or with a novel clinical approach to elevating cancer cell ceramide, are worthy of further study in the treatment of CML.
In addition to the large body of work examining sphingolipids as key in CML resistance to TKIs, ceramide generation has been shown to occur downstream of multiple other TKIs. Gefitinib inhibits EGFR by competing at the ATP-binding site of the EGFR tyrosine kinase domain (Albitar et al., 2010). Gefitinib combination therapy with tamoxifen and etoposide (Mimeault, Venkatraman, et al., 2007) or gefitinib with cyclopamine and doxetaxel (Mimeault, Johansson, et al., 2007) in metastatic prostate cancer cells enhanced apoptotic cell death in part through ceramide accumulation. Sorafenib, a multikinase inhibitor with antiVEGF, -PDGF, and -Raf effects was shown to be effective in combination with the SK2 inhibitor ABC294640 in hepatocellular carcinoma by antagonizing ERK signaling (Beljanski, Knaak, Zhuang, & Smith, 2011). Sunitinib, a VEGFR/PDGFR kinase inhibitor approved for the treatment of renal cell carcinoma and gastrointestinal tumors, in combination with FTY720, was shown to potentiate the sunitinib response in breast cancer models, with the combination therapy decreasing tumor growth compared to single agent controls (Mousseau et al., 2011). This effect is perhaps due to further suppression of PDGFR by FTY720, as S1P receptor 3 crosstalks with PDGFR (Brunati et al., 2008). The FTY720-mediated enhancement in TKI cell killing may be able to be more broadly applied to the other TKIs, and further evaluation of mechanisms of resistance to TKIs may uncover conservation of ceramide metabolism as an approach to potentiating therapy.
4.2. Histone deacetylase inhibitors
HDACIs are a class of newly developed antineoplastic agents that inhibit histone deacetylases and thereby modulate gene expression by affecting the relative accessibility of targeted regions of DNA to transcription machinery (Imanishi et al., 2002). HDACIs have been heavily investigated in the treatment of cancer. Vorinostat is clinically approved for the treatment of T-cell lymphomas, and many other agents are in clinical trials. Interestingly, links have been found between chemotherapy with HDACIs and sphingolipids. In vitro HDACI treatment with MS-275 enhanced leukemia cell killing with fludarabine, with a large increase in ceramide over single agent treatment (Maggio et al., 2004). Delivery of exogenous cell permeable C6 ceramide and the HDACI trichostatin A synergistically enhanced cell death and decreased tumor proliferation in vivo and in vitro in ovarian and pancreatic models (Zhu et al., 2011). Interestingly, trichostatin A itself elicited ceramide generation that failed to result in apoptosis due to rapid glycosylation. Treatment with PDMP, the GCS inhibitor, markedly increased trichostatin A-induced ceramide accumulation and potentiated cell death. In another study which combined vorinistat with the Akt inhibitor Perifosine, it was shown that efficacy of the combination in human leukemia cells was dependent on ASMase-generated ceramide, as inhibition of this enzyme prevented ceramide accumulation and downstream reactive oxygen species generation and cell death (Rahmani et al., 2005).
Combination regimens with TKIs and HDACIs have also been evaluated. Low dose sorafenib and vorinostat treatment enhanced cell killing and inhibited tumor growth in vivo in renal cell carcinoma, hepatocellular carcinoma, and pancreatic adenocarcinomas (Park et al., 2010, 2008; Walker et al., 2009). Sorafenib and vorinostat cotreatment was initially found to activate CD95, an effect that was found to be the result of ceramide generation through ASMase and de novo ceramide synthesis. Interestingly, it appears that LASS6, the gene for CerS6, was hyperacetylated with vorinostat treatment, suggesting a mechanism by which ceramide and dihydroceramide were generated in the combination therapy. Inhibition of ASMase and de novo ceramide generation reduced the reduced ROS production, intracellular calcium accumulation, and synergistic cell death. This combination is currently being evaluated in a Phase I clinical trial for renal cell carcinoma and non-small cell carcinoma.
4.3. Monoclonal antibodies
mAbs are a mechanism to target and neutralize specific cellular antigens. These therapies are advantageous in that they allow very specific targeting of proteins or molecules, and thus can be tumor specific. An excellent example is trastuzumab which inhibits the her2/neu translocation kinase that drives many breast cancers. While relatively little is known about sphingolipid metabolism in response to treatment with approved mAbs, it is worth noting that rituximab, an antiCD20 mAb used to treat B-cell dyscrasias, results in a rapid and transient increase in ceramide associated with ASMase activation and colocalization with the CD20 receptor (Bezombes et al., 2002). Additional work conducted using milatuzumab, an antiCD74 mAb in Phase I/II clinical trials, demonstrated synergistic killing with FTY720 in mantle cell lymphoma (Alinari, Mahoney, et al., 2011; Alinari, Yu, et al., 2011). These studies uncovered an interesting mechanism through which FTY720 disrupted autophagy resulting in cell death through leakage of lysosomal hydrolases into the cytoplasm. Disruption of autophagy led to an accumulation of CD74, which is normally recycled in the lysosome, thus mechanistically explaining potentiation of milatuzumab effectiveness by FTY720.
