SUMMARY
The PI3K/AKT/mTOR pathway is commonly activated in non-small-cell lung cancer. It plays important roles in promoting oncogenesis in lung cancer and mediating resistance to EGF receptor tyrosine kinase inhibitors. Targeted agents against the components of this pathway are currently in development and their clinical benefits remain to be defined. This review provides an overview of the pathway dysregulation and novel agents targeting the pathway in lung cancer. In addition, potential predictive biomarkers guiding patient selection for targeted PI3K/AKT/mTOR inhibition is also discussed.
Lung cancer is the leading cause of cancer-related deaths in the USA with approximately 226,160 cases and 160,340 deaths per year [1]. Despite recent advances in diagnosis and treatment, the 5-year survival remains to be 16% for non-small-cell lung cancer (NSCLC). The treatment paradigm has recently been shifted with targeted therapy being standard treatment for molecular subsets of lung cancers harboring distinct molecular genetic alterations or driver mutations, such as EGF receptor (EGFR), ALK, HER2, RET and ROS1 [2]. Those distinct molecular changes serve as good predictive biomarkers to their specific targeted therapy.
Emerging evidence suggests that PI3K/ AKT/mTOR signaling is frequently activated in NSCLC and plays important roles in the oncogenesis through promoting cell survival, growth, proliferation and migration (Figure 1). Therapy targeting this axis is being exploited in clinical settings and has showed some promise. The current review is focused on pathway activation, novel agents targeting the cascade and potential predictive biomarkers of targeted inhibitors in lung cancer.
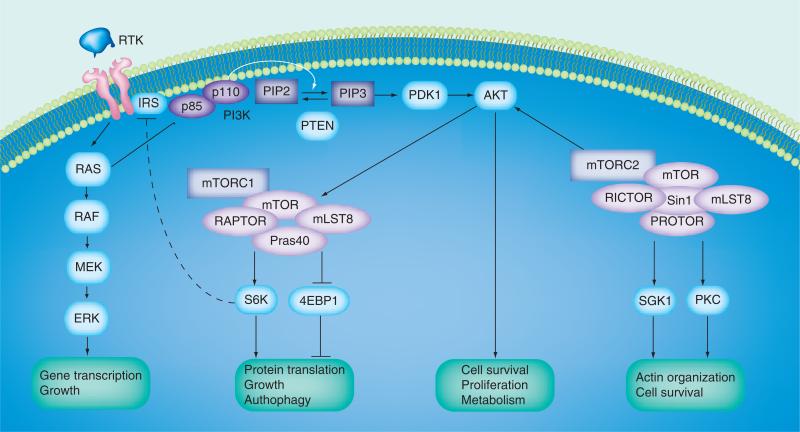
Figure 1. PI3K/AKT/mTOR signaling pathway.
PI3K consists of a family of lipid kinases and class IA PI3Ks are made up of a catalytic p110 subunit and a regulatory p85 subunit. RTKs are the principle proteins upstream of PI3Ks. Following ligand binding and RTK activation, p110 subunit is free to catalyze the phosphorylation of PIP2 to PIP3, which subsequently locates AKT to the plasma membrane. AKT is fully activated when its T308 and S473 are phosphorylated by PDK1 and mTORC2. Once activated, AKT separates from the plasma membrane and phosphorylates a wave of targets to promote cell survival, proliferation, mobility and metabolism. The mTOR is a serine/threonine kinase. It associates with different proteins to form two structurally and functionally distinct complexes, mTORC1 and mTORC2. The key components of mTORC1 include mTOR, RAPTOR, mLST8 and PRAS40. The mTORC1 signaling can be initiated by AKT, ERK1/2 and changes to the energy status of cells. The mTORC1 pathway promotes protein translation and cell growth predominantly through activating S6K and inhibiting eIF4E. The mTORC2 complex is mainly made up of mTOR, RICTOR, mLST8, PROTOR1 and mSIN1. The principle downstream targets of mTORC2 include AKT, SGK1 and PKC. The PI3K/AKT/mTOR signaling is controlled by several negative regulators. For example, PI3K phosphorylates PIP2 to active PIP3, whereas the tumor suppressor PTEN converts PIP3 to inactive PIP2 and, thereby, impairs AKT activation. As a downstream target of mTORC1, S6K also mediates negative feedback through degradation of IRS-1 and IRS-2 and subsequently weakens the PI3K activation by IGF receptor signaling. The PI3K/AKT/mTOR axis communicates with other pathways, such as the RAS/RAF/MEK/ERK cascade and PI3K can also be activated through RAS. Arrows represent activation, whereas bars represent inhibition.
IRS: Insulin receptor substrate; mTORC2: mTOR complex 2; PDK1: Phosphoinositide-dependent kinase 1; PIP2: Phosphatidylinositol bisphosphate; PIP3: Phosphatidylinositol triphosphate; PTEN: Phosphatase and tensin homolog; RTK: Receptor tyrosine kinase; SGK1: Serum/glucocorticoid-regulated kinase 1.
