Abstract
Morquio A (Mucopolysaccharidosis IVA; MPS IVA) is an autosomal recessive lysosomal storage disorder caused by partial or total deficiency of the enzyme galactosamine-6-sulfate sulfatase (GALNS; also known as N-acetylgalactosamine-6-sulfate sulfatase) encoded by the GALNS gene. Patients who inherit two mutated GALNS gene alleles produce protein with decreased ability to degrade the glycosaminoglycans (GAGs) keratan sulfate and chondroitin 6-sulfate, thereby causing GAG accumulation within lysosomes and consequently pleiotropic disease. GALNS mutations occur throughout the gene and many mutations are identified only in single patients or families, causing difficulties both in mutation detection and interpretation. In this study, molecular analysis of 163 patients with Morquio A identified 99 unique mutations in the GALNS gene believed to negatively impact GALNS protein function, of which 39 are previously unpublished, together with 26 single-nucleotide polymorphisms. Recommendations for the molecular testing of patients, clear reporting of sequence findings, and interpretation of sequencing data are provided.
Keywords: MPS IVA, Morquio A, Mucopolysaccharidosis type IVA, GALNS, Lysosomal storage disorder, Mutation
1. Introduction
Morquio A syndrome (also known as mucopolysaccharidosis type IV A or MPS IVA) is a member of a group of inherited metabolic disorders collectively termed mucopolysaccharidoses (MPSs). An MPS disorder is caused by a deficiency of one of 11 different lysosomal enzymes required for the degradation of mucopolysaccharides or glycosaminoglycans (GAGs). Morquio A is caused by deficiency of galactosamine-6-sulfatase (N-acetyl-galactosamine-6-sulfate sulfatase; GALNS). GALNS deficiency leads to the accumulation of the GAGs keratan sulfate (KS) and chondroitin-6-sulfate (C6S) in lysosomes and results in prominent skeletal and connective tissue abnormalities. In addition to skeletal and connective tissue abnormalities, patients also experience joint hypermobility (Aslam et al., 2013), muscle weakness, pulmonary and cardiac manifestations of the disease, all of which can result in reduced endurance and impact both quality of life and mortality. Compared with patients with other MPS disorders, those with Morquio A often show joint hypermobility in contrast to joint stiffness. Morquio A often shows prominent spine involvement (Solanki et al., 2013), but patients are typically reported to be intellectually normal. The clinical presentation of Morquio A varies among patients in both the specific features observed and their severity, making any single metric an incomplete description of disease burden (Harmatz et al., 2013; Hendriksz et al., 2013; Montano et al., 2007b; Tomatsu et al., 2011).
Morquio A is a rare disorder, with incidence estimated to range from 1 in 76,000 to 1 in 640,000 live births in different populations (Hendriksz et al., 2013). The incidence has been reported as one in 76,000 in Northern Ireland, one in 640,000 in Western Australia, one in 450,000 in the Netherlands, and one in 450,000 in Portugal (Meikle et al., 1999; Nelson, 1997; Nelson et al., 2003; Pinto et al., 2003; Poorthuis et al., 1999). More accurate data about the incidence of Morquio A can be obtained in the future if screening of newborns is introduced, as this occurs for other lysosomal storage disorders such as Pompe and Fabry disease (Labrousse et al., 2010; Nakamura et al., 2011; Scott et al., 2013).
Diagnosis of Morquio A begins with clinical suspicion, followed by screening tests (which are sometimes omitted if there is a known family history). The Morquio A diagnostic algorithm recommends a GALNS enzyme activity assay performed in leukocytes or fibroblasts as the gold standard for diagnosis of Morquio A (Wood et al., 2013); diagnosis of Morquio A can be supported by molecular analysis of the GALNS gene (Wood et al., 2013). Screening tests that may also be used for Morquio A are urinary GAG analysis and/or enzyme activity analysis performed on dried blood spots. Urinary GAG analysis measures either the total accumulation of all urinary GAGs (quantitative assay) or the relative abundance of each of the GAGs (qualitative assay). It is recommended to perform both quantitative and qualitative urinary GAG analyses in parallel, because quantitative GAGs are not always elevated in Morquio A patients and both tests are susceptible to false-negative results due to low KS excretion (relative to other GAGs) in teenagers and adults (Tomatsu et al., 2004d; Whitley et al., 1989a; Whitley et al., 1989b; Wood et al., 2013). Enzyme assays performed on dried blood spot samples are an alternative screening tool (Camelier et al., 2011) but are not recommended or Morquio A diagnosis where alternatives exist, since assay robustness and sample quality are potential concerns (Wood et al., 2013). A liquid chromatography/tandem mass spectrometry-based approach may also be used to measure levels of keratanase II-digested mono- and di-sulfated KS disaccharides, providing a means to measure KS both quantitatively and qualitatively at the same time (Hintze et al., 2011; Martell et al., 2011; Oguma et al., 2007; Tomatsu et al., 2010; Tomatsu et al., 2013; Oguma et al., 2007). A diagnosis of Morquio A is established if GALNS enzyme activity is markedly decreased in fibroblasts or leukocytes and control enzymes display wild-type activity (Wood et al., 2013). Additional reference enzyme measurements are critical to confirm sample integrity and exclude other disorders, such as MPS VI (caused by loss of arylsulfatase B activity; patients with Morquio A have been misdiagnosed with MPS VI), Morquio B (caused by deficiency of β-galactosidase due to mutations in GLB1; patients with Morquio B have been misdiagnosed with Morquio A), multiple sulfatase deficiency (mutations in the SUMF1 gene result in reduced activity of multiple sulfatases, including GALNS), and mucolipidoses types II/III (leads to mislocalization of GALNS and other lysosomal enzymes in some tissues).
The GALNS gene is approximately 50kb long and contains 14 exons, producing a 2339-bp mRNA that encodes a 522-amino acid protein (Nakashima et al., 1994; Tomatsu et al., 1991). The protein structure of the human GALNS protein has recently been solved (Rivera-Colón et al., 2012). The GALNS active site is a large trench containing a catalytic formylglycine aldehyde, derived from a cysteine residue by action of the formylglycine-generating enzyme (FGE) (Cosma et al., 2003; Dierks et al., 1997; Dierks et al., 2003; Rivera-Colón et al., 2012). The GALNS protein is found as a homodimer (Pshezhetsky and Potier, 1996) and is described as occurring in a multiprotein complex with other lysosomal enzymes (Adzhubei et al., 2010; Pshezhetsky and Potier, 1996).
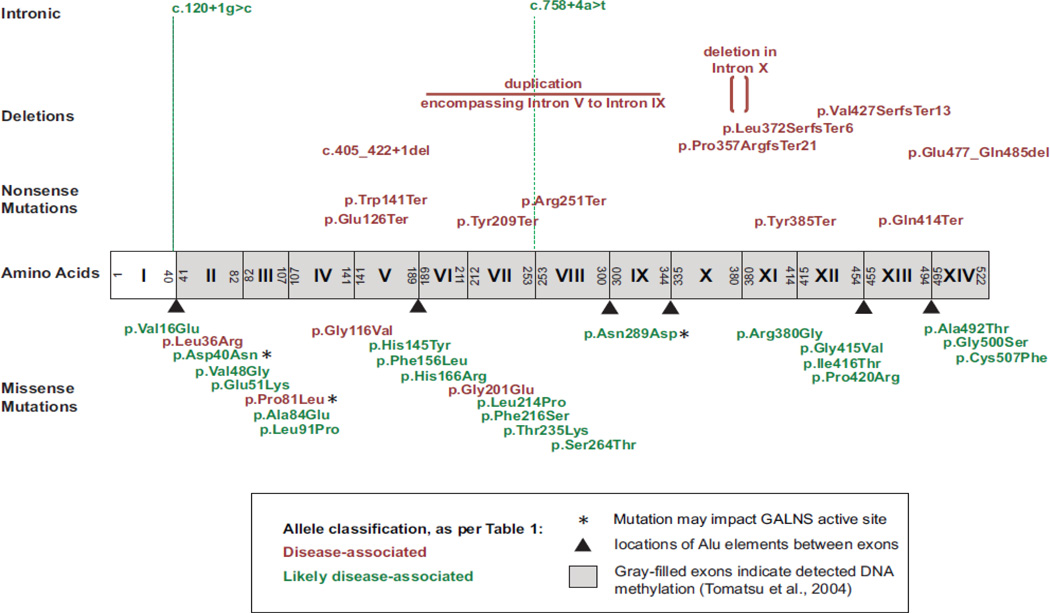
The GALNS mutations that cause Morquio A are very heterogeneous and are detected throughout the gene (Tomatsu et al., 2005). Even the most frequently detected mutations are relatively uncommon (Tomatsu et al., 2005); however, founder effects can greatly alter GALNS allele frequencies in individual populations (Kato et al., 1997; Wood et al., 2013; Yamada et al., 1998). DNA methylation at CpG sites occurs in every exon but one and inappropriate repair is thought to lead to transition mutations at these sites (Tomatsu et al., 2004c). Multiple introns contain Alu repetitive elements, which can undergo recombination and lead to large deletions and/or rearrangements (http://genome.ucsc.edu/; February 2009 assembly; Meyer et al., 2013). This mutational heterogeneity can lead to difficulties in interpretation of molecular testing results, as novel mutations/variants of unknown significance may be detected relatively frequently.