Targeted therapies are likely to play a large role in the future of cancer therapy as techniques to identify specific molecular alterations that drive patient-specific malignancies improve. The oncogene era has defined hundreds of potential targets for cancer therapy, so the task remains to develop methods to attack those targets as well as to predict and respond to mechanism of resistance. The potential for interdiction of sphingolipid metabolism to be useful in the treatment of cancer extends beyond cytotoxic chemotherapy and radiation into the new age of targeted therapies. Preclinical evidence shows that these therapies frequently upregulate ceramide generation and that resistance often occurs through ceramide consumption. Development of clinical agents that inhibit sphingosine kinases, ceramidases, GCS, and perhaps other ceramide consumers holds promise in the potentiation of targeted cancer therapy.
5. CONCLUDING REMARKS
Decades of study have shown that ceramide is a critical mediator of apoptosis in response to standard cancer therapies including radiation, chemotherapy, and emerging targeted inhibitors. As we have delineated in this review, it is critical that the mechanisms that result in its accumulation of ceramide remain intact for fully efficacious therapy, as altered metabolism of sphingolipids can cause treatment resistance and result in poor therapeutic outcomes. Interdiction of sphingolipid metabolism represents a unique opportunity to sensitize cancers to standard therapy for several reasons:
Consumption of ceramide and generation of S1P are mechanisms of resistance to diverse therapies. We have outlined generation of ceramide as a critical part of therapy-induced cell death in response to cytotoxic chemotherapies, radiation therapy, and newer targeted therapies. This confirms a conserved role for sphingolipids in apoptosis.
Ceramide induces apoptosis through redundant mechanism. Ceramides mediates apoptosis through effectors upstream of multiple Bcl-2 family members, Akt, Myc, SR proteins, Rb, PKC, and JNK, among others. Therefore, in contrast to therapies that signal specifically at distal points in cell death cascades, ceramides have the potential to be effective even in cells with multiple apoptosis signaling defects by affecting intact pathways.
Multiple sphingolipid metabolizing enzymes have been identified as mechanisms of resistance to cancer therapy. Inhibition of acid ceramidase, sphingosine kinases, and GCS is well supported as means of sensitizing cancer cells to standard treatment.
The challenge is to develop feasible ways to inhibit these enzymes in patients. Clinically tested agents that target sphingolipid metabolism are few. As discussed above, there is an SK2 inhibitor in clinical trials, and multiple groups are actively searching for clinically relevant inhibitors of ceramidase (Draper et al., 2011), sphingosine kinases (French et al., 2003), and GCS (Nietupski et al., 2012). Successful development of these therapies is an exciting prospect for the future of cancer therapy. With decades of study justifying cotargeting sphingolipid metabolism with standard therapies, the time has come to test these ideas in human disease.
REFERENCES
- Akao Y, Banno Y, Nakagawa Y, Hasegawa N, Kim T-J, Murate T, et al. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochemical and Biophysical Research Communications. 2006;342:1284–1290. doi: 10.1016/j.bbrc.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Albitar L, Pickett G, Morgan M, Wilken JA, Maihle NJ, Leslie KK. EGFR isoforms and gene regulation in human endometrial cancer cells. Molecular Cancer. 2010;9:166. doi: 10.1186/1476-4598-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: A tumor suppressor with lipid phosphatase activity. Journal of the National Cancer Institute. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, et al. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–6903. doi: 10.1182/blood-2011-06-363879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117:4530–4541. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphonse G, Aloy MT, Broquet P, Gerard JP, Louisot P, Rousson R, et al. Ceramide induces activation of the mitochondrial/caspases pathway in Jurkat and SCC61 cells sensitive to gamma-radiation but activation of this sequence is defective in radioresistant SQ20B cells. International Journal of Radiation Biology. 2002;78:821–835. doi: 10.1080/09553000210153943. [DOI] [PubMed] [Google Scholar]
- Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R, Rodriguez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004;23:2703–2715. doi: 10.1038/sj.onc.1207357. [DOI] [PubMed] [Google Scholar]