PI3K/AKT/mTOR signaling pathway
PI3K consists of a family of lipid kinases, mainly including three classes with class IA PI3K being most commonly indicated in cancer [3–5] Class IA PI3Ks are made up of a catalytic p110 subunit and a regulatory p85 subunit. There are three isoforms for p110 subunits: p110α, p110β and p110δ, encoded by PI3KCA, PIK3CB and PIK3D, respectively. Receptor tyrosine kinases (RTK), such as EGFR, HER2 and IGF receptor (IGFR), are the principle proteins upstream of PI3Ks. Following ligand binding and RTK activation, p110 subunit is free to catalyze the phosphorylation of phosphatidylinositol bisphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3), which subsequently locates AKT to the plasma membrane. AKT is fully activated when its T308 and S473 are phosphorylated by PDK1 and mTORC2. Once activated, AKT separates from the plasma membrane and phosphorylates a wave of targets, such as the FOXO family of transcription factors and cell cycle regulatory proteins, to promote cell survival, proliferation, mobility and metabolism [6].
mTOR is a serine/threonine kinase. It associates with different proteins to form two structurally and functionally distinct complexes, mTORC1 and mTORC2 [4,7]. The key components of mTORC1 include mTOR, RAPTOR, mLST8 and PRAS40. The mTORC1 signaling can be initiated by AKT, ERK1/2 and changes of energy status of cells. The mTORC1 pathway promotes protein translation and cell growth predominantly through activating S6K and inhibiting eIF4E. The mTORC2 complex is mainly made up of mTOR, RICTOR, mLST8, PROTOR1 and mSIN1. Although the precise upstream regulation of mTORC2 remains to be defined, studies suggest that a direct association with ribosome is required for mTORC2 activation and insulin-stimulated PI3K signaling stimulates mTORC2-ribosome binding [8]. The principle downstream targets of mTORC2 include AKT, SGK1 and PKC [7,9].
The PI3K/AKT/mTOR signaling is controlled by several negative regulators. For example, PI3K phosphorylates PIP2 to active PIP3, whereas the tumor suppressor phosphatase and tensin homolog (PTEN) converts PIP3 to inactive PIP2 and thereby impairs AKT activation [10]. As a downstream target of mTORC1, S6K also mediates negative feedback through degradation of IRS-1 and IRS-2 and subsequently weakens the PI3K activation by IGFR signaling [11].
The PI3K/AKT/mTOR axis communicates with other pathways, such as the RAS/RAF/ MEK/ERK cascade [12,13]. For example, PI3K can also be activated through RAS, while mTORC1 can be triggered through ERK and RAF can be inhibited by AKT. The dysregulation and crosstalk of those signaling pathways play essential roles in lung cancer tumorigenesis.
PI3K/AKT/mTOR signaling in NSCLC
PI3K/AKT/mTOR signaling is activated in a large proportion of NSCLC [5]. For example, the alteration of its upstream regulators, such as activating EGFR mutations (10–20% of NSCLC) and KRAS mutations (8–21% of NSCLC), can lead to constitutive stimulation of the cascade. Among the components of the pathway, studies indicated that phosphorylated AKT was observed in most NSCLC tumor specimens (50–73%) and was associated with poor prognosis [5,14]. Moreover, PI3KCA and AKT1 mutations have been observed in 2–5% and 1–2% of NSCLC, respectively. In addition, down-regulation of PTEN, the negative regulator of the pathway, by an inactivating mutation (4–5% of NSCLC) or loss/reduced expression of PTEN (~70% of NSCLC) is quite common in NSCLC and also related with poor prognosis [5,15].
On the other hand, downstream activation of the PI3K/AKT/mTOR pathway has been indicated to play crucial roles in acquired resistance to EGFR-targeted therapy [13]. Furthermore, MET amplification, a resistance mechanism to EGFR tyrosine kinase inhibitor (TKI), can lead to activation of PI3K/AKT/mTOR axis through its downstream ERK. Preclinical studies showed that PI3K pathway inhibitors could overcome EGFR TKI resistance mediated by the HGF–MET cascade [13,16].
Drugs targeting the PI3K/AKT/mTOR pathway in NSCLC
There is great interest in exploring agents targeting the PI3K/AKT/mTOR pathway for cancer treatment. Several categories of inhibitors with distinct targets have been developed (Figure 2). In most lung cancer studies involving unselected patients, the single-agent application of those inhibitors is associated with stable disease and tolerable toxicities. Their common side effects include fatigue, rash, metabolic abnormality (e.g., hyperglycemia) and transaminase elevation.