Molecular analysis can confirm the Morquio A diagnosis and aid in genetic counseling by detecting causative mutations in the GALNS gene. Morquio A is an autosomal recessive disorder, so for disease to occur, both GALNS alleles must carry mutations that decrease or eliminate GALNS enzyme activity. In standard DNA sequencing approaches, separate PCR reactions amplify the GALNS exons and short stretches of the adjacent sequence, followed by sequencing of the individual PCR products. However, standard sequencing approaches can yield misleading results when one patient allele contains deletions or point mutations that eliminate PCR primer binding sites, resulting in apparent homozygosity due to allele dropout (Landsverk et al., 2012). Parental testing can confirm biallelic inheritance and indicate cases where deletion/duplication testing is necessary or uniparental isodisomy (UPD) may be a possibility (Catarzi et al., 2012). However, not all mutations can be detected by standard molecular approaches, and sometimes the clinical significance of newly detected sequence alterations will not be clear. For example, standard GALNS sequencing approaches do not sequence deep within most introns, and the functional consequences of deep intronic mutations can be difficult to assess. Molecular analysis reports should clearly state which conclusions are possible for any detected sequence alterations.
In this study, we report the molecular testing that led to the identification of genetic lesions in GALNS in 163 patients with Morquio A. We identified 99 mutations believed to be disease associated or that are likely disease associated, of which 39 are previously unpublished. Of the 39 novel mutations, 25 are missense mutations, six are nonsense mutations, five are small deletions, two are intronic mutations that likely affect splicing sites, and one is a large deletion-duplication. We also propose guidelines for the interpretation and reporting of GALNS mutations based on the supporting information available. Using these classification guidelines, 16 of the 39 novel changes are “disease associated” and 23 are “likely disease associated”. In addition to the 39 novel changes believed to be disease associated or that are likely disease associated, two novel changes were detected that are classified as “variants of unknown significance”. We also identified 26 separate single-nucleotide polymorphisms (SNPs). Finally, we provide guidelines and recommendations for molecular testing and highlight situations where inaccurate genotyping conclusions may occur.
2. Materials and Methods
Individuals genotyped in this study had received a diagnosis of Morquio A prior to and independent of any molecular testing results, and thus, no power calculations were performed or required for the purposes of this study. All detected mutations in the GALNS gene were checked against reported SNPs. At three of the six institutions reporting novel alterations in GALNS, novel alterations were shown to be absent from the GALNS sequences of at least 100 unaffected individuals; at the remaining three institutions, this was not routinely performed for GALNS alterations identified in patients with a diagnosis of Morquio A.
The results reported here 1) have been reviewed and approved by a duly constituted ethics committee (Hospital Clínic, Barcelona, Spain; and Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil), 2) are from samples referred as diagnostic samples, which do not require ethical approval at this institution (Willink Biochemical Genetics Unit, Manchester, United Kingdom), or 3) are from retrospective case reports that do not require ethics committee approval at these institutions (Unidade de Bioquímica Genética, Porto, Portugal; Children’s Hospital of Orange County, Orange, California, United States; King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia; and SA Pathology, North Adelaide, South Australia, Australia)
2.1 Patient Material and Molecular Analysis
Genomic DNA was isolated from fibroblasts or peripheral blood cells using standard protocols. GALNS exons and adjacent intron regions were amplified by PCR reactions and then sequenced; in some cases, a single-strand conformation polymorphism assay was also performed, all using standard protocols (see Supplementary Methods). Molecular testing was performed at the laboratories included in this publication and no data from BioMarin Pharmaceutical-sponsored studies are reported.
The DNA and protein sequence numbering was based on the GALNS cDNA sequence (GenBank entry NM_000512.4), with the sequence position +1 corresponding to the A of the initial ATG in the reference sequence. Novel mutations are defined as nucleotide changes in the GALNS gene that have not previously been described and published; they are classified by the supporting evidence available per Table 1.
Table 1.
Suggested GALNS Mutation Reporting Guidelines
| Mutation disease associated |
Mutation likely disease associated | Variant of unknown significance (VUS) |
Likely benign variant |
|---|---|---|---|
| Disease diagnosis confirmed | |||
| Known to cause disease and found in more than one family in association with enzyme defect |
AND | ||
| Unlikely to be benign variant | |||
| OR | AND ONE OF BELOW:
|
Ambiguous/unclear effect on protein |
Nucleotide change present at high frequency in population |
| Clear-cut effect of mutation on protein:
|
|
Rare in population | OR |
|
Reported in single individual/family with inadequate clinical or segregation information |
Accurate published evidence characterizes as benign polymorphism |
|
| |||
Mutation reporting based on recommendations of Emory Genetics Laboratory (Richards et al., 2008; Ogino et al., 2007) and the Human Genome Variation Society (den Dunnen and Antonarakis, 2000)(http://www.hgvs.org/mutnomen/).
2.2 GALNS Enzyme Assay and Urinary GAG Testing
GALNS enzyme activity was measured in leukocytes or cultured fibroblasts using either fluorogenic (Zhao et al., 1990) or radiolabelled substrates (Hopwood and Elliott, 1983); assays were performed according to the manufacturer’s protocols.
Total urinary GAGs were quantified using the Alcian blue or dimethylmethylene blue (DMB) method (Panin et al., 1986). Qualitative GAG analysis for the detection of KS was carried out by one dimensional or two dimensional low voltage electrophoresis of extracted GAGs, or by thin layer chromatography (Hopwood and Harrison, 1982; Humbel et al., 1972).
3. Results
3.1 Detected alterations in GALNS
In this study, molecular testing was performed on 163 patients with Morquio A (Supp. Table S1). A total of 99 predicted sequence alterations suspected of negatively impacting GALNS function were identified, of which 39 are previously unpublished: 25 missense changes, six nonsense changes, five small deletions (one in-frame deletion, three frameshifts, and one deletion that encompasses exonic and intronic sequence), one complex large deletion-duplication, and two intronic changes affecting consensus splice sites (Fig 1, Fig 2, Table 2, Supp. Table S2; information from all patients in Supp. Table S1). Six of these novel changes were detected in more than one patient: c.347G>T (p.Gly116Val, found in seven patients), c.107T>G (p.Leu36Arg, found in three patients), c.602G>A (p.Gly201Glu, found in three patients, of whom two are siblings), c.422G>A (p.Trp141Ter, found in two patients), c.242C>T (p.Pro81Leu, found in two patients), and c.1155C>A (p.Tyr385Ter, found in two patients); all other novel mutations were only detected in a single patient. In addition to the 39 novel GALNS alterations associated with Morquio A, we also detected two missense variants of unknown significance (Table 2, Supp. Table S1, Supp. Table S2) and 26 separate SNPs (Supp. Table S3).
Figure 1. Location of 39 novel mutations detected in the GALNS gene.
Exons are depicted as boxes and labeled with roman numerals. Novel alleles are color coded by their classification per Table 1.
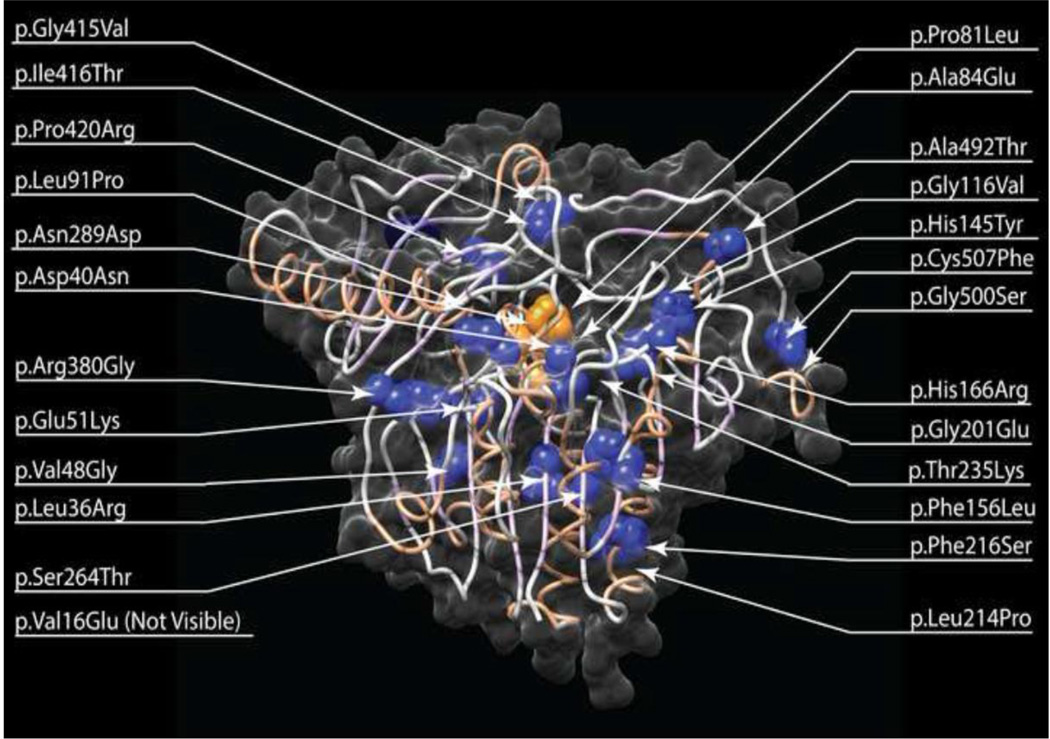
Figure 2. Location of novel Morquio A missense mutations mapped on the protein structure of the human GALNS protein.
Novel missense mutations identified in this study are mapped on the human GALNS protein structure (pdb:4FDI). Mutations identified were mapped to the structure and visualized using UCSF Chimera (http://www.cgl.ucsf.edu/chimera). The protein chain is represented as a ribbon showing beta sheets as light purple and alpha helices as light red, surrounded by a transparent solvent excluded surface. Mutation positions are represented as spheres for the wild type side chains. Note that position 16 is in the N-terminal disordered region. Mutations colored in orange are predicted to affect GALNS active site primary residues; all others are colored in blue.
Table 2.
