Abstract
Non-inflammatory subsynovial connective tissue (SSCT) fibrosis with nerve compression is a prominent feature of carpal tunnel syndrome (CTS). Studies have shown that SSCT matrix synthesis and material property changes in CTS are associated with increased activity of transforming growth factor β (TGF-β1). This study’s aims were to 1) investigate the ability of SSCT fibroblasts from CTS patients and unaffected individuals to contract a collagen gel ring and 2) determine how the addition of TGF-β1 affects this ability. SSCT fibroblasts from 3 normal cadavers and 3 age-matched female patients who had undergone surgery for CTS were used. Results showed patient cell seeded gels had a significantly higher contraction rate (p<0.001) than control cells and fully contracted gel rings possessed a significantly higher tensile strength (p=0.003) and stiffness (p<0.001). Furthermore, TGF-β1 significantly intensified contraction rate (p<0.001), tensile strength (p<0.001) and stiffness (p<0.001). In conclusion, SSCT cells from normal donors and CTS patients contract collagen gel rings differently, and this ability is affected by TGF-β1 treatment. This cell-seeded collagen gel model may be useful for developing methods of stopping or eliminating the effect of TGF-β1 effects on the SSCT fibroblasts and surrounding matrix, which might aid identification of medical treatments of CTS.
Keywords: Carpal Tunnel Syndrome, Subsynovial Connective Tissue, Collagen Gel Contraction, TGF-β1, Fibrosis
INTRODUCTION
Carpal tunnel syndrome (CTS) is one of the most common clinical hand conditions, affecting nearly 10 million people annually in the United States.1,2 Surgery is the most effective treatment, with as many as 500,000 carpal tunnel release procedures performed annually in the United States.2,3 CTS can be associated with repetitive manual activities,4,5 anatomic anomalies,6,7 autoimmune or hematologic disorders,8,9 arthritis,10 trauma11 or neoplasms.12 While etiology is unknown in most cases,13,14 studies have shown that symptoms and subsequent work-related disabilities15 are created by increased pressure within the carpal tunnel, which causes compression and ischemia of the median nerve.16-18
Non-inflammatory subsynovial connective tissue (SSCT) fibrosis is the most common histopathologic finding reported in patients with CTS.19,20 Moreover, studies of median nerve motion in idiopathic CTS patients and control subjects have clearly shown that nerve mobility is hampered in patients.17,21 SSCT fibrosis is also known to be associated with elevated activity of transforming growth factor β (TGF-β1), which can upregulate fibroblast proliferation and activation. TGF-β1 stimulates membrane-cytoskeletal structural protein formation, which mechanically generates and transmits contractile force to the extracellular matrix (ECM).14,22,23 Moreover, many studies have demonstrated that TGF-β1 signaling plays a central role in fibrosis. TGF-β1 is one of the most common profibrogenic cytokines and can induce and regulate ECM synthesis through a variety of signaling pathways.24,25
The gel contraction model, using a collagen lattice matrix with embedded cells is useful to study mechanisms of ECM contraction.26 The gel contraction process has characteristics similar to wound healing in vivo and also provides a convenient and quantitative method for examining the contractibility of cells in vitro.22,26,27
In this study, we used a cell-seeded collagen gel contraction model to assess the gel contraction rate and the material properties of the contracted gel ring. Our main objectives were 1) to compare the interactions of patient SSCT fibroblasts in a collagen type I matrix compared to those of normal human SSCT fibroblasts and 2) to determine the effect of treatment with TGF-β1 on these interactions.
MATERIALS AND METHODS
Harvesting and Culturing of SSCT Cells
Tissue harvest procedures in this study were conducted with approval of our Institutional Review Board. SSCT tissue, the source of fibroblast cells for this study, was harvested from the operated hands of three female patients with idiopathic CTS, aged 52, 59 and 63 years. Tissue was similarly harvested from the hands of fresh female cadavers with no antecedent history of CTS, with donor ages of 55, 57 and 67. Cadaver tissues were acquired within 12 hours after death. Exclusion criteria for both patients and cadavers included a history of prior carpal tunnel release, volar wrist surgery, corticosteroid injection into the carpal tunnel or known hand/wrist tumor or deformity. Patients and cadavers were also excluded if they had any of the following clinical diagnoses or conditions: cervical radiculopathy, inflammatory arthritis, osteoarthritis in the wrist, flexor tendinitis, hemodialysis, obesity (body mass index >30 kg/m2), sarcoidosis, peripheral nerve disease, metabolic disorder, amyloidosis, or major trauma to the ipsilateral wrist.