Pan-PI3K inhibitors
Pan-PI3K inhibitors bind to the catalytic p110 subunits of class IA PI3Ks, PI3Kα, PI3Kβ, PI3Kδ and PI3Kγ [17]. GDC 0941, a potent oral pan-PI3K inhibitor, was first tested in a Phase I study and was associated with stable disease [18]. It is now being investigated in advanced tumors either alone or in combination with other agents [17]. When tested with MEK inhibitor GDC 0973 in a Phase Ib trial, two patients with KRAS mutant NSCLC had 13–18% shrinkage of target lesions [19]. In another Phase Ib study of NSCLC patients, it was combined with carbo platin and paclitaxel with or without bevacizumab and was associated with an objective relative risk of 75% in squamous histology and 66% in nonsquamous NSCLC [20]. BKM 120, another oral pan-PI3K inhibitor derived from pyrimidine, had significant antitumor effects on several xeno graft models of lung cancer [21,22]. Lung cancer patients involved in a Phase I trial of BKM 120 experienced stable disease [22]. BAY 80-6946 is an intravenous pan-PI3K inhibitor. In a Phase I dose escalation study, BAY 80-6946 led to about 40% of fluorodeoxyglucose uptake decrease in a NSCLC patient [23]. PX866 is an potent oral irreversible pan-PI3K inhibitor, developed from natural fungal product wortmannin. When tested in Phase I dose escalation trial in advanced solid tumors, its best response was stable disease in seven out of 31 evaluable patients [24]. When combined with docetaxel in a Phase I/ II study (43 patients), two out of six NSCLC patients had >15% tumor shrinkage [25]. On the other hand, to prevent the toxicity associated with pan-inhibition of PI3K, isoform-specific agents are being investigated in solid malignancies, for example, PI3Kα-selective GDC 0032 mainly in breast cancer patients (NCT01296555 [101]) and PI3Kβ-selective GSK 2636771 in tumors with PTEN deficiency (NCT01458067 [102]).
AKT inhibitors
GDC 0068 which targets all three isoforms of AKT was associated with stable disease for a lung cancer patient included in a Phase I trial for advanced solid tumor [26]. GSK 2141795, an inhibitor of all three isoforms of AKT, was tested in combination with oral MEK1/2 inhibitor but the trial had no lung cancer patients [27]. MK2206, another AKT inhibitor, not only potentiated erlotinib activity NSCLC cell lines but also resensitized cells which developed erlotinib resistance through the HGF–MET axis [28]. Accordingly, MK2206 is being studied in a Phase II trial with erlotinib for patients with advanced NSCLC who progressed after previous response to erlotinib (NCT01294306 [103]). A Phase I dosing study with MK2206 and geftinib in NSCLC patients is also active (NCT01147211 [104]). Perifosine, an oral AKT inhibitor being studied in Phase III trials for colorectal cancer and multiple myeloma [29], is also under investigation in NSCLC patients in a Phase I/II trial (NCT00399789 [105]).
mTOR1 inhibitors
The mTOR1 inhibitors, such as everolimus (RAD-001), temsirolimus and ridaforolimus, bind to the FKBP12 protein that leads to engagement of the TORC1 complex and attenuation of downstream TORC1 signaling. They have been approved for the treatment of advanced renal cell carcinoma (everolimus, temsirolimus), pancreatic neuroendocrine tumors (everolimus), angiomyolipoma (everolimus), hormone receptor positive/HER2-negative advanced breast cancer (everolimus) and a rare subependymal giant cell astrocytoma (everolimus).
Everolimus is the most recognized oral mTOR1 inhibitor. The early preclinical data demonstrated its antitumor activity in vivo and in vitro [30–32]. As a single agent, it was studied in Phase I/ II trials in heavily pretreated NSCLC patients and showed only modest clinical activity [33–35]. RAD-001 was then tested in combination with EGFR TKIs given synergistic effects between these agents in preclinical work [36]. In a Phase I study for patients with previously treated advanced NSCLC, the combination of erlotinib and RAD-001 showed some promising results with good tolerability. Of the cohort of patients who were assigned to daily (vs weekly) everolimus, 12% had complete/partial response and 38% had stable disease with median duration of disease control of 9.3 months [37]. A Phase II trial of everolimus and gefitinib in patients with stage IIIB/IV NSCLC with or without prior chemotherapy showed 13% partial response [38]. It was also used in combination with sorafenib in a Phase I trial (SORAVE) in patients with relapsed solid tumors with stable disease as the best response (most noticeable in KRAS-mutated NSCLC patients). This trial has been extended to KRAS-mutated NSCLC patients (NCT00933777 [39]). A Phase II study combining everolimus with docetaxel as second- or third-line therapy for advanced NSCLC only showed par tial response in two patients (7%) [40]. Combination of everolimus with pemetrexed in patients with stage IIIB/IV NSCLC showed partial response in five out of 24 patients in a Phase I study [41].
Temsirolimus (CCI-779) has also been studied extensively in lung cancer. As a single agent, it was associated with 8% partial response and 30% stable disease in Phase II study in patients with advanced NSCLC [42]. Moreover, given promising preclinical results, temsirolimus was tested in combination with neratinib, an irreversible pan-HER TKI in HER2-mutated NSCLC. The proof-of-concept Phase I study showed tumor regression in five out of six patients [43]. A randomized Phase II study of neratinib with or without everolimus is currently ongoing. The combination of temsirolimus with sorafenib and bevacizumab in a Phase I trial showed good tolerability and stable disease for 6 months in two NSCLC patients [44]. In addition, temsirolimus had been tested in extensive stage small-cell lung cancer in a Phase II randomized study in patients’ postinduction chemotherapy but failed to reach statistical significance in progression-free survival or overall survival [45].