Genotypes of Patients With Morquio A Carrying Novel GALNS Mutations Identified in This Study
| Allele 1 | Allele 2 | GALNS enzyme activity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number |
Nucleotide change | Predicted effect on protein | Classification of novel allele(s) per Table 1 |
Nucleotide change |
Predicted effect on protein |
Reported country/ethnicity |
M/F | Age at diagnosis (years) |
Reported as low? |
% Wild- type |
Reporting institute |
| 1 | c.47T>A | p.Val16Glu | Likely disease associated |
c.47T>A | p.Val16Glu | Brazilian | F | 10 | Yes | 9 | HCPA |
| 2 | c.107T>G | p.Leu36Arg | Disease associated | c.107T>G | p.Leu36Arg | Asian-multiethnic | F | 2 | Yes | 6 | Willink |
| 3 | c.107T>G | p.Leu36Arg | Disease associated | c.107T>G | p.Leu36Arg | Asian-multiethnic | M | Yes | 4 | Willink | |
| 4 | c.107T>G | p.Leu36Arg | Disease associated | c.107T>G | p.Leu36Arg | Asian-multiethnic | F | 12 | Yes | 5 | Willink |
| 5 | c.118G>A | p.Asp40Asn | Likely disease associated |
c.118G>A | p.Asp40Asn | Asian-multiethnic | F | Willink | |||
| 6 | c.120+1g>c | - | Likely disease associated |
c.120+1g>c | - | Middle Eastern | M | 0.5 | Yes | 3 | Willink |
| 7 | c.151G>A | p.Glu51Lys | Likely disease associated |
c.151G>A | p.Glu51Lys | Brazilian | F | 4 | Yes | 0.6 | HCPA |
| 8 | c.242C>T | p.Pro81Leu | Disease associated | c.242C>T | p.Pro81Leu | Turkish | M | 13 | Yes | 6 | Willink |
| 9 | c.251C>A | p.Ala84Glu | Likely disease associated |
c.251C>A | p.Ala84Glu | Portuguese | M | 4 | Yes | 0 | CGMJM |
| 10 | c.272T>C | p.Leu91Pro | Likely disease associated |
c.272T>C | p.Leu91Pro | Portuguese | F | 3 | Yes | 0 | CGMJM |
| 11 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Asian-multiethnic | Willink | ||||
| 12 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Asian-multiethnic | F | 3 | Yes | 3 | Willink |
| 13 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Asian-multiethnic | M | 3 | Yes | 2 | Willink |
| 14 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Asian-multiethnic | F | Willink | |||
| 15 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Asian-multiethnic | F | 0.08 | Yes | 2 | Willink |
| 16 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Asian-multiethnic | M | 1 | Yes | 5 | Willink |
| 17 | c.347G>T | p.Gly116Val | Disease associated | c.347G>T | p.Gly116Val | Norwegian | M | Yes | Willink | ||
| 18 | c.376G>T | p.Glu126Ter | Disease associated | c.865A>G | p.Asn289Asp | Australian | 4.4 | Yes | 1.7 | SA Path | |
| 19 | c.422G>A | p.Trp141Ter | Disease associated | c.422G>A | p.Trp141Ter | UK | M | 3 | Yes | 6 | Willink |
| 20 | c.433C>T | p.His145Tyr | Likely disease associated |
c.433C>T | p.His145Tyr | Canadian | 4.7 | Yes | SA Path | ||
| 21 | c.466T>C | p.Phe156Leu | Likely disease associated |
c.466T>C | p.Phe156Leu | Middle Eastern | F | 2 | Yes | KFSH&RC | |
| 22 | c.497A>G | p.His166Arg | Likely disease associated |
c.497A>G | p.His166Arg | UK | F | Yes | Willink | ||
| 23 | c.602G>A | p.Gly201Glu | Disease associated | c.602G>A | p.Gly201Glu | Middle Eastern | Yes | Willink | |||
| 24 | c.602G>A | p.Gly201Glu | Disease associated | c.602G>A | p.Gly201Glu | Middle Eastern | 5.2 | SA Path | |||
| 25 | c.602G>A | p.Gly201Glu | Disease associated | c.602G>A | p.Gly201Glu | Middle Eastern | Yes | SA Path | |||
| 26 | c.627C>G | p.Tyr209Ter | Disease associated | c.627C>G | p.Tyr209Ter | F | 4 | Yes | Willink | ||
| 27 | c.641T>C | p.Leu214Pro | Likely disease associated |
c.641T>C | p.Leu214Pro | Turkish | Yes | 4 | Willink | ||
| 28 | c.647T>C | p.Phe216Ser | Likely disease associated |
c.647T>C | p.Phe216Ser | Indian | Yes | 8 | Willink | ||
| 29 | c.791G>C | p.Ser264Thr | Likely disease associated |
c.791G>C | p.Ser264Thr | UK | M | 2 | Yes | 4 | Willink |
| 30 | c.1070delC | p.Pro357ArgfsTer21 | Disease associated | c.1070delC | p.Pro357ArgfsTer21 | Middle Eastern | M | 1 | KFSH&RC | ||
| 31 | c.1114delC | p.Leu372SerfsTer6 | Disease associated | c.1114delC | p.Leu372SerfsTer6 | Portuguese | M | 10 | Yes | 0 | CGMJM |
| 32 | c.1138A>G | p.Arg380Gly | Likely disease associated |
c.1138A>G | p.Arg380Gly | Cape Verdean | M | 3 | Yes | 0 | CGMJM |
| 33 | c.1244G>T | p.Gly415Val | Likely disease associated |
c.1244G>T | p.Gly415Val | Portuguese | M | 15 | Yes | 0 | CGMJM |
| 34 | c.1247T>C | p.Ile416Thr | Likely disease associated |
c.1247T>C | p.Ile416Thr | Asian-multiethnic | M | Yes | Willink | ||
| 35 | c.1259C>G | p.Pro420Arg | Likely disease associated |
c.1259C>G | p.Pro420Arg | Asian-multiethnic | F | Yes | Willink | ||
| 36 | c.1429 1455del27 | p.Glu477 Gln485del | Disease associated | c.1429 1455del27 | p.Glu477 Gln485del | Middle Eastern | F | 13 | KFSH&RC | ||
| 37 | c.1474G>A | p.Ala492Thr | Likely disease associated |
c.1474G>A | p.Ala492Thr | Middle Eastern | M | 9 | Yes | KFSH&RC | |
| 38 | c.1498G>A | p.Gly500Ser | Likely disease associated |
c.1498G>A | p.Gly500Ser | Moroccan | F | 11 | Yes | 0 | CGMJM |
| 39 | c.143T>G | p.Val48Gly | Likely disease associated |
c.604delG | p.Glu202LysfsTer115 | UK | M | Yes | Willink | ||
| 40 | c.242C>T | p.Pro81Leu | Disease associated | c.1156C>T | p.Arg386Cys | Other | M | Yes | Willink | ||
| 41 |
c.[566+? 1003-?dup; 1139+? 1140-?del] |
complex del-dup: duplication from intron 5 to intron 9, deletion in intron 10 |
Disease associated | NF | NF | Middle Eastern | F | 12 | KFSH&RC | ||
| 42 | c.751C>T | p.Arg251Ter | Disease associated | c.268C>T | p.Arg90Trp | UK | F | 4 | Yes | 6 | Willink |
| 43 | c.1171A>G | p.Met391Val | Disease associated | c.405 422+1del | - | French Canadian | 48 | SA Path | |||
| 44 | c.925G>A | p.Gly309Arg | Disease associated | c.422G>A | p.Trp141Ter | UK | M | Willink | |||
| 45 | c.1171A>G | p.Met391Val | Likely disease associated |
c.704C>A | p.Thr235Lys | French Canadian | 8.9 | Yes | SA Path | ||
| 46 | c.860C>T | p.Ser287Leu | Likely disease associated |
c.758+4a>t | - | Greek | M | Yes | Willink | ||
| 47 | c.740G>A | p.Glu247Asp | VUS, VUS |
c.[937A>G; 977G>C] |
p.[Thr313Ala; Trp326Ser] |
Australian | 1.1 | Yes | 0.8 | SA Path | |
| 48 | c.752G>A | p.Arg251Gln | Disease associated | c.1155C>A | p.Tyr385Ter | UK | F | Yes | Willink | ||
| 49 | c.752G>A | p.Arg251Gln | Disease associated | c.1155C>A | p.Tyr385Ter | UK | M | Yes | Willink | ||
| 50 | c.776G>A | p.Arg259Gln | Likely disease associated |
c.1520G>T | p.Cys507Phe | Hispanic | F | Yes | 7 | CHOC | |
| 51 | c.850T>G | p.Phe284Val | Disease associated | c.1275delA | p.Val427SerfsTer13 | UK | M | 5 | Yes | 4 | Willink |
| 52 | c.860C>T | p.Ser287Leu | Disease associated | c.1240C>T | p.Gln414Ter | New Zealander | 1.8 | Yes | 0 | SA Path | |
Novel alleles in bold. Where data were not provided to the lab for the given patient, the field is left blank. The field “Classification of novel allele per Table 1” refers to the novel allele(s), if any; when the novel alleles differ, in this study both happened to share the same classification.
CGMJM, Unidade de Bioquímica Genética, Centro de Genética Médica Jacinto Magalhães (CGMJM) do Centro Hospitalar do Porto (CHP), Porto, Portugal; CHOC, Children’s Hospital of Orange County, Orange, California, United States; HCPA, Laboratório de Genética Molecular, Serviço de Genética Médica, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, Brazil; KFSH&RC, Department of Medical Genetics, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia; SA Path, SA
Pathology, Women’s and Children's Hospital, North Adelaide, South Australia, Australia; Willink, Willink Biochemical Genetics Unit, Department of Genetic Medicine, Saint Mary’s Hospital, Manchester, United Kingdom.
