Abstract
A reactive oxygen species (ROS)-degradable scaffold is fabricated by crosslinking biocompatible, hydrolytically-degradable poly(ε-caprolactone) (PCL) with a ROS-degradable oligoproline peptide, KP7K. The ROS-mediated degradability triggers favorable host responses of the scaffold including improved cell infiltration and angiogenesis in vivo, indicating its unique advantages for tissue engineering applications.
Recent progress in biomaterial development emphasizes the advanced function that enables target-specific therapeutic delivery through a stimuli-responsive structural change in a spatiotemporally controlled manner. These materials have been designed to activate the advanced functions in response to various physiological parameters, such as pH,2 temperature,3 protease activities, and redox balance4to target a local pathological event in the body. One such biological stimulus that is drawing keen attention recently is reactive oxygen species (ROS) which include hydroxyl radicals(OH.), hydrogen peroxides(H2O2), peroxynitrites(ONOO-) and superoxides(O2-) among others.5While low levels of ROS are part of normal cell metabolism, excessive amounts of ROS cause oxidative stress and damage critical components of cells at all levels including DNA, 6 proteins,7 and lipids8 by oxidation. Moreover, chronically increased levels of ROS are present locally in cancer,9 atherosclerosis,10 diabetes,11 infections,12 inflammatory diseases,13 and even in the aging process14 where ROS levels can be 10- to 100-times the normal levels. For example, in the vicinity of activated macrophages that are prevalent in inflammation and implantation sites, the hydrogen peroxide concentration can reach 10 – 100 μM.15 Therefore, ROS can be considered as a critical parameter indicating local occurrence or progression of such diseases, and ROS-responsive materials are needed to enable site-specific delivery of therapeutics and imaging agents as they can undergo structural changes (e.g., degradation) and release the payload in response to locally overproduced ROS in vivo.
Starting with polypropylene sulfide (PPS) developed in 2004, ROS-sensitive materials are still relatively new, but have been continuously developed due to the increasing demand for various biomedical applications. While numerous other studies focused on designing synthetic polymers that rapidly respond to ROS in nanoscale delivery formats 16,17,18, we investigated a peptide oligomerasa ROS-responsive unitfor long-term tissue engineering applications. Previous studies have shown that proteins containing aspartic acid, glutamic acid orproline residues are especially prone to oxidative degradation. 19 ROS-sensitive degradability of proline residues has demonstrated particular promise.20, 21
In a previous study, we synthesized proline oligomers as a ROS-cleavable crosslinker and extensively characterized its fabrication and response to ROS in vitro.22 In particular, oligoproline peptides with 5 or 7 or 10 proline residues were tested for ROS-mediated degradation by reactingin 5 mM H2O2 with 50 μMCu(II) at 37°C. All proline residues were cleaved away after 6 days, confirming relatively slow ROS-mediated degradation, compared to PPS that degrades in several hours under oxidative environment.16
Therefore, in the current study, we investigated the potential of using an oligoproline-crosslinked poly(ε-caprolactone) (PCL)-based scaffold in an in vivo model for long-term tissue engineering applications. PCL was chosen as the base polymer,as it is approved by the Food and Drug Administration (FDA) for biomedical applications such as tissue engineering and drug delivery.23 Furthermore, PCL is known for slow in vivo degradation over several years by hydrolysis of ester bonds, thereby providing a material format where we can impart faster-acting ROS-degradability to the scaffold. We hypothesized that such ROS-degradability of scaffolds would allow for favourable interactions with host cells in vivo where the initial inflammatory host response would degrade the implanted scaffold by excess ROS production and encourage cell infiltration into the scaffold, leading to improved neovascularization and engraftment within the site of implantation.24 Towards this end, first we modified and improved the chemistry involved to efficiently crosslink PCL with ROS-degradable oligoproline peptides, and studied their chemical and thermal properties as well as their in vitro ROS-degradability. Finally, we implanted the scaffolds subcutaneously in mice to test our hypothesis for the effect of ROS-degradability of scaffold on host-material interaction with an emphasis on vascularization of the implanted scaffold.
