Abstract
Although there has been great interest in the evolutionary approach to cooperative breeding species, few studies actually directly compare fathers and mothers on their motivation to parent offspring. We tested the responsiveness of common marmoset mothers and fathers to vocal and olfactory cues from their own and other infants using a two-chamber test apparatus designed to evaluate responses in the absence of competition from other caregivers within the family. We tested parentally experienced mothers and fathers living with young infants and former parents with no current offspring to address the following questions: (1) do mothers and fathers respond equally to sensory cues of infants; (2) do parents discriminate cues of their own offspring when the infants are highly dependent and when the infants are more independent; and (3) are parents responsive to both auditory and olfactory cues? Mothers and fathers reacted similarly in all tests. Parents responded equally to isolation calls from their own and unfamiliar dependent infants and there was minimal response to olfactory cues. Responses to infant vocal cues were significantly stronger when infants were dependent upon direct parental care. There was no difference in response between parents whose infants were no longer dependent and former parents with no current offspring. The results show that both parents are highly responsive to infant vocal cues when their own infants are dependent on parental care, supporting an effect of hormonal priming. However, parents only showed behavioural discrimination between vocalizations from their own and unfamiliar infants when their infants were mostly independent.
Keywords: common marmoset, distress vocalizations, fathers, infant care, infant dependency, isolation calls, mothers, odours
In some species, mothers are assisted by fathers and by nonbreeding helpers (cooperative breeders; Clutton-Brock, 2009; Russell, 2004). Cooperative breeding systems are uncommon in mammals, but there are some clear exceptions such as the Callitrichidae (New World monkeys), Canidae (dogs), Herpestidae (mongooses), Bathyergidae (mole-rats), Castoridae (beavers), Hystricidae (porcupines), Muridae (mice and rats) and terrestrial Sciuridae (squirrels) (Lukas & Clutton-Brock, 2012). Callitrichids live in groups of 3–15 individuals in the wild, composed mainly of related individuals (Yamamoto, Arruda, Alencar, de Sousa & Araujo, 2009). There is usually only one reproductive female who has a high reproductive rate owing to twin births as well as postpartum ovulations occurring 10–20 days after birth (Lunn & McNeilly, 1982; Ziegler, Savage, Scheffler & Snowdon, 1987), and who requires help from all family members for infant care (Snowdon & Ziegler, 2007). Cooperative infant care in callitrichids occurs especially through carrying and food sharing but also through grooming, protecting and other types of care (Snowdon & Ziegler, 2007).
From an evolutionary point of view cooperative breeding has been associated with biparental, socially monogamous mating systems in mammals (Lukas & Clutton-Brock, 2012) and birds (Cornwallis, West, Davis & Griffin, 2010). Biparental care is part of cooperative breeding although it is also found in many other species without cooperative care. Among mammals biparental care has been described in some rodents (prairie voles, Microtus ochrogaster, California mice, Peromyscus californicus, Mongolian gerbils, Meriones unguiculatus, and Djungarian hamsters, Phodopus campbelli; Wynne-Edwards, 2001) and in nonhuman New World primates such as the titi monkey, Callicebus spp., owl monkey, Aotus spp., Goeldi's monkey, Callimico goeldii, marmosets, Callithrix spp. and tamarins, Saguinus spp. and Leontopithecus spp.
Biparental care in callitrichids involves a complex communication system between the breeding female and the expectant father during the female's gestation (Ziegler, 2013). This has led to males being hormonally prepared to take the major role in infant care at the time of birth (Ziegler, 2013). Cottontop tamarin, Saguinus oedipus, females have a midgestation increase in cortisol that remains elevated to the end of pregnancy and parentally experienced fathers show a peak of glucocorticoid excretion generally during the week following their mate's onset of cortisol elevation at midpregnancy (Ziegler, Washabaugh & Snowdon, 2004a). Expectant fathers gain weight during their mate's pregnancy (Ziegler, Prudom, Schultz-Darken, Kurian & Snowdon, 2006; Sánchez, Peláez, Fidalgo, Morcillo & Caperos, 2008a, b), and increase levels of reproductive hormones by the end of the pregnancy, perhaps preparing fathers to take care of their infants (Ziegler et al., 2004a). This hormonal priming may have evolved into a signalling system for biparental species leading to anticipation of the birth by fathers to ensure they will remain with the female throughout pregnancy, birth and beyond (Ziegler et al., 2004a). Common marmoset fathers carry infants on the first day after birth and may continue carrying their offspring for more than 3 months postpartum (Mills, Windle, Baker & Ridley, 2004).
Zahed, Prudom, Snowdon and Ziegler (2008) demonstrated that experienced fathers with infants currently present in their family responded the same to their own live infant and to a recording of their distress vocalizations (also called isolation calls or cries). Also, experienced fathers responded similarly to same-age unfamiliar infants or their distress vocalizations and cries of their own infant. The study used a two-chamber test in which males had to cross a bridge from the start chamber to the stimulus chamber to interact with the infant or its cues. When fathers were tested in a small nestbox with scents from 5- to 10-dayold infants, either the male's own or a novel infant of the same age, their androgens decreased and their oestrogens increased (Ziegler, Peterson, Sosa & Barnard, 2011). However, they responded only to the odours of their own infants. When the fathers were tested again when the same infants were 3 months of age and no longer carried, fathers showed no hormonal response to either their own or unfamiliar infants (Ziegler et al. 2011). Fathers may only show an endocrine response when the infants are totally reliant on them (Ziegler 2013). In species such as rats, mice, sheep, goats and rabbits, as well as in humans, olfaction is involved in the regulation of maternal care (Fleming, Corter, Frank, Surbey, Schneider & Steiner, 1993; Lévy, Keller & Poindron, 2004; Gonzalez-Mariscal & Poindron, 2002; Numan & Insel, 2003; Stern, 1989) and even in songbirds (zebra finches, Taeniopygia guttata), olfaction seems to play a role related to nest recognition (Caspers, Hoffman, Kohlmeier, Krüger & Krause, 2013). The chemical/olfactory communication in marmosets provides an important mechanism for maintaining the bonds between individuals within their family groups (Ziegler, 2013). It is unknown whether fathers are able to recognize the individually distinct features in their infant's calls (Zahed et al. 2008). Thus, there is some apparent contradiction in these results in relation to fathers’ responsiveness but very different test conditions were involved and in Ziegler et al. (2011) only hormonal measures were reported. In all of the studies described above only fathers were tested. We have little information that directly compares responses of mothers and fathers to the same infant cues using the same test apparatus.