Ridaforolimus (AP23573; MK-8669) is a newer and highly potent oral mTOR1 inhibitor. In preclinical studies, it has been shown to inhibit the growth of NSCLC cells and xenograft models harboring KRAS mutations [46]. As a single agent, partial response was observed in only one patient and minor tumor regression in three patients in a Phase I study of 32 patients with solid tumors [47]. When used in combination with cetuximab in NSCLC patients in Phase I trial, it showed stable disease as the best response [48].
Dual mTOR1/2 inhibitors
Prior studies demonstrated that treatment with mTORC1 inhibitors can lead to activation of mTORC2 signaling via p70S6K-IRS1 negative feedback, thus limiting their efficacy [49,50]. Dual inhibition of mTORC1 and mTORC2 showed more prominent antitumor effects through additional reduction of HIF1-α expression, VEGF secretion and tumor angiogenesis [51,52]. As a dual mTOR1/2 inhibitor, OSI-027, an oral agent, competitively binds to the ATP-binding domain of mTOR. Phase I trial of OSI-027 in 31 patients with advanced solid tumors or lymphoma showed disease stabilization in eight patients (NCT00698243 [53]). Among other mTOR1/2 inhibitors, INK128 is being studied in hematologic malignancies [54] and RET-mutated thyroid cancer, and AZD8055 is being evaluated in Phase I/II solid tumors (NCT00731263) with promising results [55].
Dual PI3K/mTOR inhibitors
Dual PI3K/mTOR inhibitors were developed to enhance pI3K/AKT/mTOR pathway blockade. Among them, XL765 was the first oral dual PI3K/mTOR inhibitor that showed anti-tumor activity in Phase I clinical trial: five out of 19 patients showed stable disease [56]. Sub-sequently, it has been studied in combination with erlotinib in Phase 1 study in patients with advanced NSCLC with pending results, including those who had been previously treated with erlotinib or gefitinib (NCT00777699 [106]).
BEZ235 is an oral PI3K/mTOR inhibitor with good antitumor activity against lung cancer cell lines independent of EGFR amplification and EGFR, KRAS, PI3K and AKT mutation [57]. It is being investigated in combination with everolimus in an ongoing Phase Ib/II trial in patients with advanced malignancies, including NSCLC patients (NCT01508104) [58]. Recent preclinical data also suggest that BEZ235 might help overcome erlotinib resistance in EGFR mutant NSCLC cancer [59]
Another oral dual PI3K/mTOR inhibitor, BGT226, specifically inhibits p110α, β, δ and γ, with a preference for the α-isoform (wild-type and mutated) and mTOR. It was evaluated in a Phase I trial in 57 patients with advanced solid tumors with stable disease as the best overall response in 17 (30%) of patients (no lung cancer patients) [60]. A Phase II trial in patients with advanced cancers has been conducted (NCT00600275 [107]).
In preclinical studies, PF-04691502 demonstrated good antitumor activity for NSCLC cell lines harboring PIK3CA mutation/PTEN deletion and/or a KRAS mutation [61]. It is currently in Phase I clinical trial in patients with advanced solid tumors (NCT00927823 [108]). Another intravenous PI3K/mTOR inhibitor PF-05212384 is currently under investigation in a two-arm Phase I study in combination with an oral MEK inhibitor or in combination with irinotecan in patients with advanced solid tumors (mostly gastrointestinal; NCT01347866 [109]).
GDC-0980, a newer oral dual PI3K/mTOR inhibitor, is being evaluated in a Phase Ib study in combination with carboplatin and paclitaxel with or without bevacizumab in advanced malignancies (NCT01301716 [110]). Other agents in this category of drugs are also currently under investigation.
Potential predictive biomarkers
In most lung cancer studies involving unselected patients, the single-agent application of inhibitors targeting the PI3K/AKT/mTOR signaling is associated with stable disease. It is essential to identify predictive biomarkers for better selection of patients who would benefit most from this class of therapy. Several biomarkers have been proposed based on preclinical and early clinical studies in various tumor types. For instance, preclinical studies indicate that activating PIK3CA mutation, PTEN mutation or loss and HER2 amplification could predict sensitivities to targeted agents against the PI3K/AKT/mTOR pathway, although correlative studies in the clinical trials have been inconsistent [62,63]. On the other hand, the presence of KRAS mutation is associated with resistance to the mTORC1 inhibitor everolimus [63]. In addition, the baseline expression levels of pAKT, p4EBP1 and pS6, indicators of pathway activation, have also been suggested to be determinants of sensitivity of PI3K/AKT/mTOR inhibitors [62,64]. Nevertheless, clinical validation of those candidate biomarkers is needed.
Previous studies indicate that inhibitors of the PI3K/AKT/mTOR pathway are associated with metabolic toxicities specific to hyperglycemia and hyperlipidemia [65]. It is well-known that skin toxicity is associated with clinical benefits of EGFR-targeted therapy in NSCLC [66]. Similarly, hyperlipidemia (increase in cholesterol) has been implicated in predicting clinical benefits of temsirolimus in renal cell cancer [67]. Again, validation studies are needed to clarify the predictive values of those drug-induced metabolic toxicities.