To incorporate crosslinkers, PCL was functionalized with carboxyl groups (Fig. 1A).25 The ratio of the integral peaks for carboxylated PCL (CPCL) (3.4 and 9.2 ppm) and unmodified PCL (4.1 ppm) from the 1H-NMR spectrum revealed a molar composition of 70%PCL-30% CPCL (Fig. S1). Of note, the extent of carboxylation can be varied from 5 - 60% by varying the duration of the reaction (data not shown).The molecular weight of 70% PCL-30% CPCL (number-average molecular weight (Mn) = 95.5 kDa; polydispersity (PDI = Mw/Mn) of 1.40) is comparable to the unmodified starting material, 100%PCL (Mn = 87.0 kDa; PDI = 1.28), indicating no significant hydrolysis of polyester chains during the reaction with LDA. Fig. 1B shows the ROS-cleavable KP7K peptide crosslinker and poly(ethylene glycol) (PEG)-dihydrazide crosslinker used as a control in this study. Dicyclohexylcarbodiimide (DCC)/N-hydroxy-succinimide ester (NHS) was used to crosslink 70% PCL-30% CPCL with the crosslinkers. Rapid crosslinking was observed asthe mixture began gelling immediately.
Fig. 1.
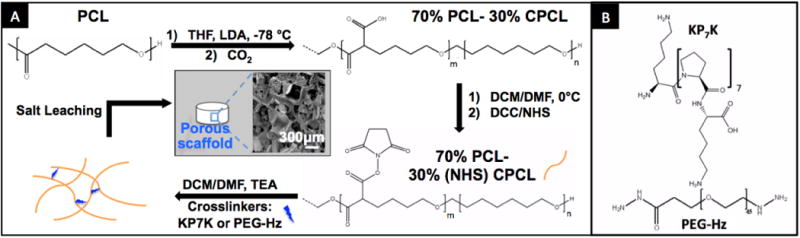
(A) Synthesis and fabrication of porous KP7K or PEG-crosslinked 70%PCL-30%CPCL scaffolds with a representative SEM image. (B) ROS-cleavable peptide KP7K1 and PEG-dihydrazide (control) crosslinkers.
We then verified and characterized the crosslinking. Successful crosslinking was first confirmed by FTIR, where amide (I) C=O band at ∼1620 cm-1 was observed for both PEG-dihydrazide and KP7K-crosslinked 70% PCL-30% CPCL, as well as increased absorbance for N-H stretching and O-H stretching from water molecules around 3200∼3700 cm-1 (Fig. 2A).26 Next, gel content measurement further characterized the degree of crosslinking, where PEG-dihydrazide and KP7K-crosslinked scaffolds exhibited 73±7.1% and 82±5.9% in gel contents with THF washes, respectively, indicating a high degree of crosslinking within these scaffolds.
Fig. 2.
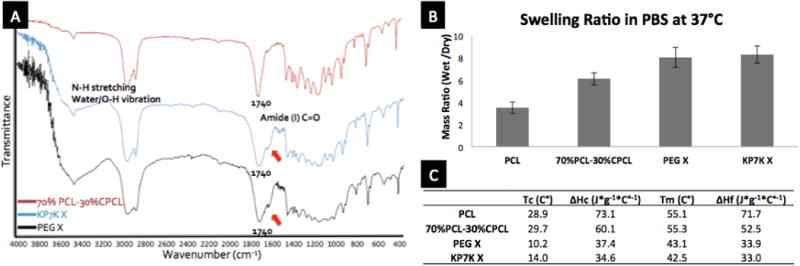
Characterization of scaffolds. (A) FTIR spec for 70%PCL-30%CPCL crosslinked with either KP7K (KP7K X) or PEG-Hz (PEG X). (B) Swelling ratios of porous scaffolds. Error bar= ±1 SD. (C) Thermal characterization of porous scaffolds byDSC.