Considering the hormonal, physical and behavioural changes in males during their mate's pregnancy and infant care period and the similarity of these changes with the changes in pregnant females, it is likely that there are shared neuroendocrine mechanisms and similarities in mothers and fathers of species with biparental care, and, specifically, in the common marmoset. This led us to predict similar behavioural responsiveness in experienced fathers and mothers of common marmosets to the sensory cues of dependent infants. Thus, the first aim of this study was to evaluate the behavioural responses of experienced fathers and mothers to distress vocalizations and olfactory stimuli of dependent infants. Additionally, as experienced fathers behave similarly to their own and to unrelated infant distress vocalizations but show different physiological responses to their own and unrelated infant odours, we predicted that both parents would distinguish differences in olfactory cues from their own and unrelated infants. Finally, we tested whether experienced parents were behaviourally responsive to signals of their own infants, not only when the infants were dependent on parental care, but also at a time when infants were primarily independent of direct parental care. We also tested experienced parents who no longer had infants present in the group to determine whether the hormonal priming of parenting has a long-term effect on responses to infant cues.
METHODS
Subjects
Common marmosets were socially housed at the Wisconsin National Primate Research Center, U.S.A. The subjects included six experienced breeding pairs that were currently breeding (six males and six females) and an additional six experienced breeding pairs that were not currently breeding (six males and six females). The breeding pairs were tested for their responsiveness to their own and unrelated infant stimuli twice: (1) in week 3 after the infants’ birth, when infants were nursing and totally dependent (‘dependent infant’) and (2) at 4 months when infants were mostly independent (‘independent infant’). Only two of the six experienced breeding females from the pairs currently breeding became pregnant again before the 4th month of their infant's life. The additional six experienced breeding pairs that were not currently breeding (‘no infant’) were used as a control sample. At the onset of testing, current breeders’ ages were 2.3–7.2 years (mean 5.14 + 1.70 SD) and they had previously reared one to four litters. Currently nonbreeding animals were 5.1–11.6 years old (mean 7.35 + 1.91 SD) and they had previously reared three to seven litters. The time elapsed since the last birth for these pairs was 1.3–5.8 years (mean 3.6 + 30.4 SD).
Marmoset families were housed in enclosures that measured either 122 x 61 cm and 183 cm high or 61 x 91 cm and 183 cm high, were made of coated wire mesh, reached the ground providing vertical space for these arboreal primates, had wood shavings on the floor and were equipped with a metal nestbox, wooden dowels for locomotion and other tactile items. None of the subjects lived alone and all subjects were allowed auditory, visual and olfactory contact with other marmosets outside their families. There were two to eight animals per cage. Diets and husbandry details have been reported previously for this colony (Woller, Sosa, Chiang, Prudom, Keelty & Moore, 2011). Subjects were fed their allotment of standardized daily diet (Mazuri Callithrichid High Fiber Diet 5MI6; Purina Mills, St Louis, MO, U.S.A.; 268 kJ day animal) plus an additional daily enrichment food (fruits, mealworms, vegetables). Tap water was available ad libitum. Lighting was regulated on a 12:12 h light:dark cycle, temperature was maintained at 27 °C and the humidity at approximately 40%. Marmosets used for this study were reused for other experiments. Housing conditions and infant stimuli response testing met the US Department of Agriculture Guidelines for Care and Housing of Nonhuman Primates and were approved by the Animal Care and Use Committee (IACUC) at the University of Wisconsin Graduate School (protocol number G00526). The research adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
Vocal and Odour Stimulus Collection
In all cases, the vocal recordings and odour collection were made from 15–17- dayold marmosets. No infant was removed from the family until it was old enough to thermoregulate. Infants were isolated from their natal group in an adjoining room in the same conditions of humidity and temperature for no longer than 10 min. The infant was placed into an open Styrofoam box on a soft, warm cotton cloth and, with a digital voice recorder (Olympus DS-2, Center Valley, PA, U.S.A.) inside the box, we recorded all the vocalizations infants produced during 5 min (5 min recordings were looped to repeat for the full 10 min of the test). We refer to these vocalizations as distress vocalizations (isolation calls or cries; Lingle, Wyman, Kotrba, Teichroeb & Romanow, 2102). After recording infants’ isolation calls, we collected their scents as urogenital rubs as described by Ziegler, Schultz-Darken, Scott, Snowdon and Ferris (2005). Ground glass stoppers were rubbed lightly over the urogenital area of the infant, avoiding any faecal residue; 300 l of ethanol:water degassed solution was washed over the collected scent and pipetted into a microcentrifuge tube for storage. The infant was then put in the carrier in the nestbox and returned to the family group. Tubes with the odour solution were collected and kept cold on dry ice/wet ice until stored at– 80 °C. Vocalization recordings and odour collection for testing responsiveness to stimuli from unrelated infants were done using the same methods as for familiar infants. To make a homogeneous unfamiliar infant scent we combined scents from several unfamiliar infants at 15–17 days of age as was done in Ziegler et al. (2011).
Behavioural Tests
Responsiveness of parents was evaluated using the standardized method developed by Zahed et al. (2008) to evaluate motivational behaviour of both parents in response to infant stimuli in the common marmoset in the absence of competition from other group members. The infant response apparatus consisted of two chambers (60 x 90 cm and 183 cm high) 60 cm apart but connected by a mesh ‘bridge’ attached 117 cm above the floor. These components were made with the same material and style of the marmoset home cages. The bridge allowed free movement between the home chamber and the stimulus chamber. The chamber in which the test subject was placed inside its ‘home nestbox’ was termed the ‘home chamber’ and the adjoining cage containing the stimulus was called the ‘stimulus chamber’. A horizontal divider was placed midway up the stimulus chamber so that only the top portion was used inside of which the stimulus was placed inside a metal ‘stimulus nestbox’. For the tests we removed the test subject from its family cage by encouraging it to enter the metal nestbox located in its cage, which was then removed, carried to the test room and placed in the test cage located in a separate room with no auditory, visual or olfactory cues from other marmosets, other than the specific stimuli provided for the test. The test animal was placed in the home chamber. The odour or vocal stimulus, respectively, was placed in the stimulus chamber inside the nestbox. Each individual received a 10 min habituation session for 3 consecutive days followed by 7 continuous days of testing (in the case of current breeders with the ‘dependent infant’ and with the ‘independent infant’) or 5 continuous days of testing (in the case of not currently breeding marmosets, ‘no infants’), alternating between stimulus tests and control tests. On control days, the marmoset was placed in the test apparatus for 10 min and its behaviour was recorded without the presence of any infant stimulus. On test days, marmosets were presented with one of six stimuli in the case of current breeders: control vocalization (CV; a repeated synthesized human ‘e’ vowel sound with the same tempo as the infant cry), own infant vocalization (OV), unrelated infant vocalization (UV), control odour (CO, the ethanol:water vehicle in which scents were dissolved), own infant odour (OO) and unrelated infant odour (UO), in a randomized order without replacement. Not currently breeding marmosets were presented only with the unrelated infant stimuli (UV and UO) and their respective controls (CV and CO). As soon as the test was finished marmosets were again placed in their metal nestbox and returned to their families. The procedure took no longer than 15 min.
The test began when the home nestbox door was opened and the marmoset could move freely about the chambers in response to the stimulus. Focal behaviours were recorded for 10 min, after which time the stimulus was removed from the test cage. Two trained observers, one of them blind to the purpose of the test (mean interclass correlations for the different behaviours = 0.946 + 0.07 SD), continuously recorded stimulus-oriented behaviour (look at bridge, stimulus cage, stimulus nestbox), stimulus exploratory behaviour (sniff, touch, bite or attempt to retrieve stimulus or search in stimulus nestbox), enter bridge, enter stimulus cage and enter stimulus nestbox.