- Ardail D, Maalouf M, Boivin A, Chapet O, Bodennec J, Rousson R, et al. Diversity and complexity of ceramide generation after exposure of jurkat leukemia cells to irradiation. International Journal of Radiation Oncology, Biology, Physics. 2009;73:1211–1218. doi: 10.1016/j.ijrobp.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Bai A, Szulc ZM, Bielawski J, Mayroo N, Liu X, Norris J, et al. Synthesis and bioevaluation of [omega]-N-amino analogs of B13. Bioorganic & Medicinal Chemistry. 2009;17:1840–1848. doi: 10.1016/j.bmc.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y, Bielawski J, Gunduz U, Ogretmen B. Targeting glucosylceramide synthase sensitizes imatinib-resistant chronic myeloid leukemia cells via endogenous ceramide accumulation. Journal of Cancer Research and Clinical Oncology. 2011;137:1535–1544. doi: 10.1007/s00432-011-1016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, et al. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. The Journal of Biological Chemistry. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Martin ML, de Almeida RF, Noguera-Salva MA, Marcilla-Etxenike A, Guardiola-Serrano F, et al. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19569–19574. doi: 10.1073/pnas.1115484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Lu P, Cheng JC, Zhao D, Turner LS, Zhang X, et al. Acid ceramidase-mediated production of sphingosine 1-phosphate promotes prostate cancer invasion through upregulation of cathepsin B. International Journal of Cancer. 2012;131:2034–2043. doi: 10.1002/ijc.27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Zhuang Y, Smith CD. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Investigational New Drugs. 2011;29:1132–1142. doi: 10.1007/s10637-010-9452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezombes C, de Thonel A, Apostolou A, Louat T, Jaffrezou J-P, Laurent G, et al. Overexpression of protein kinase czeta confers protection against antileukemic drugs by inhibiting the redox-dependent sphingomyelinase activation. Molecular Pharmacology. 2002;62:1446–1455. doi: 10.1124/mol.62.6.1446. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee Y. Devastation in Japan. Candidate radiation drugs inch forward. Science. 2011;331:1505. doi: 10.1126/science.331.6024.1505. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, et al. Novel analogs of D-e-MAPP and B13. Part 2: Signature effects on bioactive sphingolipids. Bioorganic & Medicinal Chemistry. 2008;16:1032–1045. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bionda C, Hadchity E, Alphonse G, Chapet O, Rousson R, Rodriguez-Lafrasse C, et al. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radical Biology & Medicine. 2007;43:681–694. doi: 10.1016/j.freeradbiomed.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Bold R, Chandra J, McConkey D. Gemcitabine-Induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Annals of Surgical Oncology. 1999;6:279–285. doi: 10.1007/s10434-999-0279-x. [DOI] [PubMed] [Google Scholar]
- Bonhoure E, Lauret A, Barnes DJ, Martin C, Malavaud B, Kohama T, et al. Sphingosine kinase-1 is a downstream regulator of imatinib-induced apoptosis in chronic myeloid leukemia cells. Leukemia. 2008;22:971–979. doi: 10.1038/leu.2008.95. [DOI] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Brunati AM, Tibaldi E, Carraro A, Gringeri E, D’Amico F, Jr, Toninello A, et al. Cross-talk between PDGF and S1P signalling elucidates the inhibitory effect and potential antifibrotic action of the immunomodulator FTY720 in activated HSC-cultures. Biochimica et Biophysica Acta. 2008;1783:347–359. doi: 10.1016/j.bbamcr.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Bruno AP, Laurent G, Averbeck D, Demur C, Bonnet J, Bettaieb A, et al. Lack of ceramide generation in TF-1 human myeloid leukemic cells resistant to ionizing radiation. Cell Death and Differentiation. 1998;5:172–182. doi: 10.1038/sj.cdd.4400330. [DOI] [PubMed] [Google Scholar]
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, et al. Frequent Inactivation of PTEN/MMAC1 in Primary Prostate Cancer. Cancer Research. 1997;57:4997–5000. [PubMed] [Google Scholar]
- Camgoz A, Gencer EB, Ural AU, Avcu F, Baran Y. Roles of ceramide synthase and ceramide clearence genes in nilotinib-induced cell death in chronic myeloid leukemia cells. Leukemia & Lymphoma. 2011;52:1574–1584. doi: 10.3109/10428194.2011.568653. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. The Journal of Biological Chemistry. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. The Journal of Biological Chemistry. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- Chauvier D, Morjani H, Manfait M. Ceramide involvement in homocamptothecin- and camptothecin-induced cytotoxicity and apoptosis in colon HT29 cells. International Journal of Oncology. 2002;20:855–863. [PubMed] [Google Scholar]
- Chmura SJ, Mauceri HJ, Advani S, Heimann R, Beckett MA, Nodzenski E, et al. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor killing by ionizing radiation. Cancer Research. 1997;57:4340–4347. [PubMed] [Google Scholar]
- Chmura SJ, Nodzenski E, Beckett MA, Kufe DW, Quintans J, Weichselbaum RR. Loss of ceramide production confers resistance to radiation-induced apoptosis. Cancer Research. 1997;57:1270–1275. [PubMed] [Google Scholar]