All cell cultures, harvested tissues and treatments were grown with minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY), supplemented with 10% fetal bovine serum (FBS) (GIBCO) and 1% antibiotics (Antibiotic-Antimycotic, GIBCO). Harvested tissue was minced and plated in tissue culture dishes (10 cm2) at 37°C, 5% CO2 humidified atmosphere. Media was changed every 3 days. At near confluence, the cells were passaged. Cells of passages 3 to 5 were used in all experiments.
Preparation of SSCT Cell-populated Collagen Gel
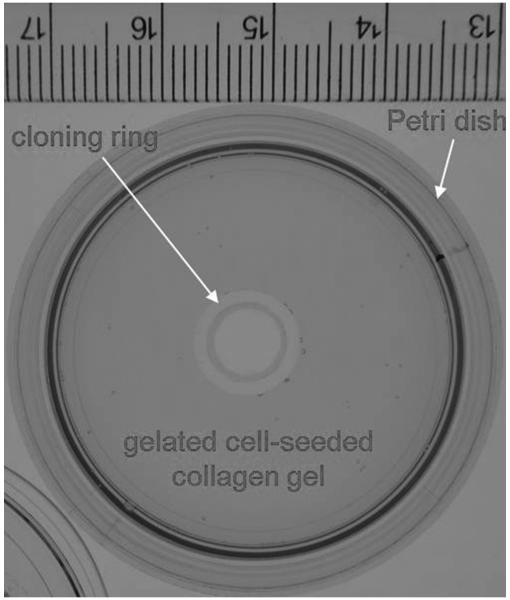
The undersides of small, sterile cloning rings (8-mm outer diameter, 8-mm height) were coated with sterile vacuum grease (Dow Corning Corporation, Midland, MI) and affixed to the center of 3.5-cm Petri dishes for future use. Collagen gels were prepared as previously described.28 Briefly, 1.88 ml of sterile, chilled Vitrogen sterile type I bovine dermal collagen (Cohesion Technologies, Palo Alto, CA) was mixed with 1.2 ml of sterile 5X MEM and 2.92 ml of distilled water to make a 1.0 mg/ml collagen/MEM solution at a pH of 7.4 ± 0.2 on ice. Based on a previous study,29 SSCT fibroblast cells were seeded and mixed into the Vitrogen collagen to make a final cell-seeded collagen solution with1.0 × 106 SSCT cells/ml in 0.5 mg/ml collagen/MEM solution at room temperature. Aliquots of 2 ml of the cell-seeded collagen solution were added to each of 4 dishes, and the solution was placed in the space between the inner wall of the well and the outer wall of the inner cloning ring, to create a ring-shaped gel within each well. The collagen gel rings were incubated at 37°C in a 5% CO2 humidified incubator for one hour to allow for gelation (Figure 1). After gelation, the cell-seeded gel was then physically detached from the outer well wall by running a sterile scalpel along the plate wall to release the gel to facilitate subsequent contraction. Concurrently, 5 ng/ml TGF-β1-supplemented media (recombinant human TGF-β1, R&D Systems, Minneapolis, MN) were also prepared for future use. Gels were treated with 5 ng/mL TGF-β1 or vehicle media. Briefly, 2 ml of media without TGF-β1 supplementation was added to two dishes of each cell-seeded collagen gel sample set, and 2 ml of 5 ng/ml TGF-β1-supplemented media were added to an additional two dishes of each cell-seeded collagen gel sample set. Gels were incubated at 37°C in a 5% CO2 humidified incubator with media changed every other day.
Figure 1.
Petri dish with central cloning ring and gelated cell-seeded collagen gel.