Data summary and analyses
For the analyses we used the following measures: (1) latency of each of the categories of the behaviours described above, (2) the combined frequency of all the above behaviours (investigatory behaviour) and (3) the time spent in proximity to the stimulus (in the bridge, stimulus chamber and stimulus nestbox).
We used nonparametric statistics. We evaluated differences in responsiveness between males and females of each category of breeders (‘dependent infant’, ‘independent infant’ and ‘no infant’), and also compared responsiveness between current breeders (‘dependent infant’ and ‘independent infant’) with not currently breeding animals using Mann–Whitney U tests. We evaluated responsiveness of current breeders when their infants were dependent and when the infants were independent, using the Wilcoxon signed-ranks test. For comparisons of behavioural responsiveness for each category of breeders across stimulus tests we used Friedman's ANOVA by ranks (k=4) for current breeders with dependent infants and also for independent infants (vocal tests: C, CV, OV, UV; odour tests: C, CO, OO, UO); k=3 for noncurrent breeders (vocal tests: C, CV, UV; odour tests: C, CO, UO). When significant Friedman's ANOVAs were found, we used the Tukey test for nonparametric multiple comparisons based on mean ranks (Zar, 1984) to determine between which conditions differences occurred. The H0 was rejected if qT>q0.05,k,∞, where q0.05, k, ∞= 3.633 if k=4 and q0.05,k, ∞= 3.314 if k=3. We report two effect size measures: Cohen's d for two means comparisons and η2 for Friedman analysis. All tests were two tailed and differences were considered significant when P<0.05. Statistical analyses were performed with Statistica 6.0 and SPSS Statistics 21.
RESULTS
Between the sexes
We found no differences between the sexes either in the latency of any of the categories of behaviour or in the frequency of all the investigatory behaviours considered together or in the time spent near the stimulus during any conditions in any sample (Mann– Whitney U test: N1=N2=6, P>0.05 in all cases). See Tables 1, 2 and 3 for the statistics of the comparisons. Thus mothers and fathers reacted similarly in all tests and their data were combined in subsequent analyses.
Table 1.
Comparison of responsiveness between fathers and mothers with dependent infants to the different stimulus tests
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | U | P | Cohen's d | ||
| OV | Latency | 325.1 | (301.1) | 407.6 | (239.6) | 13.5 | 0.480 | −1.470 |
| Frequency | 86.3 | (74.6) | 55.1 | (26.9) | 10.0 | 0.240 | 0.274 | |
| Time | 334.5 | (240.9) | 282.6 | (279.4) | 15.5 | 0.699 | 0.198 | |
| UV | Latency | 271.1 | (263.7) | 484.3.6 | (208.2) | 10.0 | 0.240 | −1.106 |
| Frequency | 16.5 | (16.6) | 30.8 | (26.9) | 14.0 | 0.484 | −0.195 | |
| Time | 380.1 | (210.5) | 259.5 | (153.9) | 9.0 | 0.937 | 0.654 | |
| OO | Latency | 600.0 | -- | 600.0 | -- | -- | -- | -- |
| Frequency | 52.0 | (81.5) | 32.1 | (32.0) | 13.0 | 0.588 | 0.477 | |
| Time | 54.0 | (88.3) | 37 | (55.6) | 17.0 | 0.937 | 0.230 | |
| UO | Latency | 600.0 | -- | 600.0 | -- | -- | -- | -- |
| Frequency | 10.6 | (10.8) | 20.1 | (14.5) | 14.0 | 0.588 | −0.134 | |
| Time | 91.1 | (88.39 | 66.6 | (105.6) | 15.0 | 0.699 | 0.251 | |
Stimuli were own infant vocalization (OV), unfamiliar infant vocalization (UV), own infant odour (OO) and unfamiliar infant odour (UO). Mann–Whitney U tests were used to compare responses between the sexes. Cohen's d for effect size in comparison of two means is reported. All Ps two tailed. Latency= latency of stimulus exploratory behaviour. Frequency= frequency of investigatory behaviour (the combined frequency of stimulus oriented, stimulus exploratory, enter bridge, enter stimulus cage and enter stimulus nestbox). Time= time spent near the stimulus (the combined time in the bridge, stimulus chamber and stimulus nestbox).
Table 2.
Comparison of responsiveness between fathers and mothers with independent infants to the different stimulus tests
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | U | P | Cohen's d | ||
| OV | Latency | 569.3 | (75.11) | 600.0 | -- | 15.0 | 0.699 | −0.547 |
| Frequency | 300.5 | (97.2) | 225.6 | (105.0) | 13.0 | 0.484 | 0.317 | |
| Time | 101.0 | (127.7) | 117.3 | (202.1) | 18.0 | 1.000 | −0.096 | |
| UV | Latency | 600.0 | -- | 600.0 | -- | -- | -- | -- |
| Frequency | 202.5 | (132.5) | 139.3 | (70.4) | 12.0 | 0.393 | 0.288 | |
| Time | 112.8 | (125.7) | 101.5 | (230.6) | 16.5 | 0.818 | 0.060 | |
| OO | Latency | 586.1 | (33.8) | 600.0 | -- | 15.0 | 0.699 | −0.247 |
| Frequency | 69.0 | (71.7) | 72.1 | (64.7) | 15.0 | 0.699 | −0.039 | |
| Time | 97.1 | (188.5) | 108 | (230.6) | 18.0 | 1.000 | −0.051 | |
| UO | Latency | 600.0 | -- | 600.0 | -- | -- | -- | -- |
| Frequency | 64.1 | (46.5) | 54.1 | (42.9) | 9.50 | 0.179 | 0.577 | |
| Time | 34.6 | (84.9) | 124.8 | (169.3) | 18.0 | 0.588 | −0.673 | |
Stimuli were own infant vocalization (OV), unfamiliar infant vocalization (UV), own infant odour (OO) and unfamiliar infant odour (UO). Mann–Whitney U tests were used to compare responses between the sexes. Cohen's d for effect size in comparison of two means is reported. All Ps two tailed. Latency= latency of stimulus exploratory behaviour. Frequency= frequency of investigatory behaviour (the combined frequency of stimulus oriented, stimulus exploratory, enter bridge, enter stimulus cage and enter stimulus nestbox). Time= time spent near the stimulus (the combined time in the bridge, stimulus chamber and stimulus nestbox).
Table 3.
