Abstract
Background & Aim
Acid sphingomyelinase (ASMase) is activated in nonalcoholic steatohepatitis (NASH). However, ASMase’s contribution to NASH is poorly understood and limited to hepatic steatosis and glucose metabolism. Here we examined ASMase’s role in high fat diet (HFD)-induced NASH.
Methods
Autophagy, endoplasmic reticulum (ER) stress and lysosomal membrane permeabilization (LMP) were determined in ASMase−/− mice fed HFD. The impact of pharmacological ASMase inhibition on NASH was analyzed in wild type mice fed HFD.
Results
ASMase deficiency determined resistance to HFD or methionine and choline deficient diet-mediated hepatic steatosis. ASMase−/− mice were resistant to HFD-induced hepatic ER stress, but sensitive to tunicamycin-mediated ER stress and steatosis, indicating selectivity in the resistance of ASMase−/− mice to ER stress. Autophagic flux determined in the presence of rapamycin and/or chloroquine was lower in primary mouse hepatocytes (PMH) from ASMase−/− mice and accompanied by increased p62 levels, suggesting autophagic impairment. Moreover, autophagy suppression by chloroquine and brefeldinA caused ER stress in PMH from ASMase+/+ mice but not ASMase−/− mice. ASMase−/− PMH exhibited increased lysosomal cholesterol loading, decreased LMP and apoptosis resistance induced by O-methyl-serine dodecylamide hydrochloride or palmitic acid, effects that were reversed by decreasing cholesterol levels by the oxysterol 25-hydroxycholesterol. In vivo pharmacological ASMase inhibition by amitriptyline, a widely used tricyclic antidepressant, protected wild type mice against HFD-induced hepatic steatosis, fibrosis, and liver damage, effects indicative of early-stage NASH.
Conclusions
These findings underscore a critical role for ASMase in diet-induced NASH and suggest the potential of amitriptyline as a treatment for patients with NASH.
Keywords: Ceramide, fatty liver, endoplasmic reticulum stress, autophagy, lysosomal membrane permeabilization
INTRODUCTION
Nonalcoholic steatohepatitis (NASH) is an advanced stage of fatty liver disease characterized by steatosis, oxidative stress, fibrosis and hepatocellular death, which can progress to cirrhosis and hepatocellular carcinoma [1, 2]. NASH is a major health concern due to its association with obesity, insulin resistance and type-2 diabetes. NASH pathogenesis is still incompletely understood, which has limited the availability of effective treatments.
Ceramide regulates many cellular processes, including differentiation, apoptosis and metabolism. Cells generate ceramide via de novo synthesis in the endoplasmic reticulum (ER) beginning with the condensation of palmitic acid (PA) with serine catalyzed by serine palmitoyl transferase (SPT) [3–5]. SPT inhibition prevents genetic and diet-induced hepatic steatosis and de novo ceramide synthesis modulates insulin sensitivity [6,7]. Furthermore, sphingomyelin (SM) hydrolysis by sphingomyelinases (SMases) generates ceramide [3–5]. Acid SMase (ASMase) is of particular relevance in metabolic liver diseases, as it is required for TNF-induced hepatocellular apoptosis [8–10]. ASMase overexpression has been reported in adipose tissue of ob/ob mice [11], in mice fed a methionine and choline deficient diet (MCD) [12] and in liver of patients with NASH [13]. Moreover, ASMase promotes liver fibrosis by regulating lysosomal cathepsins in hepatic stellate cells [14, 15]. ASMase’s contribution to NASH is incompletely understood. To the best of our knowledge only two studies have focused on the role of ASMase in glucose/lipid homeostasis with controversial findings [16, 17]. For example, ASMase deletion superimposed on the genetic background of LDL receptor deficiency (ASMase/LDL receptor double knockout mice, ALDLRDKO) prevented diet-induced hyperglycemia [16]. Intriguingly, these findings were accompanied by a paradoxical increase in hepatic ceramide and de novo ceramide synthesis due to SPT activation [16]. In contrast, ASMase overexpression improved glucose metabolism in diabetic db/db mice, and accordingly, ASMase−/− mice exhibited higher blood glucose levels than wild type mice upon glucose tolerance tests [17]. Moreover, although ALDLRDKO mice are resistant to high fat diet (HFD) induced steatosis [16], the role of ASMase per se in HFD-mediated steatosis has not been addressed. Furthermore, ASMase’s impact in key features of NASH, including ER stress and autophagy, critical players in lipid and glucose metabolism [18–20], and lysosomal membrane permeabilization (LMP), an important mechanism of saturated fatty acid-mediated lipotoxicity [21], has not been previously examined. Here, we characterized the impact of HFD on hepatic steatosis, ER stress, autophagy and LMP-mediated apoptosis in ASMase−/− mice. Moreover, in vivo treatment of wild type mice with amitriptyline, a tricyclic antidepressant widely prescribed for depression or neuropathic pain that inhibits the proteolytic processing of pro-ASMase in endolysosomes, prevented HFD-induced obesity, glucose intolerance and NASH. These findings suggest the potential of amitriptyline as an effective therapy in human NASH.