Challenges in the drug development & cotargeting strategy
As aforementioned, the single-agent utilization of targeted inhibitors against PI3K/AKT/mTOR is mostly associated with stable disease. The lack of clinical benefits in unresponsive patients may be attributed to development of drug resistance. On the other hand, the PI3K/AKT/mTOR pathway consists of a complex signaling network, which further communicates with other parallel cascades, such as RAS/RAF/MEK/ERK pathway (Figure 1). The coexistence of molecular aberrations of PI3K/AKT/mTOR pathway with other driver oncogenes, such as EGFR, HER2 and KRAS has been reported in lung cancers [68,69]. Although the mechanisms of resistance to PI3K/AKT/mTOR inhibitors remain to be an area of active research, the release of feedback loops and the activation of compensatory/ parallel signaling have been suggested to play important roles. Thus, one strategy to improve the efficacy of these targeted inhibitors is combination therapy, cotargeting the potential bypass tracts [70]. For example, to overwhelm the activation of MEK pathway by PI3K inhibitors, combined therapy with both PI3K inhibitors and MEK inhibitors has been tested in preclinical lung cancer model and showed some promising activity. Clinical trials as proof-of-concepts are currently ongoing [70].
Conclusion & future perspective
Lung cancer is no longer a single disease entity and its treatment paradigm has greatly evolved with molecular therapy. The PI3K/AKT/mTOR signaling cascade plays key roles in the tumori-genesis of lung cancer and also contributes to EGFR TKI resistance. Targeted agents against this pathway are currently being investigated in early clinical trials for lung cancer management with promising results. Future studies are needed to identify and validate predictive bio-markers to optimize the clinical benefits of this intriguing class of anticancer agents.
Practice Points.
□ The PI3K/AKT/mTOR pathway is frequently activated in non-small-cell lung cancer.
□ Targeted agents against the PI3K/AKT/mTOR pathway are currently in clinical development for lung cancer management.
□ In most series of lung cancer studies involving unselected patients, the single-agent usage of those targeted agents is associated with stable disease and tolerable toxicities.
□ One of the key challenges is to identify predictive biomarkers that can potentially guide patient selection and optimize the utility of agents targeting this oncogenic pathway.
□ Metabolic toxicity, such as hyperglycemia and hyperlipidemia, may represent a class side effect and may be explored as a surrogate predictive biomarker.
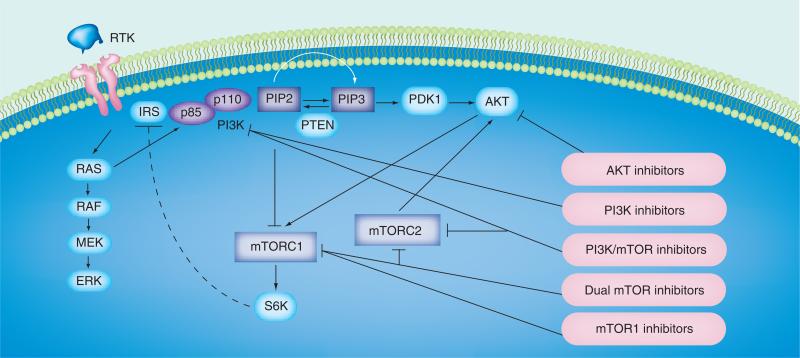
Figure 2. Targeted inhibitors of PI3K/AKT/mTOR signaling pathway.
Arrows represent activation, whereas the dashed bar represents negative feedback and the solid bars indicate the targets of pharmacological inhibitors.
IRS: Insulin receptor substrate; mTORC2: mTOR complex 2; mTORC3: mTOR complex 3; PDK1: Phosphoinositide-dependent kinase 1; PIP2: Phosphatidylinositol bisphosphate; PIP3: Phosphatidylinositol triphosphate; PTEN: Phosphatase and tensin homolog; RTK: Receptor tyrosine kinase.