Comparison of responsiveness between noncurrently breeding fathers and mothers to the different stimulus tests
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | U | P | Cohen's d | ||
| UV | Latency | 600.0 | -- | 600.0 | -- | -- | -- | -- |
| Frequency | 61.3 | (57.2) | 144.0 | (86.0) | 11.5 | 0.309 | −0.567 | |
| Time | 52.6 | (111.0) | 25 | (38.9) | 17.0 | 0.937 | 0.331 | |
| UO | Latency | 538.3 | (151.0) | 544.0 | (137.1) | 17.5 | 0.937 | −0.083 |
| Frequency | 94.1 | (120.2) | 161.6 | (334.1) | 17.00 | 0.937 | −0.029 | |
| Time | 91.8 | (186.7) | 83.8 | (205.3) | 13.5 | 0.484 | 0.040 | |
Stimuli were unfamiliar infant vocalization (UV) and unfamiliar infant odour (UO). Mann–Whitney U tests were used to compare responses between the sexes. Cohen's d for effect size in comparison of two means is reported. All Ps two tailed. Latency= latency of stimulus exploratory behaviour. Frequency= frequency of investigatory behaviour (the combined frequency of stimulus oriented, stimulus exploratory, enter bridge, enter stimulus cage and enter stimulus nestbox). Time= time spent near the stimulus (the combined time in the bridge, stimulus chamber and stimulus nestbox).
Across stimulus tests
Across stimulus tests the major significant differences were found in response to infant vocalizations when infants were dependent (see Tables 4, 5). Parents with dependent infants showed shorter latencies overall across tests of both types of stimulus (see Table 4), to orient towards and explore the stimulus and to enter the stimulus box than in the respective control condition (ANOVA Friedman's test: N=12, P<0.01; see Fig. 1 for latency measures of stimulus exploratory behaviour and entering the nest). However, post hoc tests showed significance only for stimulus orientation with the control condition having longer latencies than either OV or UV conditions (qT<3.633, P<0.05 in all cases). Parents with dependent infants also showed significant differences in frequency of investigatory behaviours (ANOVA Friedman's test: N=12, P<0.01; Fig. 2a) and duration of time near the stimulus (ANOVA Friedman's test: N=12, P<0.01; Fig. 1c), with both OV and UV eliciting greater responsiveness than either control condition (no stimulus or control vocalization; qT<3.633, P<0.05 in all cases; see Table 4). Post hoc tests showed that although parents with independent infants showed more frequent investigatory behaviour when exposed to OV than UV (qT=4.19), the parents did not show significant differences between these two stimuli when infants were totally dependent (qT=1.98). When infants were independent we also found differences (ANOVA Friedman's test: N=12, P<0.01; Fig. 2b): parents showed greater investigatory behaviour to their own infant's calls than with both the control conditions and the unfamiliar infant vocalization (qT<3.633, P<0.05 in all cases; see Table 4). Finally, marmosets that were not currently breeding showed differences (ANOVA Friedman's test: N=12, P<0.01), responding with shorter latency to both the control vocalization and the unfamiliar infant vocalization compared with the no stimulus condition and more investigatory behaviour towards the unfamiliar vocalization (qT<3.633, P<0.05 in all cases; see Table 4).
Table 4.
Responsiveness of current breeders with dependent and independent infants and noncurrent breeders (no infants) across the vocal stimulus tests
| Dependent infants | Independent infants | No infants* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P | η 2 | Post hoc (qT >3.633) | P | η 2 | Post hoc (qT>3.633) | P | η 2 | Post hoc (qT>3.314) | |
| Latency to orient to stimulus | 0.01 | 0.464 | C> UV,OV | 0.19 | 0.156 | -- | 0.01 | 0.235 | C>CV,UV |
| Latency to explore stimulus | 0.01 | 0.474 | NS | 0.40 | 0.023 | -- | 0.37 | 0.083 | -- |
| Latency to enter stimulus nestbox | 0.01 | 0.515 | NS | 0.40 | 0.250 | -- | -- | -- | -- |
| Frequency of investigatory behaviour | 0.01 | 0.456 | C<UV,OV CV<UV,OV |
0.01 | 0.021 | C<OV UV<OV |
0.01 | 0.248 | C<UV |
| Time spent near stimulus | 0.01 | 0.555 | C<UV,OV CV<UV,OV |
0.20 | 0.126 | -- | 0.96 | 0.169 | -- |
Stimuli were control (C), control vocalization (CV), own infant vocalization (OV) and unfamiliar infant vocalization (UV). We used Friedman's ANOVA by ranks (k=4) for current breeders with dependent and independent infants; (k=3) for noncurrent breeders; Tukey's test for nonparametric multiple comparisons determined between which tests differences occurred. Significant post hoc test comparisons are shown. Effect size measure η2 is reported. Significant P values are shown in bold. Also see Figs 1, 2.
When the behaviour did not occur in any of the stimulus tests the statistical comparison was not performed.
Table 5.
Responsiveness of current breeders with dependent and independent infants and noncurrent breeders (no infants) across the odour stimulus tests
| Dependent infants | Independent infants | No infants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P< | η 2 | Post hoc qT>3.633 | P< | η 2 | Post hoc qT>3.633 | P< | η 2 | Post hoc qT>3.14 | |
| Latency to orient to stimulus | 0.01 | 0.452 | C>CO,UO | 0.23 | 0.085 | -- | 0.01 | 0.428 | C>CO,UO |
| Latency to explore stimulus | 0.96 | 0.020 | -- | 0.58 | 0.167 | -- | 0.14 | 0.159 | -- |
| Latency to enter stimulus nestbox* | -- | -- | -- | 0.58 | 0.167 | -- | 0.08 | 0.102 | -- |
| Frequency of investigatory behaviour | 0.18 | 0.223 | -- | 0.38 | 0.286 | -- | 0.18 | 0.424 | -- |
| Time spent near stimulus | 0.29 | 0.091 | -- | 0.63 | 0.104 | -- | 0.96 | 0.091 | -- |
Stimuli were control (C), control odour (CO), own infant odour (OO) and unfamiliar infant odour (UO). We used Friedman's ANOVA by ranks (k=4) for current breeders with dependent infants and independent infants; (k=3) for noncurrent breeders; Tukey's test for nonparametric multiple comparisons determined between which tests differences occurred. Significant post hoc test comparisons are shown. Effect size measure η2 is reported. Significant P values are shown in bold.
When the behaviour did not occur in any of the stimulus tests the statistical comparison was not performed.
Figure 1.
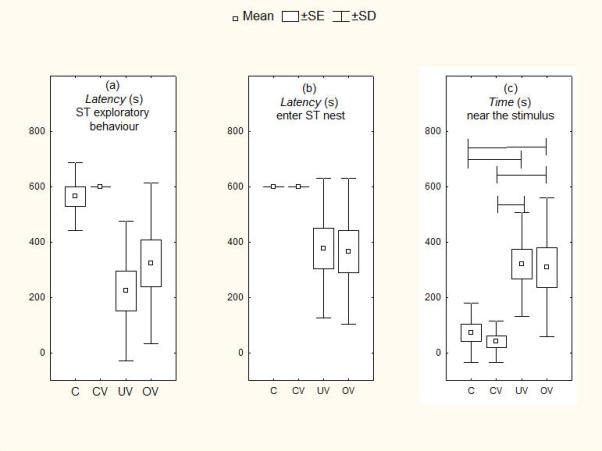
Responsiveness of current breeders with dependent infants across vocal stimulus tests. (a) Latency to explore stimulus, (b) latency to enter stimulus nestbox and (c) time spent near stimulus. Means are shown with SE (box) and SD (vertical lines). Lines spanning different histograms indicate pairs of tests between which differences were significant at P <0.05. C= no stimulus control, CV = control vocalization, UV = unfamiliar infant vocalization, OV = own infant vocalization.