The sequence alterations in GALNS associated with Morquio A are numerous, heterogeneous, and are mostly missense mutations (Hendriksz et al., 2013; Tomatsu et al., 2005; unpublished results). While some sequence alterations have clear-cut impacts on GALNS enzyme function (e.g., nonsense mutations, large deletions, or frameshifts), the interpretation of detected missense mutations can be more difficult. This poses a challenge to those who interpret sequence alterations in GALNS, since the detection of novel or poorly characterized missense mutations is a relatively common occurrence in the molecular analysis of patients with Morquio A. Here, we have created and used a GALNS sequence alteration categorization and reporting system consistent with guidelines from the Human Genome Variation Society and Emory Genetics Laboratory (Table 1). This reporting system aims to communicate succinctly what can reasonably be concluded about a detected GALNS sequence alteration. For example, reasonably confident statements can be made about sequence alterations that have clear-cut predicted effects on the GALNS protein and alterations that have been detected multiple times in independent families, while more cautious statements are appropriate for missense alterations detected in a single patient. We believe that this reporting system is useful both in publishing GALNS alterations and in communicating GALNS sequence results to clinicians.
3.1.1 Missense alleles
Among the 25 novel missense changes detected, the most frequently detected novel allele was the missense change c.347G>T (p.Gly116Val), detected 14 times in seven patients. In every case, molecular testing indicated that patients were homozygous for the c.347G>T (p.Gly116Val) allele; of these seven patients, the ethnicity of six was ‘Asian-multiethnic’ and one was Norwegian (but possibly of Asian origin). Where assayed, patients homozygous for the c.347G>T (p.Gly116Val) change were diagnosed with Morquio A at three years of age or younger and had substantial reductions in GALNS enzyme activity (6% or less of wild-type). While the specific c.347G>T (p.Gly116Val) change has not previously been published, c.346G>A (p.Gly116Ser) is a previously reported mutation associated with a severe growth phenotype (Dung et al., 2013; Rivera-Colón et al., 2012; Tomatsu et al., 2004c; Tomatsu et al., 2005). In addition to c.347G>T (p.Gly116Val), the novel alterations c.107T>G (p.Leu36Arg), c.497A>G (p.His166Arg), c.865A>G (p.Asn289Asp), c.1138A>G (p.Arg380Gly), and c.1259C>G (p.Pro420Arg) also affect amino acid residues where other missense mutations have previously been reported (Tomatsu et al., 1997; Tomatsu et al., 2005; Wang et al., 2010; Ye et al., 2013).
Three novel missense changes that directly impact the active site of the GALNS enzyme (Rivera-Colón et al., 2012) were identified: c.242C>T (p.Pro81Leu), c.118G>A (p.Asp40Asn) and c.865A>G (p.Asn289Asp) (Fig. 2). The change c.242C>T (p.Pro81Leu) alters the middle residue of the FGE recognition motif and may reduce or eliminate the FGE-dependent posttranslational generation of the GALNS catalytic residue. Consistent with this interpretation, a patient homozygous for the change c.242C>T (p.Pro81Leu) had substantially reduced levels of GALNS enzyme activity. The GALNS active site contains a Ca2+ ion coordinated by oxygen atoms from five residues (Rivera-Colón et al., 2012). The overall negative charge coordinating the calcium ion would change with c.118G>A (p.Asp40Asn) and c.865A>G (p.Asn289Asp), likely with deleterious effects on Ca2+ binding. Additionally, the primary active site residue Arg83 (Rivera-Colón et al., 2012) is adjacent to an amino acid affected by the novel missense change c.251C>A (p.Ala84Glu), which would substitute a bulky charged residue for a small nonpolar residue and may impact the structure and charge of the enzyme active site. A patient homozygous for the change c.251C>A (p.Ala84Glu) had rapidly progressing disease, as characterized by a severe growth phenotype, and no detectable GALNS enzyme activity.
3.1.2 Nonsense alleles
Six novel nonsense mutations were identified: c.376G>T (p.Glu126Ter), c.422G>A (p.Trp141Ter), c.627C>G (p.Tyr209Ter), c.751C>T (p.Arg251Ter), c.1155C>A (p.Tyr385Ter), and c.1240C>T (p.Gln414Ter). These mutations would be expected to severely affect the GALNS protein by introducing early stop codons. Additionally, the previously reported nonsense mutation c.1559G>A (Trp520Ter) (Ye et al., 2013) was detected. This mutation is of interest because it removes only the final three C-terminal amino acids but is associated with Morquio A, consistent with the role of the GALNS C-terminus in forming the enzyme’s active site pocket (Rivera-Colón et al., 2012).
3.1.3 Deletions
Five novel small deletions were identified, all of which are currently private mutations since they have been detected on only one occasion to date. Both the novel in-frame deletions and frameshift mutations detected here are associated with rapidly progressing disease, as characterized by a severe growth phenotype. Homozygosity for the frameshift mutation c.1114delC (p.Leu372SerfsTer6) was detected in a patient with rapidly progressing disease, as characterized by a severe growth phenotype, with no detectable GALNS enzyme activity. A patient found to be a compound heterozygote for the deletion c.1275delA (p.Val427SerfsTer13) and the missense mutation c.850T>G (p.Phe284Val) was diagnosed with Morquio A at five years of age with very low GALNS enzyme activity. The deletion c.405_422+1del removes sequence from the end of exon four and the beginning of intron four and may affect splicing in addition to deleting amino acid residues. A large deletion-duplication was detected by comparative genome hybridization in one patient: the apparent duplication encompassed exons 6 to exon 9 with approximate breakpoints at nucleotide position g.87,424,250 in intron 5 and g.87,430,150 in intron 9, and the apparent deletion was within intron 10 with approximate break points at nucleotides g.87,418,915 and g.87,419,462. This complex rearrangement is predicted to result in duplication of sequences between introns 5 and 9 and deletion of sequences within intron 10.
3.1.4 Alterations affecting splice sites
Two novel changes affecting intronic consensus splice sites were identified: c.120+1G>C and c.758+4A>T. While the exact consequences of these changes on the mature GALNS mRNA sequence cannot easily be predicted, a patient homozygous for the change c.120+1G>C was diagnosed with Morquio A at 6 months of age and had very low GALNS enzyme activity. Interestingly, a different mutation at the same nucleotide has previously been reported (c.120+1G>A) and is also associated with early-onset Morquio A (Laradi et al., 2006; Romdhane et al., 2012).
4. Discussion
4.1 Detected alterations
In this study, molecular testing of 163 patients with Morquio A (Supp. Table S1) identified 99 separate changes, of which 39 are previously unpublished (Supp. Table S2), together with 26 separate SNPs (Supp. Table S3). Of the novel changes in GALNS identified in this study, the novel changes c.107T>G (p.Leu36Arg), c.242C>T (p.Pro81Leu) c.422G>A (p.Trp141Ter) and c.602G>A (p.Gly201Glu) were found in more than one family. While the novel allele c.347G>T (p.Gly116Val) was detected in seven patients, we currently lack information on any potential familial relationships between the patients; the remaining novel alleles were only detected in a single individual or family (Supp. Table S2). The alleles c.376G>T (p.Glu126Ter), c.422G>A (p.Trp141Ter), c.627C>G (p.Tyr209Ter), c.751C>T (p.Arg251Ter), c1070delC (p.Pro357ArgfsTer21), c.1114delC (p.Leu372SerfsTer6), c.1155C>A (p.Tyr385Ter), c.1240C>T (p.Gln414Ter), c.1429_1455del27 (p.Glu477_Gln485del), and the complex deletion-duplication can confidently be predicted to affect protein function and have not been previously described. Further, the missense changes c.242C>T (p.Pro81Leu), c.118G>A (p.Asp40Asn) and c.865A>G (p.Asn289Asp) are all predicted to greatly alter amino acid residues characterized as “primary active site residues” (Rivera-Colón et al., 2012) and so can also be predicted to affect protein function.
This study identified 25 novel changes predicted to result in missense mutations in the GALNS protein either associated or likely to be associated with Morquio A disease; additionally, the novel missense changes c.[937A>G; 977G>C] (p.[Thr313Ala; Trp326Ser]) were found in cis in one allele of a patient with Morquio A (both in trans with the known allele c.740G>A (p.Gly247Asp)) and are both currently classified as “variants of unknown significance”. For a subset of these novel missense changes, previous studies have identified Morquio A patients with different missense changes at the same amino acid residue: p.Leu36Arg is a novel change, but p. Leu36Pro has been described (Tomatsu et al., 2005), p.Gly116Val (p.Gly116Ser (Dung et al., 2013; Tomatsu et al., 2004a)), p.Phe156Leu (p.Phe156Cys and p.Phe156Ser (Bunge et al., 1997; Tomatsu et al., 2005; Yamada et al., 1998)), p.His166Arg (p.His166Gln (Tomatsu et al., 2004c; Tomatsu et al., 2005)), p.Asn289Asp (p.Asn289Ser (Wang et al., 2010)), p.Arg380Gly (p.Arg380Ser and p.Arg380Thr (Montano et al., 2007a; Tomatsu et al., 2004a; Tomatsu et al., 2005)), and p.Pro420Arg (p.Pro420Ser (Ye et al., 2013)). These previously published missense changes at the same amino acid residues may strengthen the link between these novel changes and disease.