Crosslinking of 70% PCL-30% CPCL with hydrophilic KP7K peptide crosslinkers altered physicochemical properties of the scaffolds, as evidenced by the changes in the swelling ratios of the scaffolds. When incubated in PBS at 37 °C for 1 day, uncrosslinked 30% CPCL-70% PCL had significantly increased the swelling ratio compared to original PCL, and the highly hydrophilic nature of both crosslinkers further increased the swelling ratios of the crosslinked scaffolds (Fig. 2B). Such increases in hydrophilicity are desirable as it may improve cell attachment and infiltration compared to hydrophobic 100% PCL in vivo. 27
Similarly, the series of modifications made on PCL significantly altered the thermal properties (Fig. 2C). First, carboxylation of PCL resulted in reduced heats of crystallization (ΔHc) and melting (ΔHf), as the carboxyl groups disrupted the packing of semi-crystalline PCL chains to some degree and resulted in less crystallinity. Then, the crosslinking of KP7K or PEG-dihydrzide with 70% PCL-30% CPCL further disrupted the crystallinity of PCL and reduced the polymer chain flexibility, resulting in drastic decreases in temperatures of crystallization (Tc) and melting (Tm), as well as in ΔHc and ΔHf.28 These changes in the thermal properties resulting from carboxylation and crosslinking can significantly influence material properties, which was exhibited in elastic modulus measured by dynamic mechanical analysis (DMA). Non-porous, thin films were prepared and measured for their elastic moduli in wet conditions at 37 °C. While PCL and 70% PCL-30% CPCL were similar at 77.0 ± 6.0 MPa and 82.5 ± 4.7 MPa, respectively, both PEG- and KP7K-crosslinked conditions exhibited significantly reduced elastic moduli at 22.7 ± 2.7 MPa and 46.4 ± 0.4 MPa (each condition with N=3). The drastic decreases in elastic moduli in the crosslinked conditions are likely due to the decreases in crystallinity (Tc and ΔHc), caused by crosslinking. Thus, we have shown and proven the effects of crosslinking on PCL in several aspects.
The ROS-degradability of KP7K-crosslinked scaffold was demonstrated by changes in thermal properties (Fig. 3A). Scaffolds were incubated in PBS at 37 °C with or without daily changes of 1 mM SIN-1 for 30 days which degrades into superoxides and nitric oxides to form peroxynitrites.29 Most notably,the Tc value of the KP7K-crosslinked scaffold increased significantly with SIN-1 treatment compared to the ones of day 0 and PBS only condition on day 30, as radicals produced from SIN-1 degraded KP7K crosslinkers, and the newly freed polymer chains likely underwent reorganization and recrystallization.28 In contrast,the uncrosslinked PCL and 70%PCL-30% CPCL scaffolds showed minimal changes in the Tc. PEG-crosslinked scaffolds also degraded with SIN-1 treatment, albeit to a lesser degree than the KP7K-crosslinked scaffolds. Interestingly, Reid et al. has shown that PEG also degrades in response to ROS produced from Fenton reaction with 50% (w/v) H2O2 and 6.2 mM FeCl3 or from radicals produced by UV degradation of 30% H2O2.30 However, PEG and PEG-crosslinked scaffolds might require supraphysiological levels of ROS for degradation which may not be biologically relevant.
Fig. 3.
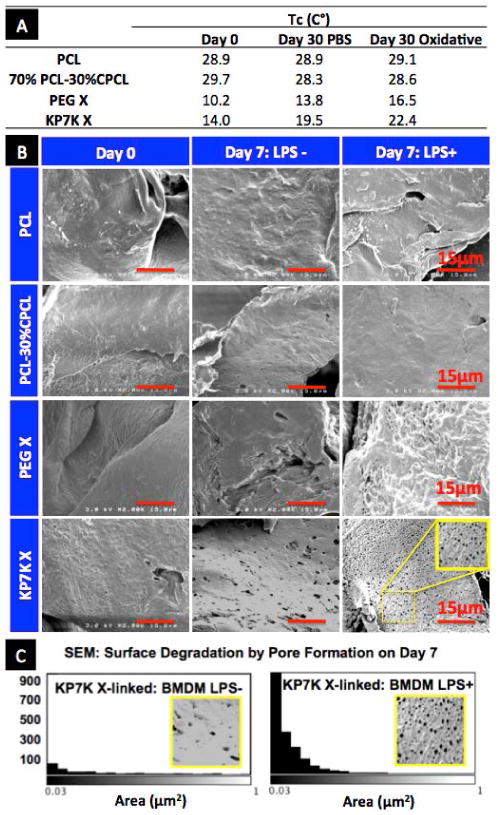
Characterization of scaffold degradation in response to ROS in vitro. (A) Tc values of scaffolds obtained from DSC after daily changes of PBS with or without 1mM SIN-1 over 30 days at 37°C. (B) SEM images of the scaffold surface after culturing mousebone marrow-derived macrophages (BMDMs)for 7 days with or without LPS stimulation. (C) Quantification of pores created by BMDMs on KP7K-crosslinked scaffold surface on day 7.