Quantification and Mechanical Testing of Gel Contraction
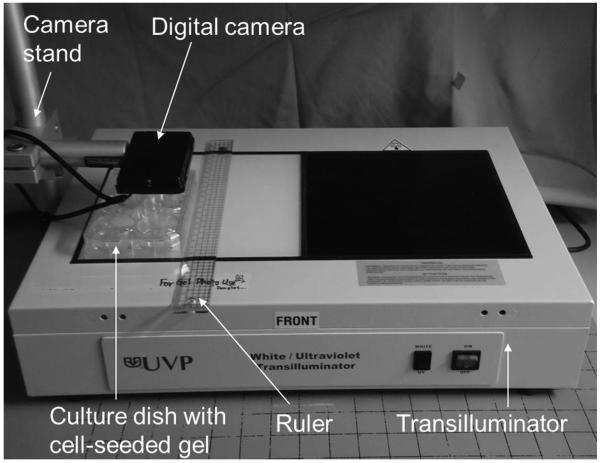
Gel contraction was assessed through measurement of the gel surface area in each dish every 4 hours for 3 days. Dishes containing gels were placed on a transilluminator (UVP, Upland, CA) along with a metric scale and photographed with a digital camera (DSC-TX9, Sony Corp., Tokyo, Japan) at a resolution of 2560 × 1920 pixels (Figure 2). Gel surface area was calculated from the digital images with a custom automatic and quantitative measurement program.30 Gel surface area was expressed as a percentage of the initial surface area, and temporal changes in the quantity were used to assess gel contraction rate. Contraction was defined as complete when the gel contracted to 5% of the initial surface area.
Figure 2.
Experimental set-up for digital image capture of contracted gel surface area.
Area data from the first reading to the point of complete contraction were modeled with linear regression and optimization to fit an exponential decay function of time, t, (Equ. 1), where in this model A0 is the initial area (t = 0); B is the decay time constant; and C is the non-zero asymptote as t→∞. The decay time constant, B, is directly proportional to the contraction rate of the gel.
| (Equ. 1) |
Since Equ.1 cannot be directly linearized for regression analysis, a linear, but parametric, system of equations was created to solve for the constants A0, B, and C (Equ. 2). The system of equations was solved iteratively by varying the value of the non-zero asymptote, C, by increments of 0.01% of the total area with the best fit identified when the squared error between the data and the regression model was minimized.
| (Equ. 2) |
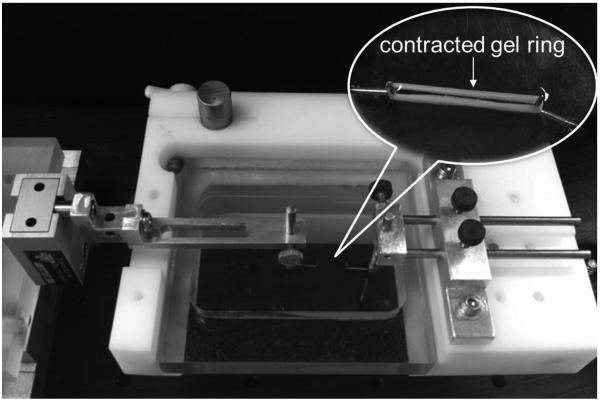
At the end of the contraction period (3rd day), the collagen ring was removed from the culture dish. The stiffness and tensile strength of the contracted gel rings were determined by uniaxial tensile testing to failure under displacement control at a distraction rate of 0.5 mm/sec. A custom-built mechanical system (Figure 3) was used to perform the tests. The test system was composed of a 150-g load cell (GSO-150, Transducer Techniques, Temecula, CA) and a stepper-motor-powered linear actuator driven by a microcontroller/driver (ACE-SDE, Arcus Technology, Livermore, CA). The collagen ring was carefully looped over two 0.6-mm-diameter hooks mounted on the testing machine. During testing, the specimen was immersed in a room-temperature buffer solution, phosphate buffered saline (PBS) (GIBCO), to retain moisture. Force and displacement data were recorded at a sample rate of 10 Hz.
Figure 3.
Configuration of custom-built mechanical test system for mechanical testing of gel ring. The gel ring was looped onto two hooks mounted on the test system.