Figure 2.
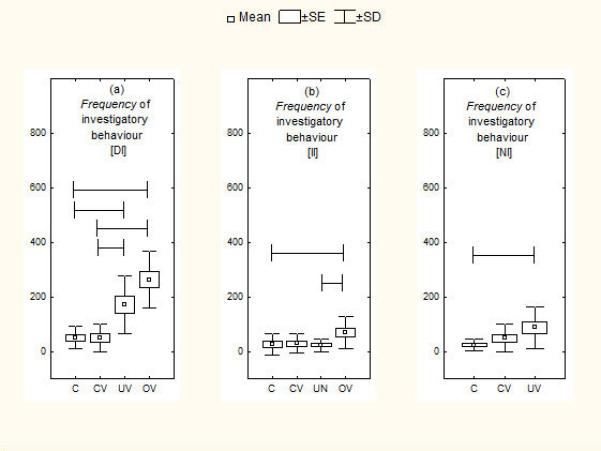
Responsiveness of current breeders with (a) dependent infants, (b) independent infants and (c) noncurrent breeders across vocal stimulus tests. Means are shown with SE (box) and SD (vertical lines). Lines spanning different histograms indicate pairs of tests between which differences were significant at P <0.05. C= no stimulus control, CV = control vocalization, UV = unfamiliar infant vocalization, OV = own infant vocalization.
In contrast to the results with vocalizations, there was little response to odour cues (see Table 5). Both the parents of dependent infants and not currently breeding parents (ANOVA Friedman's test: N=12, P<0.01 in both cases) responded with shorter latencies of investigatory behaviour to both the control odour and unfamiliar odour than to the no stimulus control condition (qT<3.633, P<0.05 in all cases), but no other results with odours were significant.
Between breeding conditions
With both own infant vocalizations and unfamiliar infant vocalizations (see Table 6 for statistics), current breeders showed significantly shorter latencies (Fig. 3a, c), increased frequencies of investigatory behaviour (Fig. 4a) and increased duration of time near the stimulus (Fig. 4c) when their infants were dependent than when they were independent. Current breeders with dependent infants also showed significantly shorter latencies (Fig. 3b, d), increased frequencies of investigatory behaviours (Fig. 4b) and increased duration of time near the stimulus (Fig. 4d) compared with marmoset parents who were not current breeders. In contrast, there were no differences between breeders with independent infants and those who were not current breeders.
Table 6.
Comparisons of responsiveness of current breeders with dependent and independent infants and noncurrent breeders (no infants) across the vocal tests
| Test | Dependent vs independent infants | Dependent infants vs no infants | Independent infants vs no infants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV | OV | UV | UV* | |||||||||
| T | P | Cohen's d | T | P | Cohen's d | U | P | Cohen's d | U | P | Cohen's d | |
| Latency to orient to stimulus | 0.0 | 0.002 | −0.624 | 0.0 | 0.002 | −0.905 | 23.5 | 0.003 | −0.633 | 47 | 0.15 | −0.077 |
| Latency to explore stimulus | 0.0 | 0.007 | −1.500 | 0.0 | 0.027 | −0.400 | 18 | 0.001 | −2.12 | -- | -- | -- |
| Latency to enter stimulus nestbox | 0.0 | 0.027 | −0.880 | 0.0 | 0.027 | −0.880 | 36 | 0.038 | −1.245 | -- | -- | -- |
| Frequency of investigatory behaviour | 0.0 | 0.001 | 1.352 | 0.0 | 0.001 | 1.998 | 38 | 0.051 | 0.900 | 39 | 0.059 | 1.16 |
| Time spent near stimulus | 0.0 | 0.003 | 1.381 | 3.0 | 0.012 | 0.863 | 12.5 | 0.001 | 1.953 | 57 | 0.409 | 0.590 |
Stimuli were own infant vocalization (OV) and unfamiliar infant vocalization (UV). Wilcoxon signed-ranks tests were used to test between different conditions in the same parents (T) and Mann–Whitney U tests were used to compare responses between different parents (U). Effect size measure Cohen's d for two comparison of two means is reported. Significant P values are shown in bold. Also see Figs 3, 4.
When the behaviour did not occur in any of the compared breeding conditions the statistical comparison was not performed.
Figure 3.
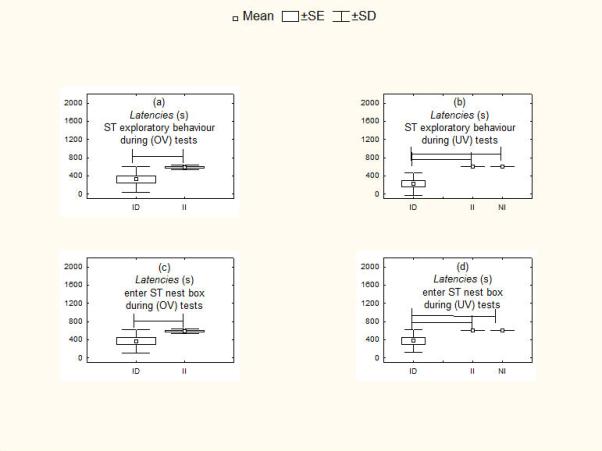
Responsiveness of current breeders with dependent infants (DI) versus independent infants (II) during (a, c) own infant vocalization tests (OV) and versus noncurrent breeders (NI) during (b, d) unfamiliar infant vocalization tests (UV). (a, b) Latency to explore stimulus. (c, d) Latency to enter stimulus nestbox. Means are shown with SE (box) and SD (vertical lines). Lines spanning different histograms indicate categories of breeders between which differences were significant at P <0.05.
Figure 4.
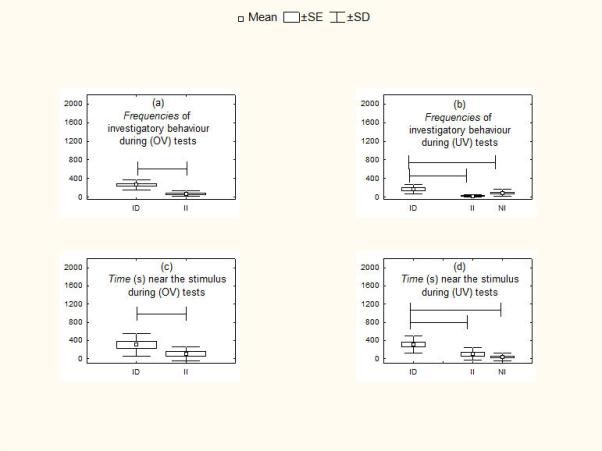
Responsiveness of current breeders with dependent infants (DI) versus independent infants (II) during (a, c) own infant vocalization tests (OV) and versus noncurrent breeders (NI) during (b, d) unfamiliar infant vocalization tests (UV). (a, b) Frequency of investigatory behaviour. (c, d) Time spent near stimulus. Means are shown with SE (box) and SD (vertical lines). Lines spanning different histograms indicate categories of breeders between which differences were significant at P <0.05.