Currently, the following mutations have only been described in one family and changes at these amino acids have not been previously noted but are associated with a GALNS enzyme defect in the patient: c.47T>A (p.Val16Glu), c.120+1G>C, c.143T>G (p.Val48Gly), c.151G>A (p.Glu51Lys), c.251C>A (p.Ala84Glu), c.272T>C (p.Leu91Pro), c.433C>T (p.His145Tyr), c.466T>C (p.Phe156Leu), c.497A>G (p.His166Arg), c.641T>C (p.Leu214Pro), c.647T>C (p.Phe216Ser), c.704C>A (p.Thr235Lys), c.758+4A>T, c.791G>C (p.Ser264Thr), c.865A>G (p.Asn289Asp), c.1138A>G (p.Arg380Gly), c.1244G>T (p.Gly415Val), c.1247T>C (p.Ile416Thr), c.1259C>G (p.Pro420Arg), c.1474G>A (p.Ala492Thr), c.1498G>A (p.Gly500Ser), and c.1520G>T (p.Cys507Phe); these changes are classified as “likely disease associated” and it is recommended that when found in a second family, patients should be verified to have a GALNS enzyme activity defect to confirm a diagnosis of Morquio A. Of particular note is the novel change c.47T>A (p.Val16Glu), which mutation predicting programs do not strongly associate with disease. The c.47T>A (pVal16Glu) allele was documented in a patient with a less severe Morquio A growth phenotype, but with accompanying detection of KS and elevation of urinary GAGs, together with a demonstrated GALNS enzyme activity defect in both dried blood spots and leukocytes and normal activity of the control enzymes β-galactosidase, α-iduronidase, arylsulfatase B, iduronate sulfatase, and β-glucuronidase; further, this patient had a pedigree indicating closely related parents. The novel splice site alterations reported here are c.120+1G>C and c.758+4A>T. While the c.120+1G>C mutation is predicted to be a clear splicing defect, the c.758+4A>T change is less well characterized as a splicing defect and we recommend, as for all changes categorized as “likely disease associated”, that if it should be identified in a second family, a defect in GALNS enzyme activity be demonstrated.
4.2 Challenges in interpretation
The high proportion of detected novel changes emphasizes the heterogeneity in GALNS mutations. This heterogeneity creates challenges in the interpretation of patient genotypes as many patients will carry novel or poorly characterized mutations. For example, some patients with identified deleterious mutations in both GALNS alleles also had additional GALNS sequence variants detected (Supp. Table S3). These variants have been reported as SNPs even if an eventual role in modulating phenotype (i.e. aberrant splicing induction) cannot be excluded. Given the potential complexity and ambiguity in interpreting the functional consequences of a mutation, we recommend standardized molecular testing reports that clearly state what conclusions can be drawn (Table 1). It is recommended that physicians collect as much as possible of a patient’s clinical phenotype data and laboratory screening results together with the age of diagnosis; however, as evidenced by our experience (see Supp. Table S1), this is often not practically possible. To aid in the interpretation of sequencing data, it is important for detected mutations to be reported to available mutation databases, such as the Human Gene Mutation Database, because independent reports that associate a particular GALNS variant with Morquio A increase confidence in its association with disease.
We recommend caution in using software programs that predict the consequences of amino acid substitutions on protein structure. Two of the more well-known software algorithms for predicting the effects of nonsynonymous SNPs are SIFT and PolyPhen-2. SIFT assumes that changes in conserved regions of a protein are more deleterious than in other regions so the availability of suitable homologs is particularly important. SIFT has a reported true-positive prediction rate of 69% using the Swiss-Prot database, with a false-positive rate of 20% (Ng and Henikoff, 2002). PolyPhen-2 makes a prediction based on 11 assessments (eight based on sequence and three on structure), with reported true-positive predictions rates of 92% and 73% on HumDiv and HumVar datasets, respectively, and a false-positive rate of 20% (Adzhubei et al., 2010). As with all statistical probabilities, the predictions are more likely to be true for the entirety of a dataset than for any specific mutation, warranting further caution in their use for the interpretation of individual novel mutations in a diagnostic setting.
Molecular testing approaches have limitations in mutation detection in patients with Morquio A. In contrast to MPS I—where it is common to identify both causative mutations in IDUA (up to 95% of the time in one study of 85 MPS I families (Beesley et al., 2001))—in Morquio A, a second mutation is not identified in up to 15% of patients (Tomatsu et al., 2005). The lower mutation detection rate in patients with Morquio A compared to those with MPS I may be due to a greater frequency of large deletions, duplications, or other rearrangements in GALNS than in IDUA, where large deletions have yet to be reported.
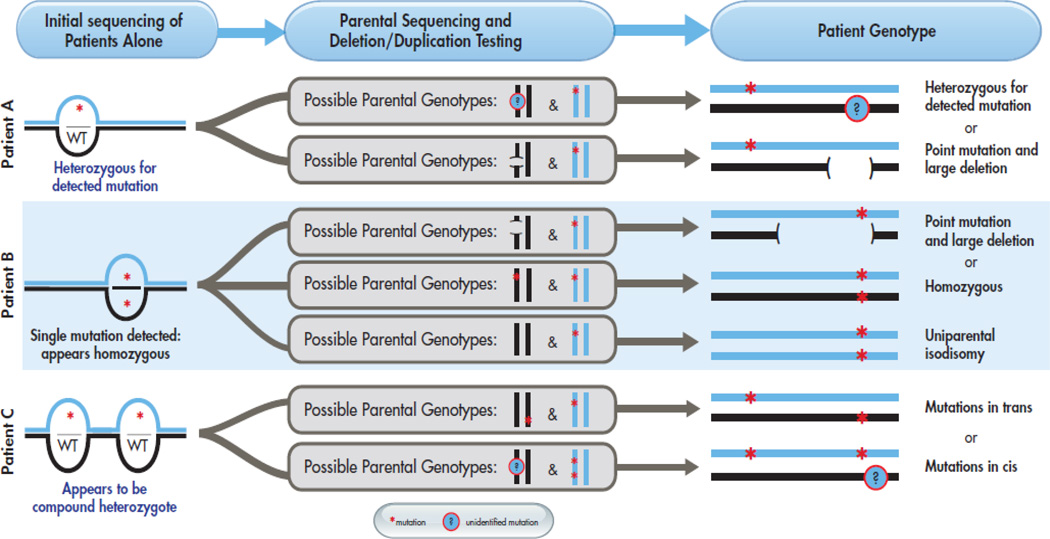
In addition to limitations in mutation detection, there are limitations in what can be concluded from molecular testing of the patient alone. For example, an inaccurate finding of homozygosity can occur when one patient allele lacks a PCR primer binding site due to deletions, point mutations, or SNPs, causing that exon to “drop out” from PCR amplification; since only one patient exon will produce a PCR product and yield sequence data, any mutations present in that exon’s PCR product will falsely appear to be homozygous. Apparent homozygosity due to allele dropout has been reported in patients with MPS VI (Villani et al., 2010), cystic fibrosis (Diana et al., 2012; Hantash et al., 2009), and familial hypercholesterolemia (Laios and Glynou, 2008). Even in patients who are genuinely homozygous for a mutation that affects GALNS function, testing of the patient alone can still result in molecular underdiagnosis since cases of UPD will not be detected; a case of Morquio A resulting from UPD has been reported (Catarzi et al., 2012).
The ideal molecular testing approach outlined in Figure 3 aims to address potential errors when genotyping patients with Morquio A. When possible, both parents should be genotyped to confirm biallelic inheritance of the causative mutations, allowing detection of situations where (1) deletions or polymorphisms at PCR primer sites could result in allele dropout and misinterpretation of patient sequencing results, (2) multiple alterations may be present in cis, or (3) homozygosity results from UPD (Fig. 3). Inaccurate and incomplete molecular diagnoses resulting from the absence of parental genotyping are potential concerns for many autosomal recessive disorders. In patients with MPS VI, multiple examples of patients with two deleterious mutations in cis have been reported (Karageorgos et al., 2007). At one molecular testing center, parental genotyping of patients with 40 different autosomal recessive disorders revealed that of 75 apparently homozygous patients, four were incorrectly assessed as homozygotes due to allele dropout and two were genuine homozygotes but resulted from UPD (Landsverk et al., 2012). Additionally, parental genotyping can aid in differentiating alleles that are genuinely associated with disease from benign polymorphisms, as recently shown in MPS VI (Zanetti et al., 2009). However, we recognize that circumstances do not always allow for parental testing to be performed. In this study, relatively few patients had parental testing performed and we recognize that this is the challenging reality we face when molecularly testing patients: it is not always feasible to test other family members or perform additional analyses.
Figure 3. Potential complexity of patient genotypes.
For three example initial genotyping results (apparently heterozygous, apparently homozygous, and apparently a compound heterozygote), illustrates how parental genotyping results can confirm the initial genotype or reveal unanticipated complexity.
4.3 Challenges in genotype/phenotype correlations
It is often desirable to make specific genotype/phenotype correlations between specific alleles and disease severity in Morquio A patients, but we believe that this practice contains two potential sources of error: first, the arbitrary classification of a complex disease state based on a few metrics (or even a single metric) and second, the consideration of a single mutation divorced from its context in the patient’s genotype. Morquio A manifests along a broad spectrum of clinical features and severity due to both the pleiotropic nature of the disease and the varied molecular consequences of its many distinct mutations; this can create difficulties in succinctly and accurately describing the disease state. The metrics most commonly used to classify Morquio A severity are patient height and growth rates. However, a patient of a given height can present with other symptoms (e.g., pulmonary or cardiac manifestations) of greater or lesser severity than might be expected; consequently, the level of dysmorphism does not fully define the disease severity (Harmatz et al., 2013; Montano et al., 2007b; Tomatsu et al., 2011; Montano et al., 2008; Tomatsu et al., 2011). A single, multivariate metric of disease severity does not currently exist, but weight, height, relative growth rate, and age at diagnosis are key markers (Hendriksz et al., 2013). Concentrations of KS measured in blood and urine, particularly in younger than pre-adolescent patients, have been found to correlate with Morquio A clinical status, suggesting their use in assessing disease severity and prognosis (Dung et al., 2013; Harmatz et al., 2013; Martell et al., 2011; Tomatsu et al., 2004d; Tomatsu et al., 2005; Tomatsu et al., 2010; Harmatz et al., 2013), and additional Morquio A biomarkers have also been proposed (Martell et al., 2011).