- Claus RA, Dorer MJ, Bunck AC, Deigner HP. Inhibition of sphingomyelin hydrolysis: targeting the lipid mediator ceramide as a key regulator of cellular fate. Current Medicinal Chemistry. 2009;16:1978–2000. doi: 10.2174/092986709788682182. [DOI] [PubMed] [Google Scholar]
- Cowart LA, Obeid LM. Yeast sphingolipids: Recent developments in understanding biosynthesis, regulation, and function. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2007;1771:421–431. doi: 10.1016/j.bbalip.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RG, Jans DA, Wagstaff KM. Microscopy: Science, technology, applications and education. Spain: Formatex Research Center; 2010. Use of fluorescence photobleaching techniques to measure the kinetics of intracellular transport; pp. 756–763. [Google Scholar]
- Deng W, Li R, Guerrera M, Liu Y, Ladisch S. Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology. 2002;12:145–152. doi: 10.1093/glycob/12.3.145. [DOI] [PubMed] [Google Scholar]
- Deng W, Li R, Ladisch S. Influence of cellular ganglioside depletion on tumor formation. Journal of the National Cancer Institute. 2000;92:912–917. doi: 10.1093/jnci/92.11.912. [DOI] [PubMed] [Google Scholar]
- Di Marzio L, Di Leo A, Cinque B, Fanini D, Agnifili A, Berloco P, et al. Detection of alkaline sphingomyelinase activity in human stool: Proposed role as a new diagnostic and prognostic marker of colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:856–862. doi: 10.1158/1055-9965.EPI-04-0434. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. The Journal of Biological Chemistry. 1993;268:15523–15530. [PubMed] [Google Scholar]
- Draper JM, Xia Z, Smith RA, Zhuang Y, Wang W, Smith CD. Discovery and evaluation of inhibitors of human ceramidase. Molecular Cancer Therapeutics. 2011;10:2052–2061. doi: 10.1158/1535-7163.MCT-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, et al. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Molecular Therapy. 2007;15:1259–1263. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- Eng C. PTEN: one gene, many syndromes. Human Mutation. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Erez-Roman R, Pienik R, Futerman AH. Increased ceramide synthase 2 and 6 mRNA levels in breast cancer tissues and correlation with sphingosine kinase expression. Biochemical and Biophysical Research Communications. 2010;391:219–223. doi: 10.1016/j.bbrc.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Facher EA, Law JC. PTEN and prostate cancer. Journal of Medical Genetics. 1998;35:790. doi: 10.1136/jmg.35.9.790-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Research. 2003;63:5962–5969. [PubMed] [Google Scholar]
- Fuks Z, Alfieri A, Haimovitz-Friedman A, Seddon A, Cordon-Cardo C. Intravenous basic fibroblast growth factor protects the lung but not mediastinal organs against radiation-induced apoptosis in vivo. The Cancer Journal from Scientific American. 1995;1:62–72. [PubMed] [Google Scholar]
- Fuks Z, Haimovitz-Friedman A, Kolesnick RN. The role of the sphingomyelin pathway and protein kinase C in radiation-induced cell kill. Important Advances in Oncology. 1995:19–31. [PubMed] [Google Scholar]
- Galadari S, Kishikawa K, Kamibayashi C, Mumby MC, Hannun YA. Purification and characterization of ceramide-activated protein phosphatases. Biochemistry. 1998;37:11232–11238. doi: 10.1021/bi980911+. [DOI] [PubMed] [Google Scholar]
- Gao P, Smith CD. Ablation of sphingosine kinase-2 inhibits tumor cell proliferation and migration. Molecular Cancer Research. 2011;9:1509–1519. doi: 10.1158/1541-7786.MCR-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzotto M, Haimovitz-Friedman A, Liao WC, White-Jones M, Huryk R, Heston WD, et al. Reversal of radiation resistance in LNCaP cells by targeting apoptosis through ceramide synthase. Cancer Research. 1999;59:5194–5201. [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Advances in Experimental Medicine and Biology. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gencer EB, Ural AU, Avcu F, Baran Y. A novel mechanism of dasatinib-induced apoptosis in chronic myeloid leukemia; ceramide synthase and ceramide clearance genes. Annals of Hematology. 2011;90:1265–1275. doi: 10.1007/s00277-011-1212-5. [DOI] [PubMed] [Google Scholar]
- Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Research. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- Guan H, Liu L, Cai J, Liu J, Ye C, Li M, et al. Sphingosine kinase 1 is over-expressed and promotes proliferation in human thyroid cancer. Molecular Endocrinology. 2011;25:1858–1866. doi: 10.1210/me.2011-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. The Journal of Experimental Medicine. 1997;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. The Journal of Experimental Medicine. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan DE, Virudachalam S, Schwartz JL, Panje N, Mustafi R, Weichselbaum RR. Inhibition of protein kinases sensitizes human tumor cells to ionizing radiation. Radiation Research. 1992;129:345–350. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Wickel M, Schneider-Brachert W, Sandberg C, Gahr J, Schwandner R, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. The EMBO Journal. 1999;18:5252–5263. doi: 10.1093/emboj/18.19.5252. [erratum appears in EMBO J 2000 Jan 17;19(2):315]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertervig E, Nilsson A, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer. 1997;79:448–453. [PubMed] [Google Scholar]