DISCUSSION
Our findings indicate that in the cooperatively breeding marmoset with biparental care, both parents respond similarly to infant stimuli. There were no differences in behavioural responses between mothers and fathers to infant odour and vocal cues under any condition. Both parents responded differently to infant cues at different stages in the developmental process with dependent infant cues eliciting the highest response. Additionally, parents with independent infants and former parents who were not currently breeding showed the same response. Under our test paradigm, parents responded much more to the infant distress vocalizations than to the odour stimuli. Therefore, we suggest that both parents are equally primed to respond to infant cues from the moment of birth and that response to these cues may diminish after infants are no longer dependent but discrimination between own and unfamiliar distress vocalizations increases.
Human fathers recognize their baby's cries just as well as mothers, providing they spend as much time with their offspring as mothers do, something that authors have interpreted to be a consequence of learning (Gustafsson, Levréro, Reby & Mathevon, 2013; Wiesenfeld, Malatesta, & Deloach, 1981). The absence of sex differences in the behavioural responsiveness to infant cues that we found could be explained at a proximate level by the similar hormonal changes before birth that prepare both parents to be ready for infant care and by the parallel neuroendocrine responses after birth that are sustained through the continuous interaction with the infant (Ziegler, Jacoris & Snowdon, 2004b; Ziegler et al., 2006). Cues from pregnant female mates or cues from offspring facilitate male parental care (Brown, 1993). Hormones, prior experience and sensory stimuli from newborns interact in complex ways to modulate the responsiveness of parental caregivers in the common marmoset (Barbosa & Mota, 2013) as well as in biparental birds (Angelier & Chastel, 2009) and mammals (Muller & Manser, 2008; meadow voles, Microtus pennsylvanicus, Storey & Joyce, 1995; striped mouse Rhabdomys pumilio, Schradin & Pillay, 2004; review by Saltzman & Maestripieri, 2011).
In our study, common marmoset parents were much more responsive to the isolation calls of their own and unrelated infants than to infant odour cues. Ziegler et al. (2011) demonstrated a decrease in androgens and an increase in oestrogens in fathers with infant odour cues only during the stage when their own infant was still dependent on paternal care, but not when the infant was mostly independent or when fathers were exposed to unfamiliar infant odours. However, in our study we found no response to infant odour cues, their own or unfamiliar ones, by either parent. The absence of responsiveness to olfactory cues from infants in our study could be explained by differences in the conditions of testing since, in contrast to Ziegler et al.'s (2011) study, the parents were not in direct contact with the odours in the current study.
Parents listening to infant cries receive very different information from parents perceiving the infant's odour under calm conditions. An infant's odour is a very different type of signal whose function is more related to the recognition and establishment of attachment. Even if under the conditions of our study, parents could detect their infant's odour, it does not necessarily follow that parents would react as urgently as to a distress vocalization. We would expect different behavioural responses to infant odours and distress vocalizations. However, we cannot disregard the fact that the absence of response to odours could be related to too great a distance between the stimulus and the subject in our test apparatus.
Distress vocalizations are a specific type of vocalization widely believed to be honest indicators of infant need or condition that require immediate response (Kilner & Johnstone 1997). An infant's cry elicits autonomic arousal in the listener that prepares an adult to react rapidly to the infant's distress, improving speed and accuracy in intentional movements (Parsons, Young, Parsons, Stein & Kringelbach, 2012). Parents in our study showed a behavioural response to infant distress vocalizations from their own and unknown infants during the period when infants were highly dependent on parental help but not at the age of 4 months when infants were mostly independent. Not currently breeding parents did not respond to the isolation calls of unrelated infants. It is unknown whether in common marmosets there are features in distress vocalizations that allow individual recognition of the infant. Many newborn animals make loud, highly tonal sounds under distress situations such as when attacked by predators (Lingle et al. 2012). Lingle et al. (2012) showed that distress vocalizations share a common acoustic structure even among infants of different vertebrate species and elicit similar response by caregivers. However, an alternative explanation for the lack of difference in the responsiveness to familiar and unfamiliar infants might be that parents are motivated to respond to any cry rather than waiting to assess specifically whether their own infant is at risk, ensuring the defence of their own offspring, even if they incur costs from occasionally defending nonoffspring (e.g. mule deer, Odocoileus hemionus, Lingle, Rendall, Wilson, DeYoung, & Pellis, 2007). Selection would favour the behaviour if the overall benefits, across contexts and over time, outweigh the overall costs of responding in this way (Sih, Bell & Johnson, 2004).
We recorded distress vocalizations during 5 min in an isolation context but the acoustic pattern of these vocalizations has not been analysed. Callitrichid infants begin their vocal activity very soon after birth and engage in long repetitive bouts of vocalizations that are part of the adult repertoire and can go on for several minutes (e.g. pygmy marmoset, Cebuella pygmaea, Snowdon & Elowson, 2001) and that elicit parental care (Snowdon 2009). In callitrichids it is unusual for same-age infants from different females to coexist in the group at the same time. In the few cases in which subordinate common marmoset females reproduce, they are successful in rearing young only when the timing of births does not overlap with the dependency period of infants born to the dominant female (Digby 1995). Ecological factors may play a role in the patterns of recognition of calls between mothers and their infants in mammals. For instance, in those with antipredator hiding strategies such as the fallow deer, Dama dama, unidirectional recognition from infant to mothers occurs (Torriani, Vannoni & McElligott 2006) but mutual recognition is necessary in the case of follower mammals such as the domestic sheep, Ovies aries (Sebe, Duboscq, Aubin, Ligout, & Poindron, 2010). In callitrichid species with a shared, and very costly, carrying pattern (Sánchez, Peláez, Gil-Burman, & Kaumanns, 1999), individual recognition of infants might be expected to occur although this may occur only after the initial response to a distress call.
The behavioural responsiveness of parents to their own and unfamiliar infant distress vocalizations when infants are highly dependent but not to the same vocalizations when infants are mostly independent could be interpreted as a consequence of the reduction in neural alterations in fathers’ brains that occurs after the birth of the infants owing to the decrease in hormonal priming (Kozorovitskiy, Hughes, Lee & Gould, 2006). Despite the common acoustic pattern in distress vocalizations, the reduced responsiveness of parents at 4 months could be the result of listening to a cry not corresponding with their current infants’ age. However, in the period in which infants were mostly independent, parents showed more interest in the distress vocalizations of their own infants than unrelated ones. This suggests that either calls do not change much from infancy to 4 months or that parents do still recognize the infant vocalizations at this later time. Hormonal priming and hormonal changes during active parenting might result in a more indiscriminate rapid response to cries of any dependent infants during the period of infant dependence. However, when infants’ needs have decreased and the transitory neuroendocrine changes have disappeared, a less intense but more selective response could occur to only the parent's own infant distress vocalizations at an independent age. This could also be explained by the continued exposure to the sensory cues of developing infants which may lead to either greater individual differentiation of infant cries over time, to parental learning of specific infant cues or a combination of both. Response to distress vocalizations may be a form of parental behaviour that is not modulated by circulating hormones (Rosenblatt, 1967; Barbosa & Mota, 2013). Successive exposure to sensory stimuli from newborns appeared to play a role in sensitizing even inexperienced common marmoset males to care-giving behaviour (Barbosa & Mota, 2013; Roberts, Jenkins, Lawler, Wegner & Newman, 2001). The successive exposure to their own infants could sensitize parents to be responsive to the specific vocalizations of their own infants, but not to unfamiliar infants, even in the absence of neuroendocrine priming.