The degradation of KP7K scaffold was accelerated in a physiologically relevant ROS environment in vitro where mouse bone marrow-derived macrophages (BMDMs, see the cell characterization in Fig. S2A) were seeded on the scaffolds with or without the stimulation with 50 μg/ml LPS (Fig. 3B-C).31 As an endotoxin, LPS activates macrophages to overproduce ROS and reactive nitrogen species (RNS) including nitric oxide (NO•), nitrite(NO2-) and peroxynitrite (ONOO-) (Fig. S2B).32 After removing cells and protein debris from the scaffold, the scaffold surface was imaged by scanning electron microscopy (SEM) (Fig. 3B). ROS-cleavable KP7K-crosslinked scaffolds evidently formed numerous pores < 1 µm2, especially when seeded with LPS activated BMDMs, while PEG-crosslinked scaffolds appeared to maintain their smooth surface over 7 day culture. Quantification of this pore formation after 7 day culture (Fig. 3C) reveals that sub-micron diameter pores increased by more than 10-folds for KP7K-crosslinked scaffolds cultured with LPS-activated BMDMs, whereas when cultured without LPS, the pore generation was minimal. These results prove that KP7K-crosslinked scaffolds degrade in response to the physiological levels of ROS produced by activated BMDMs.
PCL is known to slowly undergo hydrolytic chain scission over several years and become 6-hydroxylcaproic acids that are safely removed from the body.33 We expected that the initial host immune response to implants would degrade ROS-degradable KP7K into α-aminobutyric acids that can be used for biosynthesis, while leaving PEG crosslinkers intact due to its non-biodegradability.34 Therefore, we tested our hypothesis that ROS-degradability of the scaffolds would encourage cell infiltration in a host-responsive manner, thereby improving vascularization of the constructs in vivo (Fig. 4). Scaffolds were subcutaneously implanted in mice for 2 weeks, and sections were stained with H&E to determine host cell infiltration (Fig. 4A). While cell infiltration is observed in all the test groups, there are some noticeable differences between the materials. When the density of nuclei ismeasured per tissue/scaffold area from H&E stained sections (Fig. 4B), it is evident that PCL-30% CPCL had significantly less cells infiltrating compared to PCL, possibly due to the negative charges on carboxyl groups with degreased protein adsorption. PEG-crosslinked scaffolds showed a similar number of infiltrating host cells compared to PCL. More importantly, KP7K-crosslinked scaffolds exhibited the highest nuclei density with 50% more cells infiltrating compared to PCL. Additionally, mice were perfused through the heart with fluorescent microbeads for angiography before harvesting35 (Fig. 4A), and the implanted scaffolds were collected for gene expression analysis (Fig. 4C). While PCL-30% CPCL and PEG-crosslinked scaffolds showed similarly higher levels of neovasculature formation compared to PCL, KP7K-crosslinked scaffolds significantly promoted angiogenesis, as evidenced by abundant functional, perfusable blood vessels shown in the angiograms, compared to those of PCL scaffolds. This result was further supported by significant increases in gene expression of angiogenesis markers (CD31 and VEGFA)36assessed by quantitative RT-PCR (Fig. 4C), indicating that crosslinking with KP7K significantly increases blood vessel growth into the scaffolds upon implantation. This is likely due to the fact that early inflammatory responses resulted in degradation of KP7K-crosslinked scaffolds, which enhances cell infiltration and growth of host cells into the implanted scaffolds, thereby promoting angiogenesis, compared to other conditions that degrade slowly through hydrolysis only. It is also possible that the oligoproline peptides themselves may have collagen-mimetic functions and properties, as oligoproline peptides mimic collagen in both chemical composition and structure37. However, further studies are required to confirm this possibility.
Fig. 4.