MATERIAL AND METHODS
Mice and treatments
The experimental protocols met the guidelines of the Animal Care Committee of the Hospital Clinic-Universidad de Barcelona. ASMase−/− mice (C57BL/6 background) and their ASMase+/+ littermates were propagated using heterozygous breeding pairs as previously described [8, 9]. HFD (60%, Research Diets, Inc) was administered for 12 weeks to ASMase+/+ mice and ASMase−/− mice. Moreover, mice were fed MCD diet (TestDiet, Richmond, IN) for two weeks, as described [12]. Biochemical determinations, sphingolipids analysis, mass spectrometry, H&E, Oil red and filipin staining are described in the Supplemental Methods section.
Autophagy and lysosomal cholesterol and permeabilization assays
Primary mouse hepatocytes (PMH) were incubated with rapamycin (2µM) with or without chloroquine (50µM) to examine autophagic flux. In some cases, PMH were incubated with chloroquine and brefeldinA to block autophagy and examine the impact on of ER stress. PMH were stained with Lysotracker and filipin and analyzed by confocal imaging. PMH from ASMase−/− mice or ASMase+/+ mice fed a high cholesterol diet (HCD), as described [22], were examined for susceptibility (6–12 hr) to the lysosomotropic detergent O-methylserine dodecylamide hydrochloride (MSDH, 0–25µM) or palmitic acid (PA, 0.5–1.0mM). Further, PMH were preincubated with U18666A (0.5µg/ml, 16 hr) to induce lysosomal cholesterol accumulation and then challenged with PA. Cell death was examined by propidium iodide staining and caspase-3 activation as described [8, 9].
Statistical Analysis
Results were expressed as mean ± SD. Statistical significance of mean values was assessed using Student t-test and one-way ANOVA followed by Bonferroni post-test. Statistics were performed using GraphPad Prism 5 software.
RESULTS
Hepatic SM and ceramide homeostasis in ASMase−/− mice
We first examined the impact of ASMase deficiency on content of liver ceramide, which regulates lipid and glucose metabolism [6, 7]. HFD stimulated the expression of ASMase mRNA in ASMase+/+ mice (Supplementary Fig 1A) and increased total hepatic ceramide content (Supplementary Fig 1B), reflected mainly in long-chain ceramide C24:0 (Supplementary Fig 1C). Total hepatic ceramide levels were reduced in ASMase−/− mice fed RD or HFD but content increased slightly by HFD (Supplementary Figure 1B), particularly C16:0 and C18:1 (Supplemental Fig 1C). HFD increased hepatic SM in ASMase+/+ mice, which was associated with increased SM synthases (SMS) 1 and 2 expression (Supplementary Figure 1D, 1E). ASMase deficiency increased hepatic SM levels both with RD and HFD feeding without SMS upregulation (Supplemental Figure 1E). Hence, ASMase regulates hepatic SM homeostasis and ceramide generation by HFD.