Statistical Considerations
Each of the four groups (CTS patient cells and normal control cells treated with unsupplemented media, CTS patient cells and normal control cells treated with TGF-β1-supplemented media) included 3 samples (n=3), with duplicate gel contraction tests per group. The measured outcomes were the decay time constant, tensile strength and stiffness. All measurements were expressed as a mean and standard deviation (SD). Separate analyses were performed for each outcome. The analyses were conducted using two-factor analysis of variance in a generalized linear model utilizing generalized estimating equations (GEE) to account for the within-sample correlation (since each CTS patient or control contributed 4 samples - two to TGF-β1 and two to unsupplemented media). No significant interaction was observed between cell type and TGF-β1 treatment type for any of the outcomes; therefore, the two factors included in the final model for each outcome were cell type (patient group vs. control group), and TGF-β1 treatment type (treatment with TGF-β1 vs. treatment without TGF-β1). In addition, the student’s t-test was performed to evaluate differences between the four groups. P-values ≤ 0.05 indicated a significant test result. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc. Cary, NC).
RESULTS
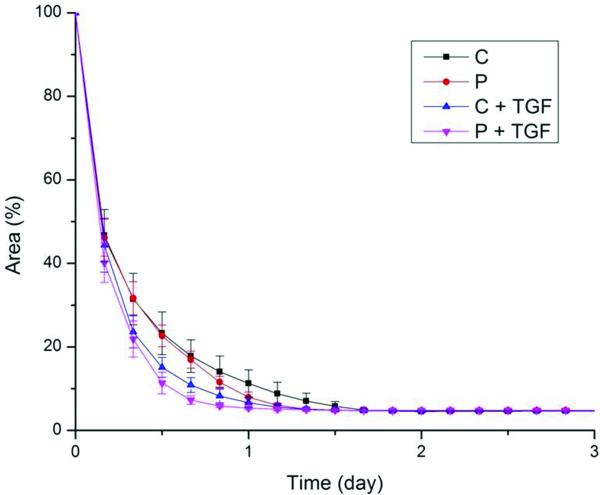
The majority of the cell-seeded collagen gels contracted completely within 2 days, as can be observed in Figure 4. Initial gels were typically translucent and covered the culture plate evenly. Gels condensed radially and became less translucent with time. By the third day, the gel had condensed tightly around the cloning ring, resembling a small rubber band.
Figure 4.
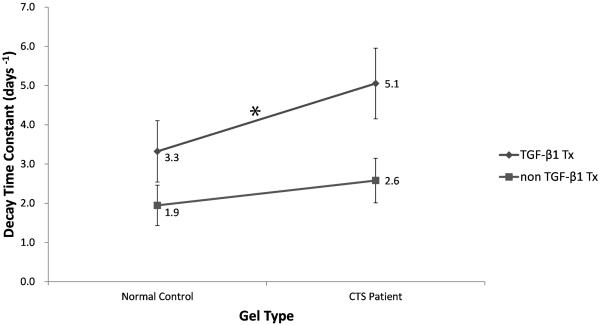
Contraction behavior of cell-seeded collagen gels for different treatment groups. The mean decay time constant was significantly higher in gels seeded with CTS patient cells than in gels seeded with normal control cells. Moreover, TGF-β1 significantly enhanced those results. (C, control group; P, patient group; C + TGF, control group with TGF-β1 treatment; P + TGF, patient group with TGF-β1 treatment). Bars represent standard deviation, SD.
The outcomes organized by group and by factors are shown in Table 1 and Table 2, respectively. Both cell type and treatment type had significant effects on the decay time constant. The mean decay time constant was higher in gels seeded with CTS patient cells than those seeded with normal control cells (3.8 days−1 vs. 2.6 days−1, p<0.001). The mean decay time constant was also higher for cells treated with TGF-β1-supplemented media than for those treated with the media lacking TGF-β1 (4.3 days−1 vs. 2.3 days−1, p<0.001).
Table 1.
Summary outcomes of analysis of cell type and treatment type, by group.
| Outcome | Group | Mean (SD) | p-value |
|---|---|---|---|
| Decay time constant (days−1) | Control without TGF-β1 | 1.9 (0.5) | 0.0707 |
| CTS patient without TGF-β1 | 2.6 (0.6) | ||
| Control with TGF-β1 | 3.3 (0.8) | 0.0052 | |
| CTS patient with TGF-β1 | 5.1 (0.9) | ||
| Tensile strength (mN) | Control without TGF-β1 | 5.1 (0.8) | 0.0059 |
| CTS patient without TGF-β1 | 7.0 (1.0) | ||
| Control with TGF-β1 | 11.1 (1.5) | 0.0282 | |
| CTS patient with TGF-β1 | 13.0 (1.1) | ||
| Stiffness (mN/mm) | Control without TGF-β1 | 1.4 (0.4) | 0.0580 |
| CTS patient without TGF-β1 | 1.8 (0.4) | ||
| Control with TGF-β1 | 3.1 (0.6) | 0.0027 | |
| CTS patient with TGF-β1 | 4.5 (0.6) |
Table 2.