These results allow us to further understand the role of biparental care in a primate with cooperative breeding. In the marmoset, in which both parents invest equally both metabolically and hormonally, the energetic burden is shared equally and allows for greater infant survival. With the rapid reproduction that occurs in this species, parents may need to reduce investment in their infants once they are independent as future infants have already been conceived and both parents need to prepare for the arrival of new offspring.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of Proyectos de Investigación, Subprograma de Proyectos de Investigación Fundamental No Orientada (PSI2012-30744), the United States National Institutes of Health (NIH RR000167) (WNPRC) and Hilldale Professorship to C.T.S. We thank Megan E. Sosa for training in marmoset work on this project and her daily care of the marmosets, Katarina Braun for her assistance with data collection and José Manuel Caperos Montalbán for his statistical advice. We are very grateful to two anonymous referees for their stimulating and valuable suggestions and especially to Susan Lingle for her very helpful feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Angelier F, Chastel O. Stress, prolactin and parental investment in birds: a review. General and Comparative Endocrinology. 2009;163:142–148. doi: 10.1016/j.ygcen.2009.03.028. http://dx.doi.org/10.1016/j.ygcen.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Barbosa MN, Mota MT. Alloparental responsiveness to newborns by nonreproductive, adult males, common marmosets (Callithrix jacchus). American Journal of Primatology. 2013;75:145–152. doi: 10.1002/ajp.22092. http://dx.doi.org/10.1002/ajp.22092. [DOI] [PubMed] [Google Scholar]
- Brown RE. Hormonal and experiential factors influencing parental behaviour in male rodents—An integrative approach. Behavioural Processes. 1993;30:1–28. doi: 10.1016/0376-6357(93)90009-G. http://dx.doi.org/10.1016/0376-6357(93)90009-G. [DOI] [PubMed] [Google Scholar]
- Caspers BA, Hoffman JI, Kohlmeier P, Krüger O, Krause T. Olfactory imprinting as a mechanism for nest odour recognition in zebra finches. Animal Behaviour. 2013;86:85–90. http://dx.doi.org/10.1016/j.anbehav.2013.04.015. [Google Scholar]
- Cornwallis CK, West SA, Davis KE, Griffin AS. Promiscuity and the evolutionary transition to complex societies. Nature. 2010;466:969–974. doi: 10.1038/nature09335. http://dx.doi.org/10.1038/nature09335. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. Structure and function in mammalian societies. Philosophical Transactions of the Royal Society. 2009;364:3229–3242. doi: 10.1098/rstb.2009.0120. http://dx.doi.org/10.1098/rstb.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby L. Infant care, infanticide, and female reproductive strategies in polygynous group of common marmosets. Behavioral Ecology and Sociobiology. 1995;37:51–60. http://dx.doi.org/10.1007/BF00173899. [Google Scholar]
- Fleming AS, Corter C, Franks P, Surbey M, Schneider B, Steiner M. Postpartum factors related to mother's attraction to newborn infant odors. Developmental Psychobiology. 1993;26:115–132. doi: 10.1002/dev.420260204. http://dx.doi.org/10.1002/dev.420260204. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Poindron P. Parental Care in Mammals: Immediate Internal and Sensory Factors of Control. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Elsevier; San Diego, CA: 2002. pp. 215–298. http://dx.doi.org/10.1016/B978-012532104-4/50005-6. [Google Scholar]
- Gustafsson E, Levréro F, Reby D, Mathevon N. Fathers are just as good as mothers at recognizing the cries of their baby. Nature Communications. 2013;4:1698. doi: 10.1038/ncomms2713. doi:10.1038/ncomms2713. [DOI] [PubMed] [Google Scholar]
- Kilner R, Johnstone RA. Begging the question: are offspring solicitation behaviours signals of needs. Trends in Ecology and Evolution. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. http://dx.doi.org/10.1016/S0169-5347(96)10061-6. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nature Neuroscience. 2006;9:1094–1095. doi: 10.1038/nn1753. http://dx.doi.org/10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Levy F, Keller M, Poindron P. Olfactory regulation of maternal behavior in mammals. Hormones and Behavior. 2004;46:284–302. doi: 10.1016/j.yhbeh.2004.02.005. http://dx.doi.org/10.1016/j.yhbeh.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Lingle S, Rendall D, Wilson WF, DeYoung RW, Pellis SM. Altruism and recognition in the antipredator defence of deer: 2. Why mule deer help nonoffspring fawns. Animal Behaviour. 2007;73:907–916. http://dx.doi.org/10.1016/j.anbehav.2006.11.004. [Google Scholar]
- Lingle S, Wyman MT, Kotrba R, Teichroeb LJ, Romanow C. What makes a cry a cry? A review of infant distress vocalizations. Current Zoology. 2012;58:698–726. [Google Scholar]
- Lukas D, Clutton-Brock T. Cooperative breeding and monogamy in mammalian societies. Proceedings of the Royal Society. 2012;279:2151–2156. doi: 10.1098/rspb.2011.2468. http://dx.doi.org/10.1098/rspb.2011.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn SF, McNeilly AS. Failure of lactation to have a consistent effect on interbirth interval in the common marmoset, Callithrix jacchus jacchus. Folia Primatologica. 1982;37:99–105. doi: 10.1159/000156023. http://dx.doi.org/10.1159/000156023. [DOI] [PubMed] [Google Scholar]
- Mills BA, Windle CP, Baker HF, Ridley RM. Analysis of infant carrying in large, well-established family group of captive marmosets (Callithrix jacchus). Primates. 2004;45:259–265. doi: 10.1007/s10329-004-0095-7. http://dx.doi.org/10.1007/s10329-004-0095-7. [DOI] [PubMed] [Google Scholar]
- Müller CA, Manser MB. Mutual recognition of pups and providers in the cooperatively breeding banded mongoose. Animal Behaviour. 2008;75:1683–1692. http://dx.doi.org/10.1016/j.anbehav.2007.10.021. [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Parsons CE, Young KS, Parsons E, Stein A, Kringelbach ML. Listening to infant distress vocalizations enhances effortful motor performance. Acta Paediatrica. 2012;101:189–191. doi: 10.1111/j.1651-2227.2011.02554.x. http://dx.doi.org/10.1111/j.1651-2227.2011.02554.x. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Jenkins KT, Lawler T, Wegner FH, Newman JD. Bromocriptine administration lowers serum prolactin and disrupts parental responsiveness in common marmosets (Callithrix jacchus). Hormones and Behavior. 2001;39:106–112. doi: 10.1006/hbeh.2000.1639. http://dx.doi.org/10.1006/hbeh.2000.1639. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. http://dx.doi.org/10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Russell AF. Mammals: comparisons and contrasts. In: Koenig WD, Dickinson JL, editors. Ecology and evolution of cooperative breeding in birds. New York: 2004. Cambridge University Press. pp. 210–227. http://dx.doi.org/10.1017/CBO9780511606816.014. [Google Scholar]