Furthermore, we believe that the possibility exists for unexpected interactions between alleles, particularly between missense alleles. Most mutations in GALNS are missense mutations (Tomatsu et al., 2005), with consequences that can potentially include reduction in GALNS enzyme activity, disruption of protein-protein interactions with other lysosomal hydrolases (Pshezhetsky and Potier, 1996), or GALNS protein destabilization and degradation. Because the GALNS enzyme is found as a homodimer (Pshezhetsky and Potier, 1996; Rivera-Colón et al., 2012), a Morquio A patient expressing two missense mutations could have three distinct GALNS dimers (aa, ab, and bb), each potentially with distinct enzymatic activity levels and protein interactions. Moreover, there are examples of pseudodeficiency alleles from other lysosomal storage disorders; these are ordinarily benign partial loss-of-function mutations that can potentially cause disease when combined with other loss-of-function mutations (Caciotti et al., 2005; Gieselmann et al., 1991; Thomas, 1994; Zlotogora and Bach, 1983). A complex example from the lysosomal storage disorder GM1 gangliosidosis describes an ordinarily benign polymorphism that increases the strength of a deleterious partial loss-of-function allele when combined in cis (Caciotti et al., 2003). While no examples of pseudodeficiency alleles or complex allelic interactions are currently described in Morquio A, we believe that the possibility warrants caution and the analysis of mutations in the context of complete and accurate patient genotypes.
4.4 Implications for Molecular Testing in Clinical Practice
From a clinical and counseling perspective, the precise definition of both causative mutations for Morquio A is particularly important. In addition to assisting in the prediction of phenotype, the detection of both mutations is required for molecular prenatal diagnosis as well as carrier testing for family members. Therefore, in patients heterozygous for two known or probable mutations, it is very important to obtain parental DNA samples to confirm biallelic inheritance of these mutations. For patients appearing homozygous for a mutation, parental samples are also necessary to show that both parents do indeed carry the mutation, confirming heterozygosity, or to indicate cases where further deletion/duplication or UPD studies are required. Unfortunately, the precise definition of large deletions or duplications using standard Sanger sequencing can often prove particularly difficult, especially when the deletion spans either end of the GALNS gene. Therefore, these studies are rarely performed as part of the initial sequence studies and in many cases will not be completed once a diagnosis has been “confirmed” by enzyme studies and the detection of a single mutation.
Furthermore, there is a significant proportion of cases (up to 15%) in which the second causative mutation is not detected by standard Sanger sequencing (Tomatsu et al., 2005). Many of these may be due to heterozygous deletions/duplications that are masked by a normal sequence in the other allele. Therefore, more extensive mutation studies are required in an attempt to define the second mutation. As for cases where deletions cause apparent homozygosity, further analysis is labor intensive and may rarely be carried out. In these cases, prenatal diagnosis using mutation studies is not feasible and must rely on enzyme studies, which are currently available in only a limited number of centers and can potentially prove problematic in cases with considerable residual GALNS enzyme activity. Also, the lack of detection of the second mutation means that conclusive carrier testing is not available for all family members. All of these are reminders that while molecular testing provides diagnostic and genetic counseling information, enzyme activity testing of GALNS, along with other enzymes, remains the standard for diagnosis of Morquio A.
Supplementary Material
Highlights.
Morquio A is an autosomal recessive disorder caused by mutations in the GALNS gene
GALNS mutations are heterogeneous and many are rare or poorly characterized
39 novel GALNS mutations were identified in study of 163 patients with Morquio A
Recommendations for molecular testing, reporting, and interpretation are provided
Acknowledgments
We thank Drs E. Pinto, H. Ribeiro, E. Silva, S. Rocha, C. Caseiro, C. Ferreira, and F. Pinto (all of the Unidade de Bioquímica Genética, Centro de Genética Médica Jacinto Magalhães [CGMJM] do Centro Hospitalar do Porto [CHP], Porto, Portugal) and Dr S. Alves (Unidade de Investigação, INSA, Porto, Portugal) for collaboration in molecular characterization, enzymatic assays, GAG analysis, and cell culture; Dr A. Gaspar (Consulta de Doenças Metabólicas, Hospital S. Maria, Centro Hospitalar de Lisboa Norte, Lisboa, Portugal), Dr L. Diogo (Consulta de Doenças Metabólicas, Centro Hospitalar Universitário de Coimbra, Coimbra, Portugal), Dr S. Sequeira (Consulta de Doenças Metabólicas, Hospital D. Estefânia, Centro Hospitalar de Lisboa Central, Lisboa, Portugal), and Dr I. Quelhas (Consulta de Desenvolvimento, Hospital Padre Américo, Centro Hospitalar Tâmega e Sousa, Penafiel, Portugal); Dr Roberto Giugliani, coordinator of the Instituto Nacional de Genética Médica Populacional of Brazil (INAGEMP) and the MPS Brazil Network; Drs J. Jarque, H. Sellés, and A.M. Valle (all of the Sección de Errores Congénitos del Metabolismo-IBC, Servicio de Bioquímica y Genética Molecular, Hospital Clínic, CIBERER, IDIBAPS, Barcelona, Spain); and Dr Emanuela Izzo and Dr Matthew Mealiffe of BioMarin Pharmaceutical Inc, Novato, California, United States. Medical writing support was provided by Dr Karl Zawadzki and Dr Sue Currie of Health Interactions, with funding provided by BioMarin Pharmaceutical Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Dr Fietz has received travel support and honoraria from BioMarin Pharmaceutical. Drs Church, Morrone, and Tylee have received consultant fees and limited travel support from BioMarin Pharmaceutical and provide a diagnostic service for MPS for samples from Turkey that is funded by BioMarin. Dr Pollard is a paid employee of the Greenwood Genetic Center, which has contracts with BioMarin. Dr Wang holds a financial interest in BioMarin. Dr Mooney serves as a consultant for BioMarin Pharmaceutical. Mrs Davidson and Dr Miller are employees of BioMarin. Drs Al-Sayed, Brusius-Facchin, Caciotti, Coll, Gort, Kubaski, Lacerda, Laranjeira, Leistner-Segal, Pajares, and Riberio declare no potential conflicts of interest.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam R, van Bommel AC, Hendriksz CJ, Jester A. Subjective and Objective Assessment of Hand Function in Mucopolysaccharidosis IVa Patients. JIMD Rep. 2013;9:59–65. doi: 10.1007/8904_2012_179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley CE, Meaney CA, Greenland G, Adams V, Vellodi A, Young EP, Winchester BG. Mutational analysis of 85 Mucopolysaccharidosis type I families: frequency of known mutations, identification of 17 novel mutations and in vitro expression of missense mutations. Hum Genet. 2001;109:503–511. doi: 10.1007/s004390100606. [DOI] [PubMed] [Google Scholar]
- Bunge S, Kleijer WJ, Tylki-Szymanska A, Steglich C, Beck M, Tomatsu S, Fukuda S, Poorthuis BJ, Czartoryska B, Orii T, Gal A. Identification of 31 novel mutations in the N-acetylgalactosamine-6-sulfatase gene reveals excessive allelic heterogeneity among patients with Morquio A syndrome. Hum Mutat. 1997;10:223–232. doi: 10.1002/(SICI)1098-1004(1997)10:3<223::AID-HUMU8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Caciotti A, Bardelli T, Cunningham J, D'Azzo A, Zammarchi E, Morrone A. Modulating action of the new polymorphism L436F detected in the GLB1 gene of a type-II GM1 gangliosidosis patient. Hum Genet. 2003;113:44–50. doi: 10.1007/s00439-003-0930-8. [DOI] [PubMed] [Google Scholar]
- Caciotti A, Donati MA, Boneh A, d'Azzo A, Federico A, Parini R, Antuzzi D, Bardelli T, Nosi D, Kimonis V, Zammarchi E, Morrone A. Role of beta-galactosidase and elastin binding protein in lysosomal and nonlysosomal complexes of patients with GM1-gangliosidosis. Hum Mutat. 2005;25:285–292. doi: 10.1002/humu.20147. [DOI] [PubMed] [Google Scholar]
- Camelier MV, Burin MG, De Mari J, Vieira TA, Marasca G, Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin Chim Acta. 2011;412:1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Carraresi L, Parini R, Filoni C, Caciotti A, Sersale G, Tomatsu S, Orlando C, Zammarchi E, Guerrini R, Donati MA, Morrone A. GALNS gene expression profiling in Morquio A patients' fibroblasts. Clin Chim Acta. 2008;397:72–76. doi: 10.1016/j.cca.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Catarzi S, Giunti L, Papadia F, Gabrielli O, Guerrini R, Donati MA, Genuardi M, Morrone A. Morquio A syndrome due to maternal uniparental isodisomy of the telomeric end of chromosome 16. Mol Genet Metab. 2012;105:438–442. doi: 10.1016/j.ymgme.2011.11.196. [DOI] [PubMed] [Google Scholar]