Summary outcomes of analysis of cell type and treatment type, by factor.
| Outcome | Factor | Level | Mean (SD) | p-value |
|---|---|---|---|---|
| Decay time constant (days−1) | Cell type | CTS patient | 3.8 (1.5) | <0.001 |
| Control | 2.6 (1.0) | |||
| Treatment type | TGF-β1 | 4.2 (1.2) | <0.001 | |
| No TGF-β1 | 2.3 (0.6) | |||
| Tensile strength (mN) | Cell type | CTS patient | 10.0 (3.3) | 0.003 |
| Control | 8.1 (3.3) | |||
| Treatment type | TGF-β1 | 12.1 (1.6) | <0.001 | |
| No TGF-β1 | 6.1 (1.4) | |||
| Stiffness (mN/mm) | Cell type | CTS patient | 3.2 (1.5) | <0.001 |
| Control | 2.2 (1.0) | |||
| Treatment type | TGF-β1 | 3.8 (0.9) | <0.001 | |
| No TGF-β1 | 1.6 (0.4) |
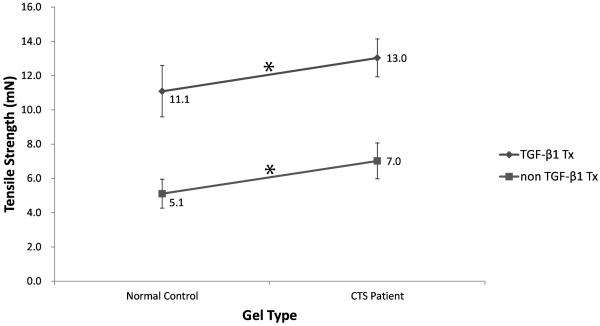
Similar findings were observed for tensile strength. Gels seeded with CTS patient cells had a significantly higher mean tensile strength than the gels seeded with normal control cells, regardless of treatment type (10.0 mN vs. 8.1 mN, p=0.003). Likewise, cells treated with TGF-β1-supplemented media had a higher mean tensile strength than cells treated with unsupplemented media (12.1 mN vs. 6.1 mN, p<0.001).
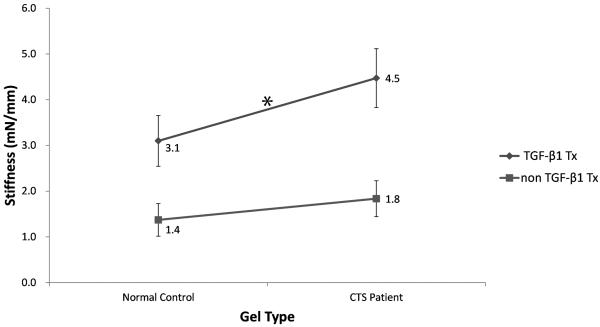
Cell type and treatment type also had significant effects on stiffness. The mean stiffness value was significantly higher in gels seeded with CTS patient cells than in gels seeded with normal control cells across both treatments (3.2 mN/mm vs. 2.2 mN/mm, p<0.001). The mean stiffness value was also significantly higher in gels treated with TGF-β1-supplemented media than in those treated with unsupplemented media for both types of cells (3.8 mN/mm vs. 1.6 mN/mm, p<0.001).
Comparisons of significant changes between the four groups (CTS patient cells and normal control cells treated with unsupplemented media and CTS patient cells and normal control cells treated with TGF-β1-supplemented media) are shown in Figures 5, 6 and 7. The rate of contraction in patient cells was higher than that in normal control cells (p=0.0707), and this ability was significantly enhanced by TGF-β1 (p=0.0052) (Fig. 5). The tensile strength of gels with patient cells were significantly higher than that of gels with normal control cells (p=0.0059), and the addition of TGF-β1 significantly increased tensile strength in both cell types (p=0.0282) (Fig 6). The stiffness of gels with patient cells were substantially higher than that of gels with normal control cells (p=0.058) and TGF-β1 treatment significantly increased the stiffness in both cell types (p=0.0027) (Fig 7).