Footnotes
Financial & competing interests disclosure
B Piperdi has received honoraria from Genentech/Roche, Celgene and Amgen (from speaker bureaus). R Perez-Soler has received honoraria from Genentech/Roche and Lilly USA LLC (from consultant and speaker bureaus). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat. Med. 2012;18(3):349–351. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 4.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr. Oncol. Rep. 2012;14(2):129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 5.Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J. Thorac. Oncol. 2012;7(8):1315–1326. doi: 10.1097/JTO.0b013e31825493eb. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7■.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [Systemic review on mTOR inhibitors.] [DOI] [PubMed] [Google Scholar]
- 8.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144(5):757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of AKT/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Stambolic V, Suzuki A, De La Pompa JL, et al. Negative regulation of PKB/AKT-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 11.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 12■.Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2(3):261–274. doi: 10.1177/1947601911408079. [Systemic review on how the RAS pathway modulates PI3K.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/AKT/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin. Lung Cancer. 2013;14(4):322–332. doi: 10.1016/j.cllc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 14■.Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of AKT activation in non-small-cell lung cancer tumors. J. Clin. Oncol. 2006;24(2):306–314. doi: 10.1200/JCO.2005.02.4133. [Evaluation of AKT activation in lung cancer.] [DOI] [PubMed] [Google Scholar]
- 15.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated AKT overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51(2):181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Donev IS, Wang W, Yamada T, et al. Transient PI3K inhibition induces apoptosis and overcomes HGF-mediated resistance to EGFR-TKIs in EGFR mutant lung cancer. Clin. Cancer Res. 2011;17(8):2260–2269. doi: 10.1158/1078-0432.CCR-10-1993. [DOI] [PubMed] [Google Scholar]
- 17.Martini M, Ciraolo E, Gulluni F, Hirsch E. Targeting PI3K in cancer: any good news? Front. Oncol. 2013;3:108. doi: 10.3389/fonc.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner AJ, Von Hoff DH, LoRusso PM, et al. A first-in-human Phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J. Clin. Oncol. 2009;27(Suppl. 15) Abstract 3501. [Google Scholar]
- 19.Shapiro G, LoRusso P, Kwak EL, et al. Clinical combination of the MEK inhibitor GDC-0973 and the PI3K inhibitor GDC-0941: a first-in-human Phase Ib study testing daily and intermittent dosing schedules in patients with advanced solid tumors. J. Clin. Oncol. 2011;29 Abstract 3005. [Google Scholar]
- 20.Besse B, Soria J, Gomez-Roca C, et al. 1A Phase Ib study to evaluate the PI3-kinase inhibitor GDC-0941 with paclitaxel (P) and carboplatin (C), with and without bevacizumab (BEV), in patients with advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2011;29 Abstract 3044. [Google Scholar]
- 21.Fruman DA, Rommel C. PI3Kδ inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov. 2011;1(7):562–572. doi: 10.1158/2159-8290.CD-11-0249. [DOI] [PubMed] [Google Scholar]
- 22.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30(3):282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 23.Patnaik A, Appleman LJ, Mountz JM, et al. A first-in-human Phase I study of intravenous PI3K inhibitor BAY 80-6946 in patients with advanced solid tumors: results of dose-escalation phase. J. Clin. Oncol. 2011;29 Abstract 3035. [Google Scholar]
- 24.Jimeno A, Herbst RS, Falchook GS, et al. Final results from a Phase I, dose-escalation study of PX-866, an irreversible, pan-isoform inhibitor of PI3 kinase. J. Clin. Oncol. 2010;28(Suppl.) Abstr 3089. [Google Scholar]
- 25.Jimeno A, Senzer NN, Rudin CM, et al. PX-866 and docetaxel in patients with advanced solid tumors. J. Clin. Oncol. 2012;30 Abstract 3024. [Google Scholar]
- 26.Tabernero J, Saura C, Roda Perez D, et al. First-in-human Phase I study evaluating the safety, pharmacokinetics (PK), and intratumor pharmacodynamics (PD) of the novel, oral, ATP-competitive AKT inhibitor GDC-0068. J. Clin. Oncol. 2011;29 Abstract 3022. [Google Scholar]
- 27.Kurzrock R, Patnaik A, Rosenstein L, et al. Phase I dose escalation of the oral MEK ½ inhibitor GSK 1120212 dosed in combination with the oral AKT inhibitor GSK 2141795. J. Clin. Oncol. 2011;29 Abstract 3085. [Google Scholar]
- 28.Mack PC, Farneth N, Mahaffey C, et al. Impact of AKT inhibitor MK-2206 on erlotinib resistance in non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2011;29 Abstract 7573. [Google Scholar]
- 29.Richardson PG, Eng C, Kolesar J, Hideshima T, Anderson KC. Perifosine, an oral, anti-cancer agent and inhibitor of the AKT pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert Opin. Drug Metab. Toxicol. 2012;8(5):623–633. doi: 10.1517/17425255.2012.681376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses AKT-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004;10(6):594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 31.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64(1):252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 32.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120(6):747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell A, Faivre S, Judson I. A Phase I study of the oral mTOR inhibitor RAD001 as monotherapy to identify the optimal biologically effective dose using toxicity, pharmacokinetic (PK) and pharmacodynamic (PD) endpoints in patients with solid tumours. Proc. Am. Soc. Clin. Oncol. 2003;22(200) Abstract 803. [Google Scholar]