ASMase−/− mice are resistant to HFD and MCD-induced liver steatosis
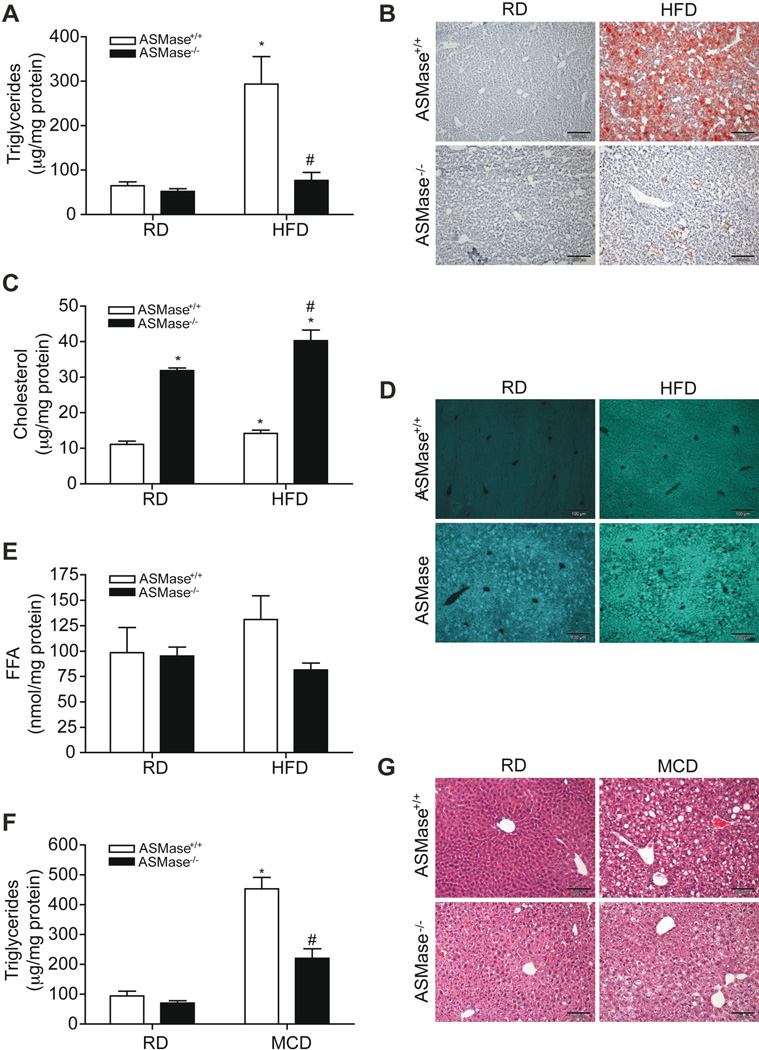
Hepatic steatosis is the first step in NASH and since the role of ASMase in diet-induced fatty liver has been only examined in ALDLRDKO mice [16], we assessed the response of ASMase−/− mice to both HFD and MCD-induced steatosis. Consistent with the findings in the ALDLRDKO mice, ASMase−/− mice were resistant to HFD-induced steatosis (Figure 1A, 1B). HFD feeding increased the levels of liver cholesterol in ASMase+/+ mice (Figure 1C). The increase in hepatic SM due to the lack of ASMase results in secondary accumulation of cholesterol and other glycosphingolipids in the liver [23]. Consequently, basal hepatic unsterified cholesterol levels increased in ASMase−/− mice as evidenced by filipin staining, which further increased upon HFD feeding (Figure 1C, 1D). Free fatty acids (FFA) increased slightly in ASMase+/+ mice fed HFD, a trend not observed in ASMase−/− mice (Figure 1E). HFD induced expression of Srebp1c, Dgat2, Fas and Acc in ASMase+/+ mice but not ASMase−/− mice, indicating that the resistance of ASMase−/− mice to HFD-induced steatosis is associated with reduced activation of diet-induced lipogenesis pathways (Supplementary Fig 2). In addition, basal mRNA levels of Srebp-2 and Hmg-CoA reductase were higher in ASMase−/− mice than in ASMase+/+, which increased with HFD (Supplementary Figure 2). Quite interestingly, feeding MCD also increased triglyceride (TG) levels and triggered liver steatosis in ASMase+/+ mice, effects that were markedly diminished in ASMase−/− mice (Figure 1F, G). Thus, ASMase deficiency ameliorates diet-induced steatosis independent of the type of diet feeding.
Figure 1. ASMase−/− mice are resistant to HFD and MCD-induced steatosis.
Mice were fed regular diet (RD) or HFD and liver samples processed for (A) liver TG, (B) oil-red staining, (C) cholesterol levels, (D) filipin staining and (E) FFA. In addition, mice were fed MCD for two weeks to examine (F) TG levels and (G) H&E staining. Results are expressed as mean ± SD (n= 5–7 mice). *p< 0.05, RD-fed ASMase+/+ mice, #p<0.05, vs HFD-fed ASMase+/+ mice or MCD-fed ASMase+/+ mice.