- Holman DH, Turner L, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemotherapy and Pharmacology. 2008;61:231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting Mitochondria. Accounts of Chemical Research. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- Huang WC, Tsai CC, Chen CL, Chen TY, Chen YP, Lin YS, et al. Glucosylceramide synthase inhibitor PDMP sensitizes chronic myeloid leukemia T315I mutant to Bcr-Abl inhibitor and cooperatively induces glycogen synthase kinase-3-regulated apoptosis. The FASEB Journal. 2011;25:3661–3673. doi: 10.1096/fj.10-180190. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Johansen B, Skarstad A, Pfeilschifter J. Ceramide binds to the CaLB domain of cytosolic phospholipase A2 and facilitates its membrane docking and arachidonic acid release. The FASEB Journal. 2001;15:7–9. doi: 10.1096/fj.00-0370fje. [DOI] [PubMed] [Google Scholar]
- Imanishi R, Ohtsuru A, Iwamatsu M, Iioka T, Namba H, Seto S, et al. A histone deacetylase inhibitor enhances killing of undifferentiated thyroid carcinoma cells by p53 gene therapy. The Journal of Clinical Endocrinology and Metabolism. 2002;87:4821–4824. doi: 10.1210/jc.2002-020877. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kitano T, Watanabe M, Kondo T, Yabu T, Taguchi Y, et al. Possible role of ceramide as an indicator of chemoresistance: decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin synthase in chemoresistant leukemia. Clinical Cancer Research. 2003;9:415–423. [PubMed] [Google Scholar]
- Jaffrezou JP, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N, et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. The EMBO Journal. 1996;15:2417–2424. [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Research. 1999;59:1606–1614. [PubMed] [Google Scholar]
- Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cellular Signalling. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossenkov AV, Vachani A, Chang C, Nichols C, Billouin S, Horng W, et al. Resection of non–small cell lung cancers reverses tumor-induced gene expression changes in the peripheral immune system. Clinical Cancer Research. 2011;17:5867–5877. doi: 10.1158/1078-0432.CCR-11-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Oskouian B, Fyrst H, Zhang M, Paris F, Saba JD. S1P lyase regulates DNA damage responses through a novel sphingolipid feedback mechanism. Cell Death & Disease. 2011;2:e119. doi: 10.1038/cddis.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour NF, Chalfant CE. Ceramide kinase and the ceramide-1-phosphate/cPLA2alpha interaction as a therapeutic target. Current Drug Targets. 2008;9:674–682. doi: 10.2174/138945008785132349. [DOI] [PubMed] [Google Scholar]
- Lavie Y, Cao HT, Volner A, Lucci A, Han TY, Geffen V, et al. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. The Journal of Biological Chemistry. 1997;272:1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Calpha. The Journal of Biological Chemistry. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao WC, et al. Mitochondrial ceramide-rich macrodomains functionalize bax upon irradiation. PLoS One. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guan H-Y, Gong L-Y, Song L-B, Zhang N, Wu J, et al. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clinical Cancer Research. 2008;14:6996–7003. doi: 10.1158/1078-0432.CCR-08-0754. [DOI] [PubMed] [Google Scholar]
- Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clinical Cancer Research. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- Liao WC, Haimovitz-Friedman A, Persaud RS, McLoughlin M, Ehleiter D, Zhang N, et al. Ataxia telangiectasia-mutated gene product inhibits DNA damage-induced apoptosis via ceramide synthase. The Journal of Biological Chemistry. 1999;274:17908–17917. doi: 10.1074/jbc.274.25.17908. [DOI] [PubMed] [Google Scholar]
- Liu X, Elojeimy S, El-Zawahry AM, Holman DH, Bielawska A, Bielawski J, et al. Modulation of ceramide metabolism enhances viral protein apoptin’s cytotoxicity in prostate cancer. Molecular Therapy. 2006;14:637–646. doi: 10.1016/j.ymthe.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. The FASEB Journal. 2001;15:719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- Liu Y-Y, Han TY, Yu JY, Bitterman A, Le A, Giuliano AE, et al. Oligonucleotides blocking glucosylceramide synthase expression selectively reverse drug resistance in cancer cells. Journal of Lipid Research. 2004;45:933–940. doi: 10.1194/jlr.M300486-JLR200. [DOI] [PubMed] [Google Scholar]