- 34.Tabernero J, Rojo F, Burris H, et al. A Phase I study with tumor molecular pharmaco-dynamic (MPD) evaluation of dose and schedule of the oral mTOR inhibitor RAD001 (RAD001) in patients (pts) with advanced solid tumors. J Clin. Oncol. 2005;23(Suppl. 16) Abstract 3007. [Google Scholar]
- 35.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann. Oncol. 2009;20(10):1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 36.Buck E, Eyzaguirre A, Brown E, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol. Cancer Ther. 2006;5(11):2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 37.Papadimitrakopoulou VA, Soria JC, Jappe A, Jehl V, Klimovsky J, Johnson BE. Everolimus and erlotinib as second- or third-line therapy in patients with advanced non-small-cell lung cancer. J. Thoracic Oncol. 2012;7(10):1594–1601. doi: 10.1097/JTO.0b013e3182614835. [DOI] [PubMed] [Google Scholar]
- 38.Price KA, Azzoli CG, Krug LM, et al. Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J. Thoracic Oncol. 2010;5(10):1623–1629. doi: 10.1097/JTO.0b013e3181ec1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nogova L, Mattonet C, Gardizi M, et al. SORAVE: sorafenib and everolimus for treatment of patients with relapsed solid tumors and with KRAS mutated NSCLC – a Phase I study. J. Clin. Oncol. 2013;31(Suppl.) Abstract 8112. [Google Scholar]
- 40.Khuri FR, Owonikoko TK, Subramanian J, et al. Everolimus, an mTOR inhibitor, in combination with docetaxel for second- or third-line therapy of advanced-stage non-small cell lung cancer: a Phase II study. J. Clin. Oncol. 2011;29 Abstract e13601. [Google Scholar]
- 41.Vansteenkiste J, Solomon B, Boyer M, et al. Everolimus in combination with pemetrexed in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a Phase I study using a novel, adaptive Bayesian dose-escalation model. J. Thoracic Oncol. 2011;6(12):2120–2129. doi: 10.1097/JTO.0b013e3182307ede. [DOI] [PubMed] [Google Scholar]
- 42.Reungwetwattana T, Molina JR, Mandrekar SJ, et al. A Phase II NCCTG ‘Window of Opportunity Front-line’ study of the mTOR inhibitor, CCI-779 (temsirolimus) given as a single agent in patients with advanced NSCLC. J. Thorac. Oncol. 2007;7(5):919–922. doi: 10.1097/JTO.0b013e31824de0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandhi L, Soria JC, Bryce R, et al. Randomized Phase II study of neratinib with or without temsirolimus in patients (pts) with non-small cell lung cancer (NSCLC) carrying HER2-activating mutations. J. Clin. Oncol. 2013;31 Abstract TPS8124. [Google Scholar]
- 44.Westin SN, Melody L, Smart ML, Pal N, et al. Phase I trial of sorafenib, bevacizumab, and temsirolimus in advanced solid tumors. J. Clin. Oncol. 2013;31 Abstract 2611. [Google Scholar]
- 45.Pandya KJ, Dahlberg S, Hidalgo M, et al. Eastern Cooperative Oncology Group (E1500). A randomized, Phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500). J. Thoracic Oncol. 2007;2(11):1036–1041. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 46.Haines B, Bittinger M, Chenard M, et al. The mTOR inhibitor deforolimus is efficacious in models of mutant KRAS lung cancer.. Presented at: American Association for Cancer Research (AACR) Annual Meeting; Denver, CO, USA. 18–22 April 2009; (Abstract 1887) [Google Scholar]
- 47.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J. Clin. Oncol. 2008;26(3):361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 48.Taber AM, Khurshid H, Perez K, et al. Phase I study of ridaforolimus with cetuximab for patients with advanced non-small cell lung cancer (NSCLC), colorectal cancer, and head and neck cancer. J. Clin. Oncol. 2013;31 Abstract 8075. [Google Scholar]
- 49.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell Biol. 2010;30(4):908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50■.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates AKT. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [Release of negative feedback loop by mTORC1 inhibition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu K, Toral-Barza L, Shi C, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69(15):6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 52.Xue Q, Hopkins B, Perruzzi C, Udayakumar D, Sherris D, Benjamin LE. Palomid 529, a novel small-molecule drug, is a TORC1/TORC2 inhibitor that reduces tumor growth, tumor angiogenesis, and vascular permeability. Cancer Res. 2008;68(22):9551–9557. doi: 10.1158/0008-5472.CAN-08-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan DS, Dumez H, Olmos D, et al. First-inhuman Phase I study exploring three schedules of OSI-027, a novel small molecule TORC1/ TORC2 inhibitor, in patients with advanced solid tumors and lymphoma. J. Clin. Oncol. 2010;28 Abstract 3006. [Google Scholar]