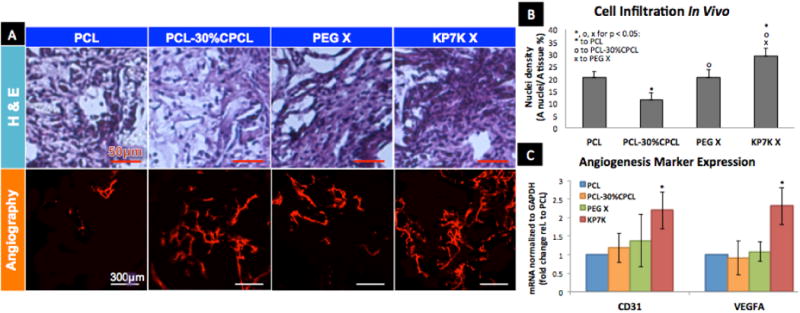
(A) After 2-week subcutaneous implantation, scaffolds were stained with H&E (purple: cytoplasm, dark blue: nuclei; scale bar = 50 μm), and imaged for angiography (scale bar = 300 μm). (B) Cell infiltration was quantified by Area (nuclei) / Area (tissue) (%) from H&E stained sections. (C) mRNA expression of angiogenesis markers by qRT-PCR (N=4; Error = ±1 SEM).
Conclusions
This study demonstrated synthesis and crosslinking of 70% PCL-30% CPCL with ROS-cleavable KP7K and PEG-dihydrazide crosslinkers via DCC/NHS coupling. To examine their ROS-mediated degradation in a physiologically relevant condition, BMDMs were cultured on the scaffolds under LPS treatment to stimulate their ROS overproduction in vitro. The KP7K-crosslinked scaffolds underwent significant surface degradation in response to overproduced ROS from activated BMDMs. Furthermore, angiogenesis was significantly promoted within the implanted KP7K-crosslinked scaffolds. This pro-angiogenic behavior can likely be explained by ROS production from host inflammatory cells which induce scaffold degradation and pore generation, resulting in enhanced host cell infiltration into the scaffolds. We have shown that KP7K-crosslinked 70%PCL-30%CPCL is 1) degradable in response to physiologically relevant ROS levels which allows for better host cell infiltration, and 2) pro-angiogenic in vivo via host-material interactions.
While various stimuli-sensitive materials have been developed innano- to microscale formats as drug delivery vehicles, applying large-scale stimuli-sensitive scaffolds for tissue engineering remain underexplored. Here, we demonstrated the unique advantages of applying ROS-responsive scaffolds in improving vascularization, which is currently an unmet need in the field of tissue engineering. Further studies are required to understand the mechanism by which ROS-degradable KP7K-crosslinked scaffolds promote angiogenesis with favorable host response. In this way, the scaffold design can be improved to achieve successful engraftment and better clinical outcomes in the future.
Supplementary Material
Acknowledgments
This study was supported by NSF CAREER CBET 1056046. This study employed the use of resources at the Vanderbilt Institute of Nanoscale Science and Engineering (VINSE), a facility renovated under NSF ARI-R2 DMR-0963361. Confocal microscopy was performed through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126). 1H-NMR was conducted in the Vanderbilt Small Molecule NMR Facility Core.