- Liu YY, Patwardhan GA, Xie P, Gu X, Giuliano AE, Cabot MC. Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. International Journal of Oncology. 2011;39:425–431. doi: 10.3892/ijo.2011.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CWY, Wang R-H, Berndt N. Protein phosphatase 1alpha activity prevents oncogenic transformation. Molecular Carcinogenesis. 2006;45:648–656. doi: 10.1002/mc.20191. [DOI] [PubMed] [Google Scholar]
- Lozano J, Menendez S, Morales A, Ehleiter D, Liao W-C, Wagman R, et al. Cell autonomous apoptosis defects in acid sphingomyelinase knockout fibroblasts. The Journal of Biological Chemistry. 2001;276:442–448. doi: 10.1074/jbc.M006353200. [DOI] [PubMed] [Google Scholar]
- Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, Cabot MC. Glucosylceramide: a marker for multiple-drug resistant cancers. Anticancer Research. 1998;18:475–480. [PubMed] [Google Scholar]
- Machaczka M, Hast R, Dahlman I, Lerner R, Klimkowska M, Engvall M, et al. Substrate reduction therapy with miglustat for type 1 Gaucher disease: a retrospective analysis from a single institution. Upsala Journal of Medical Sciences. 2012;117:28–34. doi: 10.3109/03009734.2011.641609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio SC, Rosato RR, Kramer LB, Dai Y, Rahmani M, Paik DS, et al. The histone deacetylase inhibitor MS-275 interacts synergistically with fludarabine to induce apoptosis in human leukemia cells. Cancer Research. 2004;64:2590–2600. doi: 10.1158/0008-5472.can-03-2631. [DOI] [PubMed] [Google Scholar]
- Mahdy AE, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, et al. Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Molecular Therapy. 2009;17:430–438. doi: 10.1038/mt.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavaud B, Pchejetski D, Mazerolles C, de Paiva GR, Calvet C, Doumerc N, et al. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. European Journal of Cancer. 2010;46:3417–3424. doi: 10.1016/j.ejca.2010.07.053. [DOI] [PubMed] [Google Scholar]
- Marfe G, Di Stefano C, Gambacurta A, Ottone T, Martini V, Abruzzese E, et al. Sphingosine kinase 1 overexpression is regulated by signaling through PI3K, AKT2, and mTOR in imatinib-resistant chronic myeloid leukemia cells. Experimental Hematology. 2011;39:653–665. e6. doi: 10.1016/j.exphem.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Newmeyer DD, Mathias S, Farschon DM, Wang HG, Reed JC, et al. Cell-free reconstitution of Fas-, UV radiation- and ceramide-induced apoptosis. The EMBO Journal. 1995;14:5191–5200. doi: 10.1002/j.1460-2075.1995.tb00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, et al. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. The FASEB Journal. 2011;25:3388–3400. doi: 10.1096/fj.11-183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Johansson SL, Vankatraman G, Moore E, Henichart J-P, Depreux P, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Molecular Cancer Therapeutics. 2007;6:967–978. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Venkatraman G, Johansson SL, Moore E, Henichart JP, Depreux P, et al. Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. International Journal of Cancer. 2007;120:160–169. doi: 10.1002/ijc.22268. [DOI] [PubMed] [Google Scholar]
- Mitra P, Maceyka M, Payne SG, Lamour N, Milstien S, Chalfant CE, et al. Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Letters. 2007;581:735–740. doi: 10.1016/j.febslet.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Morales A, Paris R, Villanueva A, Llacuna L, Garcia-Ruiz C, Fernandez-Checa JC. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007;26:905–916. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nature Medicine. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- Mousseau Y, Mollard S, Faucher-Durand K, Richard L, Nizou A, Cook-Moreau J, et al. Fingolimod potentiates the effects of sunitinib malate in a rat breast cancer model. Breast Cancer Research and Treatment. 2011;134:31–40. doi: 10.1007/s10549-011-1903-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. The FASEB Journal. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Hirabayashi T, Shimizu M, Murayama T. Ceramide-1-phosphate activates cytosolic phospholipase A2alpha directly and by PKC pathway. Biochemical Pharmacology. 2006;71:850–857. doi: 10.1016/j.bcp.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Molecular and Cellular Biology. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava VE, Cuvillier O, Edsall LC, Kimura K, Milstien S, Gelmann EP, et al. Sphingosine Enhances Apoptosis of Radiation-resistant Prostate Cancer Cells. Cancer Research. 2000;60:4468–4474. [PubMed] [Google Scholar]
- Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. Journal of Clinical Oncology. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietupski JB, Pacheco JJ, Chuang WL, Maratea K, Li L, Foley J, et al. Iminosugar-based inhibitors of glucosylceramide synthase prolong survival but paradoxically increase brain glucosylceramide levels in Niemann-Pick C mice. Molecular Genetics and Metabolism. 2012;105:621–628. doi: 10.1016/j.ymgme.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Norris JS, Bielawska A, Day T, El-Zawahri A, Elojeimy S, Hannun Y, et al. Combined therapeutic use of AdGFPFasL and small molecule inhibitors of ceramide metabolism in prostate and head and neck cancers: a status report. Cancer Gene Therapy. 2006;13:1045–1051. doi: 10.1038/sj.cgt.7700965. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resistance Updates. 2001;4:368–377. doi: 10.1054/drup.2001.0225. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature Reviews. Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Okahara F, Ikawa H, Kanaho Y, Maehama T. Regulation of PTEN phosphorylation and stability by a tumor suppressor candidate protein. The Journal of Biological Chemistry. 2004;279:45300–45303. doi: 10.1074/jbc.C400377200. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proceedings of the National Academy of Sciences. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris F, Grassme H, Cremesti A, Zager J, Fong Y, Haimovitz-Friedman A, et al. Natural ceramide reverses Fas resistance of acid sphingomyelinase (−/−) hepatocytes. The Journal of Biological Chemistry. 2001;276:8297–8305. doi: 10.1074/jbc.M008732200. [DOI] [PubMed] [Google Scholar]
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Haussinger D, et al. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca (2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Research. 2010;70:6313–6324. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Song JY, Kim KS, Han Y, Kim CW, Yi SY, et al. Enhancement of radiosensitivity by combined ceramide and dimethylsphingosine treatment in lung cancer cells. Experimental & Molecular Medicine. 2004;36:411–419. doi: 10.1038/emm.2004.53. [DOI] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biology & Therapy. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M, et al. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Research. 2010;70:8651–8661. doi: 10.1158/0008-5472.CAN-10-1388. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Doumerc N, Golzio M, Naymark M, Teissie J, Kohama T, et al. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Molecular Cancer Therapeutics. 2008;7:1836–1845. doi: 10.1158/1535-7163.MCT-07-2322. [DOI] [PubMed] [Google Scholar]
- Pena LA, Fuks Z, &Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Research. 2000;60:321–327. [PubMed] [Google Scholar]
- Perrotti D, Neviani P. Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer Metastasis Reviews. 2008;27:159–168. doi: 10.1007/s10555-008-9119-x. [DOI] [PubMed] [Google Scholar]
- Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. The Journal of Biological Chemistry. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- Pesche S, Latil A, Muzeau F, Cussenot O, Fournier G, Longy M, et al. PTEN/MMAC1/TEP1 involvement in primary prostate cancers. Oncogene. 1998;16:2879–2883. doi: 10.1038/sj.onc.1202081. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Reviews. Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Radford IR. Evidence for a general relationship between the induced level of DNA double-strand breakage and cell-killing after X-irradiation of mammalian cells. International Journal of Radiation Biology and Related Studies in Physics, Chemistry, and Medicine. 1986;49:611–620. doi: 10.1080/09553008514552861. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Research. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Aloy MT, Ardail D, Gerard JP, Louisot P, et al. Increasing endogenous ceramide using inhibitors of sphingolipid metabolism maximizes ionizing radiation-induced mitochondrial injury and apoptotic cell killing. International Journal of Cancer. 2002;101:589–598. doi: 10.1002/ijc.10652. [DOI] [PubMed] [Google Scholar]
- Ruckhäberle E, Karn T, Rody A, Hanker L, Gätje R, Metzler D, et al. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. Journal of Cancer Research and Clinical Oncology. 2009;135:1005–1013. doi: 10.1007/s00432-008-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, et al. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biology & Therapy. 2007;6:1455–1460. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, et al. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. The Prostate. 2004;58:382–393. doi: 10.1002/pros.10350. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Sauer L, Nunes J, Salunkhe V, Skalska L, Kohama T, Cuvillier O, et al. Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. International Journal of Cancer. 2009;125:2728–2736. doi: 10.1002/ijc.24640. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, et al. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 2000;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
- Schiffmann S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30:745–752. doi: 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