Figure 5.
Interaction diagram showing the differences in mean decay time constant by group. There were significant differences between TGF-β1 treated CTS patient and control cells (*, p<0.05) but not between cell type and TGF-β1 treatment type.
Figure 6.
Interaction diagram showing the differences in mean tensile strength by group. Values for gels seeded with normal control cells and CTS patient cells were significantly different within the TGF-β1 treatment group and within the untreated group (*, p<0.05), but not between cell type and TGF-β1 treatment type.
Figure 7.
Interaction diagram showing the differences in mean stiffness by group. Values for gels seeded with normal control cells and CTS patient cells were significantly different within the TGF-β1 treatment group (*, p<0.05), but not between cell type and TGF-β1 treatment type.
DISCUSSION
Hypertrophic collagen fibers, increased fibroblastic density, hypervascularization, and significant deposition of collagen types III and VI have been observed in the SSCT of CTS patients in several studies.14,31 In addition, evidence exists supporting the idea that a potential factor in the development of CTS is impairment of median nerve mobility, specifically restriction of the normal dorsal motion of the nerve during pinch and grip with the wrist flexed.17,21 This impairment can be correlated with the non-inflammatory fibrosis known to occur in the SSCT of CTS patients. Thus, a better understanding of the mechanisms controlling SSCT fibrosis in CTS is important. If these mechanisms are better understood, then it may be possible to arrest or reverse them with medical treatment and avoid the need for surgery. Besides, the material properties of the SSCT have been shown to differ between normal individuals and CTS patients. One study revealed increased shear modulus and pull-out force32,33 at the tissue level in CTS-affected SSCT. Clearly, the changes in these material properties are driven by the action of the cells within the SSCT. A better understanding of the factors that regulate the ability of the SSCT cells to interact with the surrounding ECM is therefore a key step in devising treatments that might arrest or reverse these interactions and restore SSCT to a more normal state. This was one of our main objectives.
Type I collagen is the most abundant protein in the human body and is one of the main components of the extracellular matrix in the SSCT.34 Type I collagen is also the major structure in the extracellular matrix that provides the matrix with its tensile strength.35 Therefore, we believe that the cell-seeded type I collagen was an appropriate matrix selection for this gel contraction study.
The contraction rate of the SSCT fibroblast-seeded gels in the present study was faster than that observed by Chen et al. in 2007,29 despite the use of nearly identical conditions, with 1.0 × 106 cells/ml solution in a 0.5 mg/ml collagen/MEM solution. We attribute this difference to cell type. SSCT fibroblasts from CTS patients and human cadavers were used in this study, while Chen et al. used dog endotenon cells.
We observed that the rate of contraction was significantly faster in the patient group than in the control group. Our mechanical testing results, which also showed that the patient group was significantly stronger and stiffer than the control group, are compatible with the results of Osamura et al. and Ettema et al., who showed that the SSCT of CTS patients was stiffer and had denser collagen networks than unaffected SSCT.32,33 Furthermore, the rate of contraction, tensile strength and stiffness were all significantly intensified by treatment with TGF-β1 in both patient and control groups. These results are consistent with those from studies on the effect of TGF-β1 on cellular processes, including synthesis and remodeling. These processes involve the enhancement of concentration and contractile machinery of fibroblasts as well as consequential force generation and transmission by ECM cytoskeletal protein synthesis and integrin expression.22,24,25,36,37
This study has some limitations. First, the sample size was small. Although small, there was a gender and age match of patient and control cells and data was normally distributed, providing confidence in our statistical comparisons. Second, this work focused only on mechanical changes in our cell-seeded contraction model. Third, we did not assess the fibrotic phenotype of our cells after passage. However, in a parallel project38 we have shown that a fibrotic phenotype is maintained after up to five passages in the CTS derived cells. Future work will explore biological mechanisms of action evaluating differences in gene expression, matrix organization and interaction with cells and matrix degradation. This will enable researchers to investigate and understand the collagen reorganization and regulation in the ECM, which may provide insight into the mechanism of SSCT pathology and treatment.