Footnotes
Electronic Supplementary Information (ESI) available: 1H-NMR and characterization of bone marrow-derived macrophages used in in vitro experiments. See DOI: 10.1039/c000000x/
References
- 1.Yu SS, Koblin RL, Zachman AL, Perrien DS, Hofmeister LH, Giorgio TD, Sung HJ. Biomacromolecules. 2011;12:4357–4366. doi: 10.1021/bm201328k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoener CA, Hutson HN, Peppas NA. Polym Int. 2012;61:874–879. doi: 10.1002/pi.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cammas S, Suzuki K, Sone C, Sakurai Y, Kataoka K, Okano T. Journal of Controlled Release. 1997;48:157–164. [Google Scholar]
- 4.Meng F, Zhong Z, Feijen J. Biomacromolecules. 2009;10:197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 5.Papa S, Skulachev VP. Molecular and cellular biochemistry. 1997;174:305–319. [PubMed] [Google Scholar]
- 6.Wiseman H, Halliwell B. Journal of biochemistry. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean RT, Fu S, Stocker R, Davies MJ. The Biochemical journal. 1997;324(Pt 1):1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aitken RJ, Clarkson JS, Fishel S. Biology of reproduction. 1989;41:183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 9.Vaccari L, Canton D, Zaffaroni N, Villa R, Tormen M, di Fabrizio E. Microelectronic Engineering. 2006;83:1598–1601. [Google Scholar]
- 10.Sugamura K, Keaney JF. Free Radical Biology & Medicine. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houstis N, Rosen ED, Lander ES. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 12.Rastew E, Vicente JB, Singh U. Int J Parasitol. 2012;42:1007–1015. doi: 10.1016/j.ijpara.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman I. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 14.Papp LV, Lu J, Holmgren A, Khanna KK. Antioxidants & redox signaling. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 15.Droge W. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 16.Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Nature materials. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 17.Wilson D, Dalmasso G, Wang L, Sitaraman S, Merlin D, Murthy N. Nautre Materials. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Gupta MK, Bang JB, Bae H, Sung HJ. Advanced Healthcare Materials. 2013;2:908–915. doi: 10.1002/adhm.201200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadtman ER, Levine RL. Amino acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 20.Garrison WM. Chem Rev. 1987;87:381–398. [Google Scholar]
- 21.Amici a, Levine RL, Tsai L, Stadtman ER. The Journal of biological chemistry. 1989;264:3341–3346. [PubMed] [Google Scholar]
- 22.Yu SS, Koblin RL, Zachman AL, Perrien DS, Hofmeister LH, Giorgio TD, Sung HJ. Biomacromolecules. 2011;12:4357–4366. doi: 10.1021/bm201328k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam CX, Hutmacher DW, Schantz JT, Woodruff MA, Teoh SH. Journal of biomedical materials research Part A. 2009;90:906–919. doi: 10.1002/jbm.a.32052. [DOI] [PubMed] [Google Scholar]
- 24.Sung HJ, Meredith C, Johnson C, Galis ZS. Biomaterials. 2004;25:5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 25.Gupta MK, Walthall JM, Venkataraman R, Crowder SW, Jung DK, Yu SS, Feaster TK, Wang X, Giorgio TD, Hong CC, Baudenbacher FJ, Hatzopoulos AK, Sung HJ. PloS one. 2011;6:e28935. doi: 10.1371/journal.pone.0028935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Movasaghi Z, Rehman S, Rehman DIur. Applied Spectroscopy Reviews. 2008;43:134–179. [Google Scholar]
- 27.Ma Z, Mao Z, Gao C. Colloids and surfaces B, Biointerfaces. 2007;60:137–157. doi: 10.1016/j.colsurfb.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Mandelkern L. Chem Rev. 1995;56:903–958. [Google Scholar]
- 29.Hogg N, Darley-Usmar V, WIlson M, Mocada S. Biochem J. 1992;281:419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid B, Gibson M, Singh A, Taube J, Furlong C, Murcia M, Elisseeff J. Journal of tissue engineering and regenerative medicine. 2013 doi: 10.1002/term.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weischenfeldt J, Porse B. CSH protocols. 2008 doi: 10.1101/pdb.prot5080. 2008, pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 32.Amatore C, Arbault S, Bouton C, Drapier JC, Ghandour H, Koh AC. Chembiochem : a European journal of chemical biology. 2008;9:1472–1480. doi: 10.1002/cbic.200700746. [DOI] [PubMed] [Google Scholar]
- 33.Woodruff MA, Hutmacher DW. Progress in Polymer Science. 2010;35:1217–1256. [Google Scholar]
- 34.Knop K, Hoogenboom R, Fischer D, Schubertf US. Angew Chem Int Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 35.Zachman AL, Crowder SW, Ortiz O, Zienkiewicz KJ, Bronikowski CM, Yu SS, Giorgio TD, Guelcher SA, Kohn J, Sung HJ. Tissue engineering Part A. 2013;19:437–447. doi: 10.1089/ten.tea.2012.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mineo TC. Journal of Clinical Pathology. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorgieva S, Kokol V. 2 Collagen- vs Gelatin. 2011 inTech. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