- Selzner M, Morse MA, Sindram D, Bielawska A, Hannun YA, Clavien P-A. Ceramide analogueB13induces apoptosis and completely inhibits growth of liver metastases from colon cancer in vivo a new strategy for the treatment of metastatic liver disease. 50th Annual American Association for the Study of Liver Disease (AAASLD) 1999:4. Abstract #1. [Google Scholar]
- Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008;112:770–781. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Tada E, Makiyama T, Yasufuku K, Moriyama Y, Fujino H, et al. Effects of ceramide, ceramidase inhibition and expression of ceramide kinase on cytosolic phospholipase A2alpha; additional role of ceramide-1-phosphate in phosphorylation and Ca2+ signaling. Cellular Signalling. 2009;21:440–447. doi: 10.1016/j.cellsig.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, et al. A Role of Sphingosine Kinase 1 in Head and Neck Carcinogenesis. Cancer Prevention Research. 2011;4:454–462. doi: 10.1158/1940-6207.CAPR-10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, et al. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. The Journal of Biological Chemistry. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- Song L, Xiong H, Li J, Liao W, Wang L, Wu J, et al. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non-small cell lung cancer. Clinical Cancer Research. 2011;17:1839–1849. doi: 10.1158/1078-0432.CCR-10-0720. [DOI] [PubMed] [Google Scholar]
- Strum JC, Small GW, Pauig SB, Daniel LW. 1-beta-D-Arabinofuranosylcytosine stimulates ceramide and diglyceride formation in HL-60 cells. The Journal of Biological Chemistry. 1994;269:15493–15497. [PubMed] [Google Scholar]
- Sun CC, Zhang Z, Zhang SY, Li J, Li ZL, Kong CZ. Up-regulation of glucosylceramide synthase in urinary bladder neoplasms. Urologic Oncology. 2010;30:444–449. doi: 10.1016/j.urolonc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, et al. The central executioner of apoptosis - multiple connections between protease activation and mitochondria in fas/apo-1/cd95- and ceramide-induced apoptosis. The Journal of Experimental Medicine. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc ZM, Mayroo N, Bai A, Bielawski J, Liu X, Norris JS, et al. Novel analogs of D-e-MAPP and B13. Part 1: Synthesis and evaluation as potential anticancer agents. Bioorganic & Medicinal Chemistry. 2008;16:1015–1031. doi: 10.1016/j.bmc.2007.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacological Reviews. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper AD, de Vries E, van Blitterswijk WJ, Borst J. Ordering of ceramide formation, caspase activation, and mitochondrial changes during CD95- and DNA damage-induced apoptosis. [erratum appears in J Clin Invest 1999 May; 103(9): 1363] The Journal of Clinical Investigation. 1999;103:971–978. doi: 10.1172/JCI5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- Turner LS, Cheng JC, Beckham TH, Keane TE, Norris JS, Liu X. Autophagy is increased in prostate cancer cells overexpressing acid ceramidase and enhances resistance to C6 ceramide. Prostate Cancer and Prostatic Diseases. 2011;14:30–37. doi: 10.1038/pcan.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Molecular and Cellular Biology. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Vit JP, Rosselli F. Role of the ceramide-signaling pathways in ionizing radiation-induced apoptosis. Oncogene. 2003;22:8645–8652. doi: 10.1038/sj.onc.1207087. [DOI] [PubMed] [Google Scholar]
- Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, et al. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Molecular Pharmacology. 2009;76:342–355. doi: 10.1124/mol.109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy. 2011;7:707–715. doi: 10.4161/auto.7.7.15154. [DOI] [PubMed] [Google Scholar]
- Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. The Journal of Biological Chemistry. 2006;281:18859–18867. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- Xu J, Yeh CH, Chen S, He L, Sensi SL, Canzoniero LM, et al. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. The Journal of Biological Chemistry. 1998;273:16521–16526. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Long J, Fu DL, Zhang QH, Ni QX. Analysis of gene expression profiles in pancreatic carcinoma by using cDNA microarray. Hepatobiliary & Pancreatic Diseases International. 2003;2:467–470. [PubMed] [Google Scholar]
- Zhu QY, Wang Z, Ji C, Cheng L, Yang YL, Ren J, et al. C6-ceramide synergistically potentiates the anti-tumor effects of histone deacetylase inhibitors via AKT dephosphorylation and alpha-tubulin hyperacetylation both in vitro and in vivo. Cell Death & Disease. 2011;2:e117. doi: 10.1038/cddis.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundel W, Giaccia A. Inhibition of the anti-apoptotic PI (3)K/Akt/Bad pathway by stress. Genes & Development. 1998;12:1941–1946. doi: 10.1101/gad.12.13.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]