ASMase−/− mice are resistant to HFD-induced ER stress
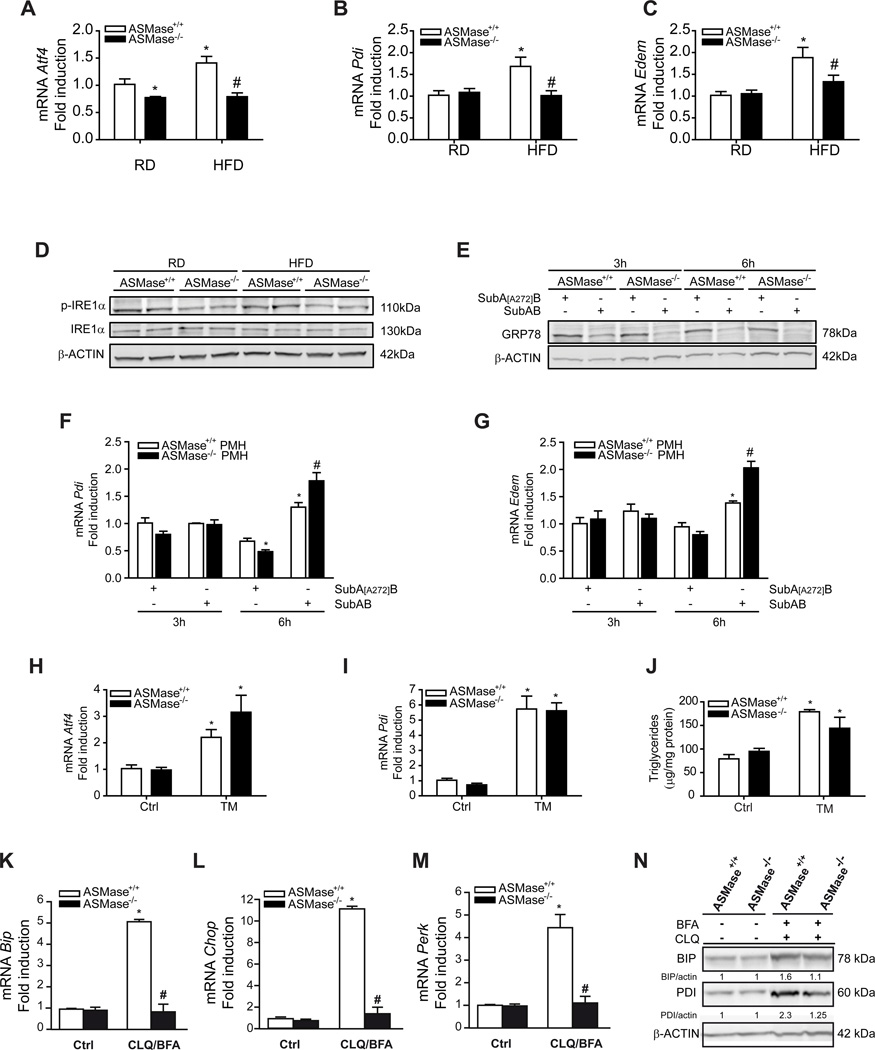
Since ER stress is a critical mechanism that promotes lipogenesis and fatty liver [18, 19], we next analyzed the sensitivity of ASMase−/− mice to HFD-induced ER stress. Unlike ASMase+/+ mice, ASMase−/− mice were resistant to HFD-mediated increased mRNA expression of Atf4, Pdi, Edem, BiP and IRE1α phosphorylation (Figure 2A-D) as well as uXBP-1, sXBP-1 and ATF6α (Supplementary Figure 3). These findings suggest that ASMase is required for the induction of the 3 arms of the UPR by HFD. Resistance was specific for HFD as PMH from ASMase+/+ and ASMase−/− mice were equally sensitive to cleavage of GRP78/BiP by SubAB (Figure 2E), a bacterial toxin that rapidly degrades GRP78 causing ER stress [24]. For both cell types, SubAB increased expression of Pdi and Edem at 6h post-treatment (Figure 2F, G). Moreover, in vivo tunicamycin (TM) administration caused enhanced expression of Atf4 and Pdi (Figure 2H, I) as well as TG accumulation (Figure 2J) and steatosis as determined by Oil-Red staining (not shown) to a similar degree in ASMase+/+ and ASMase−/− mice. Similar to HFD, palmitic acid (PA) induced ER stress in PMH from ASMase+/+ mice but not ASMase−/− mice (Supplementary Figure 4). These results indicate that the ER of ASMase−/− mice is functional and that the resistance of ASMase−/− mice to ER stress is specific for HFD. Moreover, the findings indicate that ER stress causes hepatic steatosis in both types of mice.
Figure 2. Resistance of ASMase−/− mice to HFD-mediated ER stress.
Expression of ER stress markers, (A) Atf4, (B) Pdi and (C) Edem, and (D) p-IRE1αινλιωερ from mice fed RD or HFD. Moreover, PMH were treated with SubAB to examine GRP78 degradation (E), and the expression of Pdi (F) and Edem (G). ASMase+/+ mice or ASMase−/− mice were treated with tunicamycin (TM) examining the expression of Atf4 (H), Pdi (I) and liver TG levels (J). PMH were cultured in the presence of chloroquine (CLQ) and brefeldinA (BFA) and samples processed for (K) BiP, (L) Chop, (M) Perk expression and western blot (N). Results are expressed as mean ± SD (n=5–7 mice). A–C: *p< 0.05 vs. RD-fed ASMase+/+ mice; F–G: *p<0.05 vs. SubA[A272]B-treated ASMase+/+ pmh, #p<0.05 vs SubAB-treated ASMase+/+ PMH; H–J: *p<0.05 vs. control.