- 54.Janes MR, Vu C, Mallya S, et al. Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia. 2013;27(3):586–594. doi: 10.1038/leu.2012.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S, Li Y, Hu R, et al. The mTOR inhibitor AZD8055 inhibits proliferation and glycolysis in cervical cancer cells. Oncol. Lett. 2013;5(2):717–721. doi: 10.3892/ol.2012.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LoRusso P, Markman B, Tabernero J, et al. A Phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of a novel PI3K inhibitor, XL765, administered orally to patients (pts) with advanced solid tumors. J. Clin. Oncol. 2008;26 Abstract 3510. [Google Scholar]
- 57.Herrera VA, Zeindl-Eberhart E, Jung A, Huber RM, Bergner A. The dual PI3K/ mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011;31(3):849–854. [PubMed] [Google Scholar]
- 58.Salkeni MA, Rixe O, Karim NA, et al. BEZ235 in combination with everolimus for advanced solid malignancies: preliminary results of a Phase Ib dose-escalation study. J. Clin. Oncol. 2013;31 Abstract e13518. [Google Scholar]
- 59.Sano T, Takeuchi S, Nakagawa T, et al. The novel phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor, BEZ235, circumvents erlotinib resistance of epidermal growth factor receptor mutant lung cancer cells triggered by hepatocyte growth factor. Int. J. Cancer. 2013;133(2):505–513. doi: 10.1002/ijc.28034. [DOI] [PubMed] [Google Scholar]
- 60.Markman B, Tabernero J, Krop I, et al. Phase I safety, pharmacokinetic, and pharmaco dynamic study of the oral phosphatidyl inositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann. Oncol. 2012;23(9):2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 61.Yuan J, Mehta PP, Yin MJ, et al. PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity. Mol. Cancer Ther. 2011;10(11):2189–2199. doi: 10.1158/1535-7163.MCT-11-0185. [DOI] [PubMed] [Google Scholar]
- 62.Owonikoko TK, Khuri FR. Targeting the PI3K/AKT/mTOR pathway. Am. Soc. Clin. Oncol. Educ. Book. 2013;2013:395–401. doi: 10.1200/EdBook_AM.2013.33.e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J. Clin. Invest. 2010;120(8):2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwei KA, Baker JB, Pelham RJ. Modulators of sensitivity and resistance to inhibition of PI3K identified in a pharmacogenomic screen of the NCI-60 human tumor cell line collection. PLoS ONE. 2012;7(9):e46518. doi: 10.1371/journal.pone.0046518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65■.Busaidy NL, Farooki A, Dowlati A, et al. Management of metabolic effects associated with anticancer agents targeting the PI3KAKT-mTOR pathway. J. Clin. Oncol. 2012;30(23):2919–2928. doi: 10.1200/JCO.2011.39.7356. [Evaluation and management of metabolic effects associated with inhibitors of the PI3K/AKT/mTOR pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J. Clin. Oncol. 2005;23(22):5235–5246. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- 67.Lee CK, Marschner IC, Simes RJ, et al. Increase in cholesterol predicts survival advantage in renal cell carcinoma patients treated with temsirolimus. Clin. Cancer Res. 2012;18(11):3188–3196. doi: 10.1158/1078-0432.CCR-11-3137. [DOI] [PubMed] [Google Scholar]
- 68.Chaft JE, Arcila ME, Paik PK, et al. Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling. Mol. Cancer Ther. 2012;11(2):485–491. doi: 10.1158/1535-7163.MCT-11-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70■.Heavey S, O'Byrne KJ, Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat. 2013 doi: 10.1016/j.ctrv.2013.08.006. Rev. doi:10.1016/j.ctrv.2013.08.006 (Epub ahead of print). [Systemic review of strategies for cotargeting the PI3K/AKT/mTOR signaling in lung cancer.] [DOI] [PubMed] [Google Scholar]
- 101.ClincialTrials.gov A Dose Escalation Study Evaluating the Safety and Tolerability of GDC-0032 in Patients With Locally Advanced or Metastatic Solid Tumors And in Combination With Endocrine Therapy in Patients With Locally Advanced or Metastatic Hormone Receptor-Positive Breast Cancer. http://clinicaltrials.gov/show/NCT01296555.
- 102.ClincialTrials.gov A Phase I/IIa, First Time in Human, Study of GSK2636771 in Subjects With Advanced Solid Tumors With Phosphatase and Tensin Homolog (PTEN) Deficiency. http://clinicaltrials.gov/show/NCT01458067.
- 103.ClincialTrials.gov MK2206 and Erlotinib Hydrochloride in Treating Patients With Advanced Non-Small Cell Lung Cancer Who Have Progressed After Previous Response to Erlotinib Hydrochloride Therapy. http://clinicaltrials.gov/show/NCT01294306.
- 104.ClincialTrials.gov Dose Defining Study For MK-2206 Combined With Gefitinib In Non Small Cell Lung Cancer (NSCLC) http://clinicaltrials.gov/show/NCT01147211.
- 105.ClincialTrials.gov. A Phase 1/2 Trial of Perifosine in the Treatment of Non-Small Cell Lung Cancer. http://clinicaltrials.gov/show/NCT00399789.
- 106.ClincialTrials.gov. Safety Study of XL765 (SAR245409) in Combination With Erlotinib in Adults With Solid Tumors. http://clinicaltrials.gov/show/NCT00777699.
- 107.ClincialTrials.gov. A Phase I/II Study of BGT226 in Adult Patients With Advanced Solid Malignancies Including Patients With Advanced Breast Cancer. http://clinicaltrials.gov/show/NCT00600275.
- 108.ClincialTrials.gov. A Trial To Assess Safety And Tolerability Of PF-04691502 In Cancer Patients. http://clinicaltrials.gov/show/NCT00927823.
- 109.ClincialTrials.gov. Clinical Study Of PI3K/mTOR Inhibitors In Combination With An Oral MEK Inhibitor Or Irinotecan In Patients With Advanced Cancer. http://clinicaltrials.gov/show/NCT01347866.
- 110.ClincialTrials.gov. A Study of the Safety and Pharmacology of GDC-0980 in Combination With Either Paclitaxel and Carboplatin (With or Without Bevacizumab) or Pemetrexed and Cisplatin in Patients With Solid Tumors. http://clinicaltrials.gov/show/NCT01301716.