In summary, since patient cells contracted more quickly and increased tensile strength and stiffness in the collagen matrix, a clear difference in the mechanical behavior between SSCT fibroblasts from CTS patients and normal control cells was found in this model. This phenomenon reflects the fact that CTS cells created a more tightly bound collagen matrix in this model, which is also observed in living subjects stricken with this disease. In addition, cells were treated with TGF-β1, a cytokine found over-expressed in CTS, to reproduce the condition that was upregulated in patient SSCT. This treatment was found to affect and exacerbate this response in both cell types. So we believe that this cell-seeded collagen gel model may be a useful testing platform for screening treatments that might limit pathological contraction of the SSCT that occurs during SSCT fibrosis in patients with CTS and for assessing the cellular mechanisms of SSCT cell-matrix interaction.
Acknowledgements
This study was supported by NIH/NIAMS AR49823, F32AR49823, T32AR056950 and a 2013 ASSH Basic Science Grant.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelfman R, Melton LJ, 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72(1):33–41. doi: 10.1212/01.wnl.0000338533.88960.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer DH, Hanrahan LP. Social and economic costs of carpal tunnel surgery. Instr Course Lect. 1995;44:167–172. [PubMed] [Google Scholar]
- 4.Kutluhan S, Akhan G, Demirci S, Duru S, Koyuncuoglu HR, Ozturk M, Cirak B. Carpal tunnel syndrome in carpet workers. Int Arch Occup Environ Health. 2001;74(6):454–457. doi: 10.1007/s004200100246. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong TJ. Ergonomics and cumulative trauma disorders. Hand Clin. 1986;2(3):553–565. [PubMed] [Google Scholar]
- 6.Amadio PC. Bifid Median Nerve with a Double Compartment within the Transverse Carpal Canal. J Hand Surg-Am. 1987;12A(3):366–368. doi: 10.1016/s0363-5023(87)80005-9. [DOI] [PubMed] [Google Scholar]
- 7.Barfred T, Ipsen T. Congenital carpal tunnel syndrome. J Hand Surg Am. 1985;10(2):246–248. doi: 10.1016/s0363-5023(85)80114-3. [DOI] [PubMed] [Google Scholar]
- 8.Levy M, Pauker M. Carpal-Tunnel Syndrome Due to Thrombosed Persisting Median Artery - Case-Report. Hand. 1978;10(1):65–68. doi: 10.1016/s0072-968x(78)80028-x. [DOI] [PubMed] [Google Scholar]
- 9.Sidiq M, Kirsner AB, Sheon RP. Carpal tunnel syndrome. First manifestation of systemic lupus erythematosus. JAMA. 1972;222(11):1416–1417. doi: 10.1001/jama.222.11.1416. [DOI] [PubMed] [Google Scholar]
- 10.Bardin T, Zingraff J, Shirahama T, Noel LH, Droz D, Voisin MC, Drueke T, Dryll A, Skinner M, Cohen AS. Hemodialysis-associated amyloidosis and beta-2 microglobulin. Clinical and immunohistochemical study. Am J Med. 1987;83(3):419–424. doi: 10.1016/0002-9343(87)90750-9. others. [DOI] [PubMed] [Google Scholar]
- 11.Imran D, Bainbridge LC. Carpal tunnel syndrome after distal release of the flexor digitorum profundus and subsequent retraction of the lumbrical muscle into the carpal tunnel. J Hand Surg-Br. 1999;24B(3):303–304. doi: 10.1054/jhsb.1999.0061. [DOI] [PubMed] [Google Scholar]
- 12.Amadio PC, Reiman HM, Dobyns JH. Lipofibromatous hamartoma of nerve. J Hand Surg Am. 1988;13(1):67–75. doi: 10.1016/0363-5023(88)90203-1. [DOI] [PubMed] [Google Scholar]
- 13.Stevens JC, Sun S, Beard CM, O’Fallon WM, Kurland LT. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38(1):134–138. doi: 10.1212/wnl.38.1.134. [DOI] [PubMed] [Google Scholar]
- 14.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A(7):1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Blanc PD, Faucett J, Kennedy JJ, Cisternas M, Yelin E. Self-reported carpal tunnel syndrome: predictors of work disability from the National Health Interview Survey Occupational Health Supplement. Am J Ind Med. 1996;30(3):362–368. doi: 10.1002/(SICI)1097-0274(199609)30:3<362::AID-AJIM16>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380–383. [PubMed] [Google Scholar]