Autophagy-lysosome degradation pathway in ASMase−/− mice
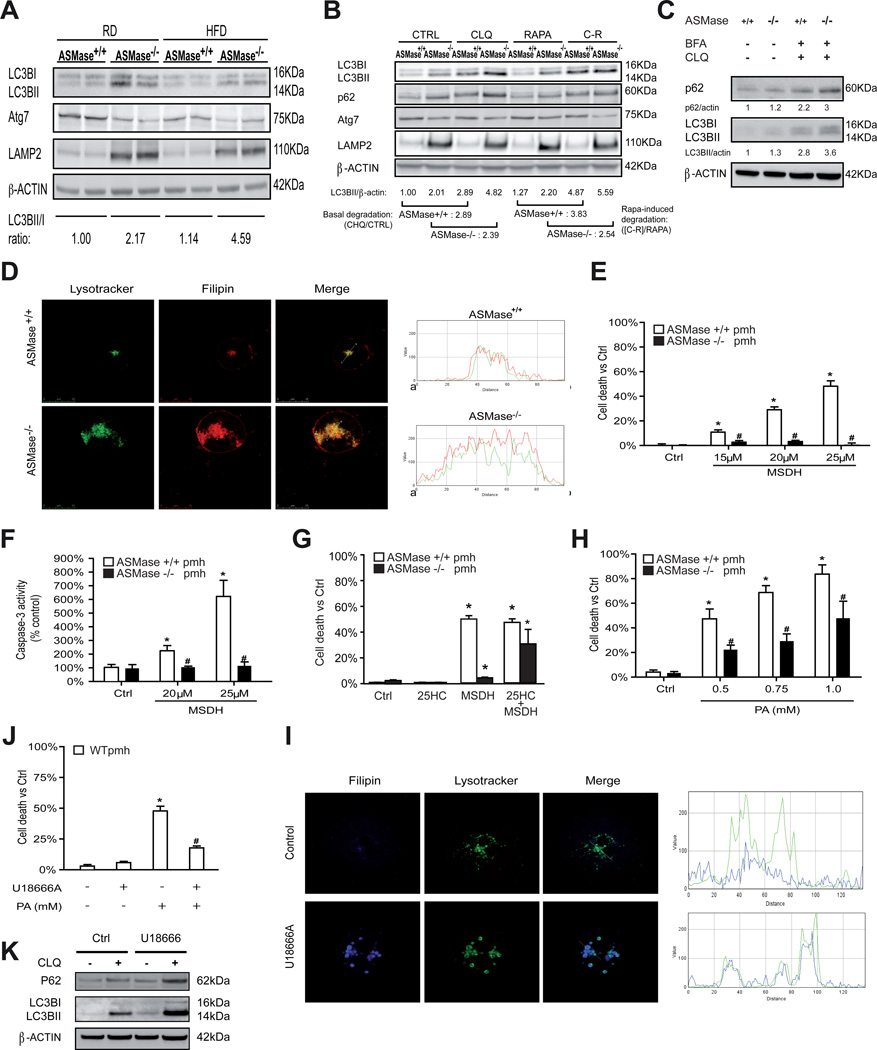
The autophagy-lysosome is a proteolytic pathway involved in the turnover of organelles and cellular debris, and has been shown to regulate lipid metabolism and steatosis [20, 25]. Given the resistance of ASMase−/− mice to HFD-induced hepatic steatosis, we next analyzed the integrity of the hepatic macroautophagy pathway. Liver extracts from ASMase−/− mice exhibited increased basal LC3BII/LC3BI ratio compared to ASMase+/+ mice, an outcome that was maintained upon HFD feeding (Figure 3A). Interestingly, livers from ASMase−/− mouse exhibited decreased expression of Atg7 (Figure 3A), an essential autophagy gene regulating conjugation of LC3 with phosphatidylethanolamine. These findings were accompanied by a higher expression of the lysosomal marker LAMP2 (Figure 3A). As the ubiquitin-independent proteasome REGγ and SIRT1 regulate autophagy [26], we examined their expression. Livers from HFD-fed ASMase−/− mice exhibited increased REGγ expression but reduced SIRT1 levels compared to ASMase+/+ samples (Supplementary Figure 5). To determine whether these effects in hepatic extracts reflect the status of parenchymal cells and to delineate the autophagic flux, we used PMH in the presence of rapamycin (RAPA) with and without chloroquine (CHQ). As expected, CHQ increased LC3BII levels in PMH from AMase+/+ mice (Figure 3B), as autophagy is blocked after autophagosome formation and before the autophagosome can fuse with lysosomes. However, the fold increase in LC3BII levels induced by CHQ (basal degradation) was lower in PMH from ASMase−/− mice compared to ASMase+/+ mice (Figure 3B). RAPA increased LC3BII levels in PMH from both types of mice, although the increase in ASMase−/− PMH was somewhat lower than that seen in ASMase+/+ PMH (Figure 3B). Moreover, RAPA-induced LC3BII degradation in the presence of CHQ was significantly lower in PMH from ASMase−/− mice compared to PMH from ASMase+/+ mice. For all conditions, PMH from ASMase−/− mice exhibited increased p62 levels, indicating impaired lysosomal degradation (Figure 3B). Consistent with liver extracts, the protein levels of Atg7 were lower in PMH from ASMase−/− mice with respect to ASMase+/+ mice (Figure 3B). Thus, ASMase deficiency impairs the autophagy-lysosomal degradation pathway.
Figure 3. Autophagy and LMP in ASMase−/− mice.