- Cole DE, Fukuda S, Gordon BA, Rip JW, LeCouteur AN, Rupar CA, Tomatsu S, Ogawa T, Sukegawa K, Orii T. Heteroallelic missense mutations of the galactosamine-6-sulfate sulfatase (GALNS) gene in a mild form of Morquio disease (MPS IVA) Am J Med Genet. 1996;63:558–565. doi: 10.1002/(SICI)1096-8628(19960628)63:4<558::AID-AJMG9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cosma MP, Pepe S, Annunziata I, Newbold RF, Grompe M, Parenti G, Ballabio A. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113:445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Diana A, Tesse R, Polizzi AM, Santostasi T, Manca A, Leonetti G, Seia M, Porcaro L, Cavallo L. A large deletion causes apparent homozygosity for the D1152H mutation in the cystic fibrosis transmembrane regulator (CFTR) gene. Gene. 2012;497:90–92. doi: 10.1016/j.gene.2012.01.061. [DOI] [PubMed] [Google Scholar]
- Dierks T, Schmidt B, Borissenko LV, Peng J, Preusser A, Mariappan M, von Figura K. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(alpha)-formylglycine generating enzyme. Cell. 2003;113:435–444. doi: 10.1016/s0092-8674(03)00347-7. [DOI] [PubMed] [Google Scholar]
- Dierks T, Schmidt B, von Figura K. Conversion of cysteine to formylglycine: a protein modification in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1997;94:11963–11968. doi: 10.1073/pnas.94.22.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dung VC, Tomatsu S, Montano AM, Gottesman G, Bober MB, Mackenzie W, Maeda M, Mitchell GA, Suzuki Y, Orii T. Mucopolysaccharidosis IVA: Correlation between genotype, phenotype and keratan sulfate levels. Mol Genet Metab. 2013;110:129–138. doi: 10.1016/j.ymgme.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Tomatsu S, Masuno M, Ogawa T, Yamagishi A, Rezvi GM, Sukegawa K, Shimozawa N, Suzuki Y, Kondo N, Imaizumi K, Kuroki Y, Okabe T, Orii T. Mucopolysaccharidosis IVA: submicroscopic deletion of 16q24.3 and a novel R386C mutation of N-acetylgalactosamine-6-sulfate sulfatase gene in a classical Morquio disease. Hum Mutat. 1996;7:123–134. doi: 10.1002/(SICI)1098-1004(1996)7:2<123::AID-HUMU6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gieselmann V, Fluharty AL, Tonnesen T, Von Figura K. Mutations in the arylsulfatase A pseudodeficiency allele causing metachromatic leukodystrophy. Am J Hum Genet. 1991;49:407–413. [PMC free article] [PubMed] [Google Scholar]
- Hantash FM, Rebuyon A, Peng M, Redman JB, Sun W, Strom CM. Apparent homozygosity of a novel frame shift mutation in the CFTR gene because of a large deletion. J Mol Diagn. 2009;11:253–256. doi: 10.2353/jmoldx.2009.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmatz P, Mengel KE, Giugliani R, Valayannopoulos V, Lin SP, Parini R, Guffon N, Burton BK, Hendriksz CJ, Mitchell J, Martins A, Jones S, Guelbert N, Vellodi A, Hollak C, Slasor P, Decker C. The Morquio A Clinical Assessment Program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109:54–61. doi: 10.1016/j.ymgme.2013.01.021. [DOI] [PubMed] [Google Scholar]
- He D, Huang Y, Ou Z, Sheng H, Li S, Zhao X, Li R, Zheng J, Liu L. Molecular genetic assay of Mucopolysaccharidosis IVA in South China. Gene. 2013;532:46–52. doi: 10.1016/j.gene.2013.08.097. [DOI] [PubMed] [Google Scholar]
- Hendriksz CJ, Harmatz P, Beck M, Jones S, Wood T, Lachman R, Gravance CG, Orii T, Tomatsu S. Review of clinical presentation and diagnosis of Mucopolysaccharidosis IVA. Mol Genet Metab. 2013;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze JP, Tomatsu S, Fujii T, Montano AM, Yamaguchi S, Suzuki Y, Fukushi M, Ishimaru T, Orii T. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark Insights. 2011;6:69–78. doi: 10.4137/BMI.S7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood JJ, Elliott H. Detection of Morquio A syndrome using radiolabelled substrates derived from keratan sulphate for the estimation of galactose 6-sulphate sulphatase. Clin Sci (Lond) 1983;65:325–331. doi: 10.1042/cs0650325. [DOI] [PubMed] [Google Scholar]
- Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal Biochem. 1982;119:120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- Humbel R, Marchal C, Fall M. Diagnosis of Morquio's disease: a simple chromatographic method for the identification of keratosulfate in urine. J Pediatr. 1972;81:107–108. doi: 10.1016/s0022-3476(72)80387-1. [DOI] [PubMed] [Google Scholar]
- Karageorgos L, Brooks DA, Pollard A, Melville EL, Hein LK, Clements PR, Ketteridge D, Swiedler SJ, Beck M, Giugliani R, Harmatz P, Wraith JE, Guffon N, Leao Teles E, Sa Miranda MC, Hopwood JJ. Mutational analysis of 105 Mucopolysaccharidosis type VI patients. Hum Mutat. 2007;28:897–903. doi: 10.1002/humu.20534. [DOI] [PubMed] [Google Scholar]
- Kato Z, Fukuda S, Tomatsu S, Vega H, Yasunaga T, Yamagishi A, Yamada N, Valencia A, Barrera LA, Sukegawa K, Orii T, Kondo N. A novel common missense mutation G301C in the N-acetylgalactosamine-6-sulfate sulfatase gene in Mucopolysaccharidosis IVA. Hum Genet. 1997;101:97–101. doi: 10.1007/s004390050594. [DOI] [PubMed] [Google Scholar]
- Kubaski F, Brusius-Facchin AC, Palhares HM, Balarin MA, Viapiana-Camelier M, Guidobono R, Burin MG, Giugliani R, Leistner-Segal S. Identification of a novel missense mutation in Brazilian patient with a severe form of Mucopolysaccharidosis type IVA. Gene. 2013;517:112–115. doi: 10.1016/j.gene.2012.12.100. [DOI] [PubMed] [Google Scholar]
- Labrousse P, Chien YH, Pomponio RJ, Keutzer J, Lee NC, Akmaev VR, Scholl T, Hwu WL. Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol Genet Metab. 2010;99:379–383. doi: 10.1016/j.ymgme.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Laios E, Glynou K. Allelic drop-out in the LDLR gene affects mutation detection in familial hypercholesterolemia. Clin Biochem. 2008;41:38–40. doi: 10.1016/j.clinbiochem.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Landsverk ML, Douglas GV, Tang S, Zhang VW, Wang GL, Wang J, Wong LJ. Diagnostic approaches to apparent homozygosity. Genet Med. 2012;14:877–882. doi: 10.1038/gim.2012.58. [DOI] [PubMed] [Google Scholar]
- Laradi S, Tukel T, Khediri S, Shabbeer J, Erazo M, Chkioua L, Chaabouni M, Ferchichi S, Miled A, Desnick RJ. Mucopolysaccharidosis type IV: N-acetylgalactosamine-6-sulfatase mutations in Tunisian patients. Mol Genet Metab. 2006;87:213–218. doi: 10.1016/j.ymgme.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Lee NH, Cho SY, Maeng SH, Jeon TY, Sohn YB, Kim SJ, Park HD, Jin DK. Clinical, radiologic, and genetic features of Korean patients with Mucopolysaccharidosis IVA. Korean J Pediatr. 2012;55:430–437. doi: 10.3345/kjp.2012.55.11.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell LA, Cunico RL, Ohh J, Fulkerson W, Furneaux R, Foehr MD. Validation of an LC-MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis. 2011;3:1855–1866. doi: 10.4155/bio.11.172. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- Meyer LR, Zweig AS, Hinrichs AS, Karolchik D, Kuhn RM, Wong M, Sloan CA, Rosenbloom KR, Roe G, Rhead B, Raney BJ, Pohl A, Malladi VS, Li CH, Lee BT, Learned K, Kirkup V, Hsu F, Heitner S, Harte RA, Haeussler M, Guruvadoo L, Goldman M, Giardine BM, Fujita PA, Dreszer TR, Diekhans M, Cline MS, Clawson H, Barber GP, Haussler D, Kent WJ. The UCSC Genome Browser database: extensions and updates 2013. Nucleic Acids Res. 2013;41:D64–D69. doi: 10.1093/nar/gks1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano AM, Kaitila I, Sukegawa K, Tomatsu S, Kato Z, Nakamura H, Fukuda S, Orii T, Kondo N. Mucopolysaccharidosis IVA: characterization of a common mutation found in Finnish patients with attenuated phenotype. Hum Genet. 2003;113:162–169. doi: 10.1007/s00439-003-0959-8. [DOI] [PubMed] [Google Scholar]
- Montano AM, Sukegawa K, Kato Z, Carrozzo R, Di Natale P, Christensen E, Orii KO, Orii T, Kondo N, Tomatsu S. Effect of 'attenuated' mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J Inherit Metab Dis. 2007a;30:758–767. doi: 10.1007/s10545-007-0702-z. [DOI] [PubMed] [Google Scholar]
- Montano AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am J Med Genet A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007b;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hattori K, Endo F. Newborn screening for lysosomal storage disorders. Am J Med Genet C Semin Med Genet. 2011;157C:63–71. doi: 10.1002/ajmg.c.30291. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Tomatsu S, Hori T, Fukuda S, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IV A: molecular cloning of the human N-acetylgalactosamine-6-sulfatase gene (GALNS) and analysis of the 5'-flanking region. Genomics. 1994;20:99–104. doi: 10.1006/geno.1994.1132. [DOI] [PubMed] [Google Scholar]
- Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A. 2003;123A:310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–446. doi: 10.1101/gr.212802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Tomatsu S, Fukuda S, Yamagishi A, Rezvi GM, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Oru T. Mucopolysaccharidosis IVA: screening and identification of mutations of the N-acetylgalactosamine-6-sulfate sulfatase gene. Hum Mol Genet. 1995;4:341–349. doi: 10.1093/hmg/4.3.341. [DOI] [PubMed] [Google Scholar]