- 17.Zhao C, Ettema AM, Berglund LJ, An KN, Amadio PC. Gliding resistance of flexor tendon associated with carpal tunnel pressure: a biomechanical cadaver study. J Orthop Res. 2011;29(1):58–61. doi: 10.1002/jor.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanz J, Lizaur A, Sanchez Del, Campo F. Postoperative changes of carpal canal pressure in carpal tunnel syndrome: a prospective study with follow-up of 1 year. J Hand Surg Br. 2005;30(6):611–614. doi: 10.1016/j.jhsb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Donato G, Galasso O, Valentino P, Conforti F, Zuccala V, Russo E, Maltese L, Perrotta I, Tripepi S, Amorosi A. Pathological findings in subsynovial connective tissue in idiopathic carpal tunnel syndrome. Clin Neuropathol. 2009;28(2):129–135. doi: 10.5414/npp28129. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs PC, Nathan PA, Myers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg Am. 1991;16(4):753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- 21.Ettema AM, Zhao C, Amadio PC, O’Byrne MM, An KN. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: a pilot study. Clin Anat. 2007;20(3):292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- 22.Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A. 1988;85(13):4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257(1):180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 24.Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14(6):681–685. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35(Pt 4):661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 26.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapuis JF, Agache P. A new technique to study the mechanical properties of collagen lattices. J Biomech. 1992;25(1):115–120. doi: 10.1016/0021-9290(92)90250-5. [DOI] [PubMed] [Google Scholar]
- 28.Cacou C, Palmer D, Lee DA, Bader DL, Shelton JC. A system for monitoring the response of uniaxial strain on cell seeded collagen gels. Med Eng Phys. 2000;22(5):327–333. doi: 10.1016/s1350-4533(00)00040-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen MY, Sun YL, Zhao CF, Zobitz ME, An KN, Moran SL, Amadio PC. Factors related to contraction and mechanical strength of collagen gels seeded with canine endotenon cells. J Biomed Mater Res B. 2007;82B(2):519–525. doi: 10.1002/jbm.b.30757. [DOI] [PubMed] [Google Scholar]
- 30.Chen H-C, Yang T-H, Thoreson AR, Zhao C, Amadio PC, Sun Y-N, Su F-C, An K-N. Automatic and quantitative measurement of collagen gel contraction using model-guided segmentation. Meas Sci Technol. 2013;24(8):085702. doi: 10.1088/0957-0233/24/8/085702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ettema AM, Amadio PC, Zhao C, Wold LE, O’Byrne MM, Moran SL, An KN. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118(6):1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 32.Ettema AM, An KN, Zhao C, O’Byrne MM, Amadio PC. Flexor tendon and synovial gliding during simultaneous and single digit flexion in idiopathic carpal tunnel syndrome. J Biomech. 2008;41(2):292–298. doi: 10.1016/j.jbiomech.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osamura N, Zhao C, Zobitz ME, An KN, Amadio PC. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech (Bristol, Avon) 2007;22(9):999–1003. doi: 10.1016/j.clinbiomech.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh J, Zhao CF, Amadio PC, An KN, Zobitz ME, Wold LE. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005;23(5):1226–1231. doi: 10.1016/j.orthres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Feng Z, Matsumoto T, Nakamura T. Measurements of the mechanical properties of contracted collagen gels populated with rat fibroblasts or cardiomyocytes. J Artif Organs. 2003;6(3):192–196. doi: 10.1007/s10047-003-0230-z. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20(4):108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown RA, Sethi KK, Gwanmesia I, Raemdonck D, Eastwood M, Mudera V. Enhanced fibroblast contraction of 3D collagen lattices and integrin expression by TGF-beta1 and - beta3: mechanoregulatory growth factors? Exp Cell Res. 2002;274(2):310–322. doi: 10.1006/excr.2002.5471. [DOI] [PubMed] [Google Scholar]
- 38.Gingery A, Yang TH, Passe SM, An KN, Zhao C, Amadio PC. TGF-β signaling regulates fibrotic expression and activity in carpal tunnel syndrome. J Orthop Res. doi: 10.1002/jor.22694. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]