A, Autophagy in liver samples from mice fed RD or HFD. B, Autophagy in PMH challenged with rapamycin (RAPA), chloroquine (CHQ) or combination RAPA+CHQ (C-R). C, p62 and LC3BI/II in PMH treated with chloroquine and brefeldinA. D, PMH from ASMase+/+ and ASMase−/− mice were stained with Lysotracker and filipin. Images shown are representative of 4–5 individual experiments with similar findings. Cell death (E) and caspase-3 activity (F) of PMH induced by the lysosomotropic detergent MSDH. G, Effect of 25-hydroxycholesterol in MSDH-induced cell death in PMH. H, PMH challenged with increasing doses of PA to induce cell death. I, confocal imaging of PMH from wild type mice treated with U18666A. J, survival of PMH from wild type mice treated with U18666A challenged with PA. K, p62 and LC3BI/II levels in PMH treated with U18666A with or without chloroquine. Results are expressed as mean ± SD of 4–6 independent experiments. *p<0.05 vs. control ASMase+/+ PMH; # p<0.05 vs. ASMase+/+ PMH.
Impaired autophagy-induced ER stress requires ASMase
In addition to the regulation of lipid metabolism and steatosis, autophagy suppression has been shown to cause ER stress [27]. This finding implies that the onset of hepatic steatosis due to defective autophagy could reflect the combination of decreased fat degradation by autophagy and increased fat synthesis by ER stress. Thus, we next examined the susceptibility of ASMase−/− mice to impaired autophagy-mediated ER stress. Chloroquine and brefeldinA increased the expression of ER stress markers in PMH from ASMase+/+ mice (Figure 2 K–N), in agreement with previous findings [27]. However, this response was blunted in PMH from ASMase−/− mice. Chloroquine/brefeldin increased p62 levels and expression of LC3BII in both types of PMH (Figure 3C). Thus, similar to HFD, ASMase is required for ER stress caused by autophagy suppression.
Lysosomal cholesterol and resistance to LMP and cell death increase in ASMase−/− mice
We next analyzed the distribution of the increased hepatic cholesterol levels observed in ASMase−/− mice (Figure 1C, 1D). Confocal imaging of ASMase−/− PMH stained with Lysotracker and filipin revealed increased lysosomal mass and a significant increase in lysosomal cholesterol content compared to ASMase+/+ PMH (Figure 3D). No colocalization of cholesterol with mitochondria was found, in agreement with previous results [28]. This outcome paralleled the increase of cholesterol in isolated lysosomes and was reproduced in PMH from wild type mice fed a high cholesterol diet (HCD) (Supplementary Figure 6). Moreover, autophagy inhibition by chloroquine in ASMase+/+ PMH increased total cholesterol levels but, unlike ASMase−/− PMH, the increase in free cholesterol did not accumulate in lysosomes (Supplementary Figure 7), in agreement with previous findings [20]. To examine the functional impact of increased lysosomal cholesterol in LMP, we challenged PMH with O-methyl-serine-dodecylamide hydrochloride (MSDH), a known lysosomotropic detergent that induces cell death following LMP [29]. MSDH dose-dependently killed ASMase+/+ PMH (Figure 3E). Importantly, ASMase−/− hepatocytes were resistant to MSDH-induced cell death (Figure 3E) and these effects were reproduced in PMH from mice fed HCD (Supplementary Figure 8). The resistance of ASMase−/− PMH or PMH from wild type mice fed HCD to MSDH correlated with decreased caspase-3 activation (Figure 3F, Supplementary Figure 8). Decreasing cholesterol content by the oxysterol 25-hydroxycholesterol [29], reversed the resistance of ASMase−/− PMH to MSDH-induced cell death (Figure 3G). Since LMP has been shown to contribute to PA-induced lipotoxicity [21], we examined the susceptibility of ASMase−/− PMH to PA. Consistent with MSDH, PMH from ASMase−/− mice were less sensitive to PA-induced cell death (Figure 3H). PA increased total cholesterol levels but did not cause lysosomal cholesterol accumulation (Supplementary Figure 9). To pinpoint whether the resistance to PA was due to lysosomal cholesterol accumulation, we explored whether U18666A, an amphiphile that disrupts cholesterol homeostasis and causes lysosomal cholesterol accumulation [30], mimics the resistance to PA in PMH from wild type mice. U18666A reproduced the lysosomal cholesterol accumulation, and more importantly, the resistance to PA observed in ASMase−/− mice (Figure 3I, 3J). In agreement with previous results [29], U18666A increased p62 and LC3BII levels (Figure 3K) amd similar effects were observed upon MSDH treatment (not shown). Consistent with these findings in PMH, ASMase−/− mice exhibited reduced liver injury determined by serum ALT levels in response to HFD as well as decreased HFD-mediated susceptibility to LPS (Supplementary Fig 10). Thus, ASMase deficiency increases lysosomal cholesterol, which determines decreased LMP and resistance to saturated fatty acid-induced lipotoxicity.