- Sánchez S, Peláez F, Gil-Burmann C, Kaumanns W. Costs of infant carrying in the cotton-top tamarin (Saguinus oedipus). American Journal of Primatology. 1999;48:99–111. doi: 10.1002/(SICI)1098-2345(1999)48:2<99::AID-AJP2>3.0.CO;2-6. http://dx.doi.org/10.1002/(SICI)1098-2345(1999)48:2%3C99::AIDAJP2%3E3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sánchez S, Peláez F, Fidalgo A, Morcillo A, Caperos J. Changes in body mass of expectant male cotton-top tamarins (Saguinus oedipus). Folia Primatologica. 2008a;79:458–462. doi: 10.1159/000151718. DOI: 10.1159/000151718. [DOI] [PubMed] [Google Scholar]
- Sánchez S, Peláez F, Fidalgo A, Morcillo A, Caperos J. Body weight increase in expectant males and helpers of cotton-top tamarin (Saguinus oedipus): A symptom of the couvade syndrome? Psicothema. 2008b;20:825–829. [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:1192–1204. doi: 10.1016/j.pnpbp.2010.09.017. http://dx.doi.org/10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schradin C, Pillay N. Prolactin levels in paternal striped mouse (Rhabdomys pumilio) fathers. Physiology and Behavior. 2004;81:43–50. doi: 10.1016/j.physbeh.2003.12.013. http://dx.doi.org/10.1016/j.physbeh.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Sebe F, Duboscq J, Aubin T, Ligout S, Poindron P. Early vocal recognition of mother by lambs: comparison of low and high-frequency vocalizations. Animal Behaviour. 2010;79:10555–1066. doi:10.1016/j.anbehav.2010.01.021. [Google Scholar]
- Sih A, Bell S, Johnson JC. Behavioral syndromes: an ecological and Evolutionary overview. Trends in Ecology and Evolution. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. http://dx.doi.org/10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Plasticity of communication in nonhuman primates. Advances in the Study of Behavior. 2009;40:239–276. DOI: 10.1016/S0065-3454(09)40007-X. [Google Scholar]
- Snowdon CT, Elowson AM. Babbling in Pygmy Marmosets: Development after infancy. Behaviour. 2001;138:1235–1248. http://dx.doi.org/10.1163/15685390152822193. [Google Scholar]
- Snowdon CT, Ziegler TE. Growing up cooperatively: family processes and infant care in marmosets and tamarins. Journal of Developmental Processes. 2007;2:40–66. [Google Scholar]
- Stern JM. Maternal behavior: sensory, hormonal, and neural determinants. In: Levine S, Brush FR, editors. Psychoendocrinology. Academic Press; New York: 1989. pp. 104–225. http://dx.doi.org/10.1016/B978-0-12-137952-0.50008-2. [Google Scholar]
- Storey AE, Joyce TL. Pup contact promotes paternal responsiveness in male meadow voles. Animal Behaviour. 1995;49:1–10. http://dx.doi.org/10.1016/0003-3472(95)80148-0. [Google Scholar]
- Torriani MVG, Vannoni E, McElligott AG. Mother-young recognition in an ungulate hider species: a unidirectional process. American Naturalist. 2006;168:412–420. doi: 10.1086/506971. http://dx.doi.org/10.1086/506971. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld AR, Malatesta CZ, Deloach LL. Differential parental response to familiar and unfamiliar infant distress signals. Infant Behavioral Development. 1981;4:281–295. http://dx.doi.org/10.1016/S0163-6383(81)80030-6. [Google Scholar]
- Woller MJ, Sosa ME, Chiang Y, Prudom SL, Keelty P, Moore JE. Differential hypothalamic secretion of neurocrines in male common marmosets: parental experience effects? Journal of Neuroendocrinology. 2011;24:413–421. doi: 10.1111/j.1365-2826.2011.02252.x. http://dx.doi.org/10.1111/j.1365-2826.2011.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Hormones and. Behavior. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. http://dx.doi.org/10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Arruda M, Alencar A, de Sousa M, Araujo A. Mating systems and female-female competition in the common marmoset, Callithrix jacchus. In: Ford SM, Porter LM, Davis LC, editors. The smallest anthropoids, the marmoset/callimico radiation. Springer; New York: 2009. pp. 119–133. http://dx.doi.org/10.1007/978-1-4419-0293-1_6. [Google Scholar]
- Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus). American Journal of Primatology. 2008;70:84–92. doi: 10.1002/ajp.20460. http://dx.doi.org/10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]
- Zar HH. Biostatistical analysis. 2nd ed. Prentice-Hall; Englewood Cliffs, NJ: 1984. [Google Scholar]
- Ziegler T, Savage A, Scheffler G, Snowdon C. The endocrinology of puberty and reproductive functioning in female cotton-top tamarins (Saguinus oedipus) under varying social conditions. Biology of Reproduction. 1987;37:618–627. doi: 10.1095/biolreprod37.3.618. http://dx.doi.org/10.1095/biolreprod37.3.618. [DOI] [PubMed] [Google Scholar]
- Ziegler TE. Social effects via olfactory sensory stimuli on reproductive function and dysfunction in cooperative breeding marmosets and tamarins. American Journal of Primatology. 2013;75:202–211. doi: 10.1002/ajp.22061. http://dx.doi.org/10.1002/ajp.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate's pregnancy. Hormones and Behavior. 2004a;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. http://dx.doi.org/10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Jacoris S, Snowdon CT. Sexual communication between breeding male and female cottontop tamarins (Saguinus oedipus) and its relationship to infant care. American Journal of Primatology. 2004b;61:57–69. doi: 10.1002/ajp.20061. http://dx.doi.org/10.1002%2Fajp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Hormones and Behavior. 2005;47:56–64. doi: 10.1016/j.yhbeh.2004.08.009. http://dx.doi.org/10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Schultz-Darken NJ, Kurian AV, Snowdon CT. Pregnancy weight gain: marmoset and tamarin dads show it too. Biology Letters. 2006;2:181–183. doi: 10.1098/rsbl.2005.0426. http://dx.doi.org/10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Peterson LJ, Sosa ME, Barnard AM. Differential endocrine responses to infant odors in common marmoset (Callithrix jacchus) fathers. Hormones and Behavior. 2011;59:265–270. doi: 10.1016/j.yhbeh.2010.12.001. http://dx.doi.org/10.1016/j.yhbeh.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