- Ogino S, Gulley ML, den Dunnen JT, Wilson RB. Association for Molecular Patholpogy Training and Education Committtee Standard mutation nomenclature in molecular diagnostics: practical and educational challenges. J Mol Diagn. 2007;9:1–6. doi: 10.2353/jmoldx.2007.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- Pajares S, Alcalde C, Couce ML, Del Toro M, Gonzalez-Meneses A, Guillen E, Pineda M, Pintos G, Gort L, Coll MJ. Molecular analysis of Mucopolysaccharidosis IVA (Morquio A) in Spain. Mol Genet Metab. 2012;106:196–201. doi: 10.1016/j.ymgme.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Panin G, Naia S, Dall'Amico R, Chiandetti L, Zachello F, Catassi C, Felici L, Coppa GV. Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulfates. Clin Chem. 1986;32:2073–2076. [PubMed] [Google Scholar]
- Park HD, Ko AR, Ki CS, Lee SY, Kim JW, Cho SY, Kim SH, Park SW, Sohn YB, Jin DK. Five novel mutations of GALNS in Korean patients with mucopolysaccharidosis IVA. Am J Med Genet A. 2013;161A:509–517. doi: 10.1002/ajmg.a.35298. [DOI] [PubMed] [Google Scholar]
- Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcao A, Ribeiro I, Lacerda L, Ribeiro G, Amaral O, Sa Miranda MC. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2003;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- Pshezhetsky AV, Potier M. Association of N-acetylgalactosamine-6-sulfate sulfatase with the multienzyme lysosomal complex of beta-galactosidase, cathepsin A, and neuraminidase. Possible implication for intralysosomal catabolism of keratan sulfate. J Biol Chem. 1996;271:28359–28365. doi: 10.1074/jbc.271.45.28359. [DOI] [PubMed] [Google Scholar]
- Qubbaj W, Al-Aqeel AI, Al-Hassnan Z, Al-Duraihim A, Awartani K, Al-Rejjal R, Coskun S. Preimplantation genetic diagnosis of Morquio disease. Prenat Diagn. 2008;28:900–903. doi: 10.1002/pd.2081. [DOI] [PubMed] [Google Scholar]
- Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, Lyon E, Ward BE. Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008;10:294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- Rivera-Colón Y, Schutsky EK, Kita AZ, Garman SC. The Structure of Human GALNS Reveals the Molecular Basis for Mucopolysaccharidosis IV A. J Mol Biol. 2012;423:736–751. doi: 10.1016/j.jmb.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romdhane L, Kefi R, Azaiez H, Ben Halim N, Dellagi K, Abdelhak S. Founder mutations in Tunisia: implications for diagnosis in North Africa and Middle East. Orphanet J Rare Dis. 2012;7 doi: 10.1186/1750-1172-7-52. 52-1172-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, Gelb MH, Turecek F. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki GA, Martin KW, Theroux MC, Lampe C, White KK, Shediac R, Lampe CG, Beck M, Mackenzie WG, Hendriksz CJ, Harmatz PR. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): presentation, diagnosis and management. J Inherit Metab Dis. 2013;36:339–355. doi: 10.1007/s10545-013-9586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzioglu M, Tokatli A, Coskun T, Emre S. Molecular analysis of Turkish mucopolysaccharidosis IVA (Morquio A) patients: identification of novel mutations in the N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene. Hum Mutat. 2002;20:477–478. doi: 10.1002/humu.9088. [DOI] [PubMed] [Google Scholar]
- Thomas GH. "Pseudodeficiencies" of lysosomal hydrolases. Am J Hum Genet. 1994;54:934–940. [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Dieter T, Schwartz IV, Sarmient P, Giugliani R, Barrera LA, Guelbert N, Kremer R, Repetto GM, Gutierrez MA, Nishioka T, Serrato OP, Montano AM, Yamaguchi S, Noguchi A. Identification of a common mutation in mucopolysaccharidosis IVA: correlation among genotype, phenotype, and keratan sulfate. J Hum Genet. 2004a;49:490–494. doi: 10.1007/s10038-004-0178-8. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Filocamo M, Orii KO, Sly WS, Gutierrez MA, Nishioka T, Serrato OP, Di Natale P, Montano AM, Yamaguchi S, Kondo N, Orii T, Noguchi A. Mucopolysaccharidosis IVA (Morquio A): identification of novel common mutations in the N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene in Italian patients. Hum Mutat. 2004b;24:187–188. doi: 10.1002/humu.9265. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, Kida K, Shibata Y, Futatsumori H, Montano AM, Mason RW, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Fukuda S, Cooper A, Wraith JE, Ferreira P, Di Natale P, Tortora P, Fujimoto A, Kato Z, Yamada N, Isogai K, Yamagishi A, Sukegawa K, Suzuki Y, Shimozawa N, Kondo N, Sly WS, Orii T. Fourteen novel mucopolysaccharidosis IVA producing mutations in GALNS gene. Hum Mutat. 1997;10:368–375. doi: 10.1002/(SICI)1098-1004(1997)10:5<368::AID-HUMU6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi GM, Yamagishi A, Yamada N, Kato Z, Isogai K, Sukegawa K. Mucopolysaccharidosis type IVA: identification of six novel mutations among non-Japanese patients. Hum Mol Genet. 1995;4:741–743. doi: 10.1093/hmg/4.4.741. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fukuda S, Masue M, Sukegawa K, Fukao T, Yamagishi A, Hori T, Iwata H, Ogawa T, Nakashima Y. Morquio disease: isolation, characterization and expression of full-length cDNA for human N-acetylgalactosamine-6-sulfate sulfatase. Biochem Biophys Res Commun. 1991;181:677–683. doi: 10.1016/0006-291x(91)91244-7. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fukuda S, Yamagishi A, Cooper A, Wraith JF, Hori T, Kato Z, Yamada N, Isogai K, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IVA: four new exonic mutations in patients with N-acetylgalactosamine-6-sulfate sulfatase deficiency. Am J Hum Genet. 1996;58:950–962. [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Kida K, Kubota M, Barrera L, Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010;333(Suppl):S35–S42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montano AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Nishioka T, Montaño AM, Gutierrez MA, Pena OS, Orii KO, Sly WS, Yamaguchi S, Orii T, Paschke E, Kircher SG, Noguchi A. Mucopolysaccharidosis IVA: identification of mutations and methylation study in GALNS gene. Journal of Medical Genetics. 2004c;41:e98–e98. doi: 10.1136/jmg.2003.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res. 2004d;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Nishioka T, Gutierrez MA, Peña OM, Tranda firescu GG, Lopez P, Yamaguchi S, Noguchi A, Orii T. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum Mutat. 2005;26:500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- Tylki-Szymanska A, Czartoryska B, Bunge S, van Diggelen OP, Kleijer WJ, Poorthuis BJ, Huijmans JG, Gorska D. Clinical, biochemical and molecular findings in a two-generation Morquio A family. Clin Genet. 1998;53:369–374. doi: 10.1111/j.1399-0004.1998.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Villani GR, Grosso M, Pontarelli G, Chierchia A, Sessa R, Sibilio M, Parenti G, Di Natale P. Large deletion involving exon 5 of the arylsulfatase B gene caused apparent homozygosity in a mucopolysaccharidosis type VI patient. Genet Test Mol Biomarkers. 2010;14:113–120. doi: 10.1089/gtmb.2009.0138. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang W, Wang Y, Meng Y, Su L, Shi H, Huang S. Mucopolysaccharidosis IVA mutations in Chinese patients: 16 novel mutations. J Hum Genet. 2010;55:534–540. doi: 10.1038/jhg.2010.65. [DOI] [PubMed] [Google Scholar]
- Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin Chem. 1989a;35:2074–2081. [PubMed] [Google Scholar]
- Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989b;35:374–379. [PubMed] [Google Scholar]
- Wood TC, Harvey K, Beck M, Burin MG, Chien YH, Church HJ, D'Almeida V, van Diggelen OP, Fietz M, Giugliani R, Harmatz P, Hawley SM, Hwu WL, Ketteridge D, Lukacs Z, Miller N, Pasquali M, Schenone A, Thompson JN, Tylee K, Yu C, Hendriksz CJ. Diagnosing mucopolysaccharidosis IVA. J Inherit Metab Dis. 2013;36:293–307. doi: 10.1007/s10545-013-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Fukuda S, Tomatsu S, Muller V, Hopwood JJ, Nelson J, Kato Z, Yamagishi A, Sukegawa K, Kondo N, Orii T. Molecular heterogeneity in mucopolysaccharidosis IVA in Australia and Northern Ireland: nine novel mutations including T312S, a common allele that confers a mild phenotype. Hum Mutat. 1998;11:202–208. doi: 10.1002/(SICI)1098-1004(1998)11:3<202::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yang CF, Tsai FJ, Lin SP, Lee CC, Wu JY. A novel in-frame deletion mutation (c106-111del) identified in a Taiwan Chinese patient with type IVA mucopolysaccharidosis. Hum Mutat. 2001;18:254. doi: 10.1002/humu.1187. [DOI] [PubMed] [Google Scholar]
- Ye J, Lei HL, Zhang HW, Qiu WJ, Han LS, Wang Y, Li XY, Gu XF. Analysis of GALNS gene mutation in thirty-eight Chinese patients with mucopolysaccharidosis type IVA. Zhonghua Er Ke Za Zhi. 2013;51:414–419. [PubMed] [Google Scholar]
- Zanetti A, Ferraresi E, Picci L, Filocamo M, Parini R, Rosano C, Tomanin R, Scarpa M. Segregation analysis in a family at risk for the Maroteaux-Lamy syndrome conclusively reveals c.1151G>A (p.S384N) as to be a polymorphism. Eur J Hum Genet. 2009;17:1160–1164. doi: 10.1038/ejhg.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Van Diggelen OP, Thoomes R, Huijmans J, Young E, Mazurczak T, Kleijer WJ. Prenatal diagnosis of Morquio disease type A using a simple fluorometric enzyme assay. Prenat Diagn. 1990;10:85–91. doi: 10.1002/pd.1970100204. [DOI] [PubMed] [Google Scholar]
- Zlotogora J, Bach G. Deficiency of lysosomal hydrolases in apparently healthy individuals. Am J Med Genet. 1983;14:73–80. doi: 10.1002/ajmg.1320140112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.