ASMase inhibition protects against HFD-induced NASH
To examine the relevance of the preceding findings on HFD-induced NASH, we explored the use of amitriptyline, which prevents the proteolytic activation of ASMase [31], to inhibit in vivo ASMase in wild type mice. HFD stimulated ASMase activity compared to mice fed control diet (from 5±0.2 to 12±1.6 pmol/min/mg protein) and amitriptyline significantly inhibited ASMase activation in both cases by 83% and 78%, respectively. Amitriptyline did not affect food intake but significantly ameliorated the body weight gain in response to HFD (Supplementary Fig 11) as well as the hepatomegaly and the epidydimal white adipose weight gain (Supplementary Fig 11). Moreover, amitriptyline normalized the hyperglycemia induced by HFD (Supplementary Fig 11) and reduced blood glucose levels during glucose (GTT) and insulin tolerance tests (ITT) following HFD feeding (Supplementary Figure 11). The beneficial effects of amitriptyline on body weight and glucose tolerance seen in HFD-fed wild type mice were abrogated in ASMase−/− mice (not shown), indicating the dependence of amitriptyline on ASMase to regulate body weight and glucose homeostasis. Interestingly, the effects of HFD feeding in blood glucose homeostasis following GTT and ITT were aggravated in ASMase−/− mice compared to ASMase+/+ mice despite similar hepatic insulin signaling in both cases (unpublished observations).
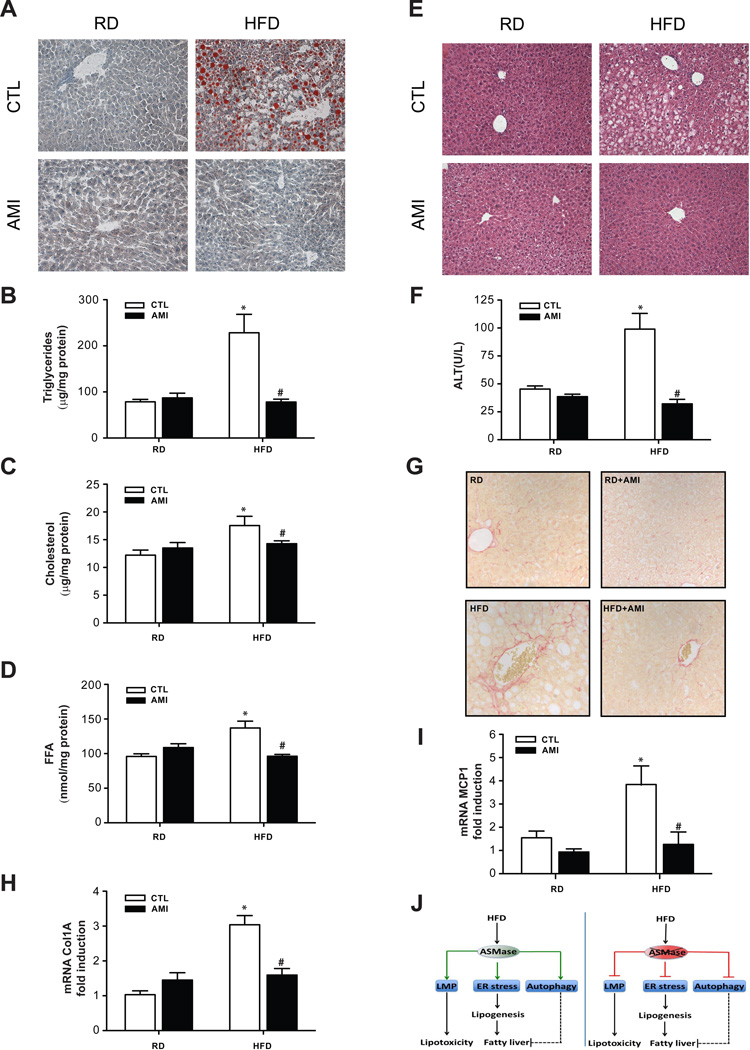
Moreover, amitriptyline abolished HFD-induced hepatic steatosis, reflected by lower Oil-red staining and the biochemical determination of liver TG and FFA (Figure 4A-D). In contrast to ASMase−/− mice, ASMase inhibition by amitriptyline reduced both liver SM content (not shown) and hepatic cholesterol levels in response to HFD (Figure 4C). Importantly, amitriptyline normalized liver histology and prevented liver injury as determined by the release of serum ALT (Figure 4E, F). Moreover, fibrosis estimated by Sirius-red staining (Figure 4G) and expression of markers such as Col1A mRNA (Figure 4H) increased in HFD-fed control mice and these effects were ameliorated by amitriptyline, in agreement with previous findings [13]. In addition, although the increase in inflammatory markers, including MCP-1 mRNA, in control mice fed HFD was prevented by amitriptyline (Figure 4I), there was not significant inflammatory infiltrates at histological examination, indicating minimal inflammation in mice fed HFD for 16 weeks. As expected HFD increased the expression of ER stress markers in control mice but not in HFD-fed mice treated with amitriptyline (Supplementary Fig 12). Thus, these findings indicate that ASMase inhibition by amitriptyline prevents diet-induced NASH.
Figure 4. Amitriptyline protects wild type mice from HFD-induced NASH.
Wild type mice were fed RD or HFD with or without amitriptyline (AMI) for the last 6 weeks to determine oil red staining (A), TG (B), cholesterol (C), FFA (D), H&E (E), serum ALT (F), Col1A mRNA levels (G), Sirius Red staining (H) or MCP-1 expression (I). J, schematic summary of the findings: ASMase promotes HFD-induced ER stress, autophagy and LMP, which in turn, contribute to steatosis and lipototoxicity. ASMase ablation prevents these effects induced by HFD. The dash line linking autophagy and fatty liver denotes that this relationship may not be direct in view of recent findings (see Discussion). Results are expressed as mean ± SD of n=10 mice per group. * p<0.05 vs. control mice fed RD; #p<0.05 vs. control mice fed HFD.
DISCUSSION
Here, we report a novel role of ASMase in key events in NASH. Specifically, ASMase regulates diet-induced steatosis and ER stress, lysosomal cholesterol homeostasis, autophagy and LMP-mediated apoptosis (Figure 4J). More importantly, ASMase inhibition in wild type mice prevents HFD induced liver injury, inflammation and fibrosis, characteristic of NASH.
Resistance to diet-induced hepatic steatosis in the absence of ASMase
A novel finding is the resistance of ASMase−/− mice not only to HFD but also to MCD-mediated fatty liver. This finding is surprising, as MCD is known to induce steatosis by preventing hepatic phosphatidylcholine (PC) synthesis, which in turn, impairs VLDL secretion to the circulation resulting in liver TG loading and subsequent fatty liver. Previous findings reported that ASMase deficiency results in higher PC levels both in brain and liver compared to ASMase+/+ tissues [32]. In line with these findings, we observed that ASMase−/− mice fed MCD exhibited higher liver PC levels than ASMase+/+ mice, suggesting that ASMase deficiency spares PC to prevent VLDL accumulation in the absence of methionine and choline. Although the increased hepatic SM is the direct consequence of ASMase deficiency, the reason for the maintenance of liver PC content in ASMase−/− mice remains to be characterized. As SM is synthesized from ceramide and PC by SMS in the Golgi, the maintenance of PC in the absence of ASMase implies that de novo SM synthesis is a major pathway of PC consumption in the liver. However, it could also reflect the lack of inhibition of PC synthesis at the CTP:cholinephosphate cytidylyltransferase level, as suggested in rat lung [33]. In line with this possibility, it has been shown that C2-ceramide inhibits PC neosynthesis in neuroblastoma cells [34].
ER stress-autophagy interplay in the regulation of hepatic steatosis
A key finding is that PMH from ASMase−/− mice exhibit defective autophagic flux, in agreement with recent findings in coronary arterial smooth muscle cells from ASMase−/− mice [35]. In both cell types, the defect of autophagy translated in increased LC3BII and enhanced p62 levels, reflecting impaired formation of autophagolysosomes. The association between defective autophagy and the resistance to diet-induced steatosis in ASMase−/− mice is intriguing. Previous findings demonstrated that lipid droplets and autophagic components colocalized during nutrient deprivation and that mice with liver Atg7 deletion (Atg7Δhep) exhibited defective autophagy and increased TG storage and hepatic steatosis [20]. In addition to this novel pathway of autophagy regulation of hepatic steatosis by fat digestion, ER stress is also known to trigger hepatic steatosis by activation of transcription factors that stimulate the expression of enzymes involved in lipogenesis [18, 19]. ER stress and autophagy exhibit mutual regulation. Autophagy antagonizes ER stress and autophagy stimulation improves ER stress and its metabolic consequences [36]; consequently, defective autophagy causes ER stress [27]. Therefore, fat degradation by autophagy opposes while fat synthesis by ER stress promotes hepatic steatosis. We show that ASMase−/− mice exhibit a selective resistance to ER stress induced by HFD, while the sensitivity of ASMase−/− PMH to ER stress caused by TM or SubAB argues that ASMase deficiency does not preclude the ER from sensing stress. Importantly, we also show that autophagy suppression by chloroquine/brefelfinA results in ER stress, as expected [27], only in ASMase+/+ PMH, indicating that in addition to HFD, ASMase is required for autophagy-induced ER stress. These findings suggest that in the context of impaired autophagy due to the lack of ASMase, defective onset of ER stress-induced lipogenesis may be more significant in the regulation of hepatic steatosis (Figure 4J). Moreover, the findings highlight a critical role for ASMase in regulating the ER stress in response to a variety of stimuli, including HFD, PA and autophagy suppression, as shown here, or chronic alcohol feeding, as described recently [28]. Diet and genetic obesity have been shown to alter ER lipid composition, which in turn, regulates SERCA-mediated ER Ca2+ homeostasis [19]. As ASMase per se induces ER stress by disrupting ER Ca2+ regulation [28], our data suggest that ASMase is required for HFD-mediated ER Ca2+ disruption and subsequent ER stress. While further understanding is needed as to how ASMase regulates ER Ca2+ homeostasis, our findings showing hepatic steatosis resistance despite autophagy impairment are in agreement with recent findings. Mice with liver-specific deletion of FIP200, a core subunit of the Atg1 complex, were protected from starvation and HFDinduced hepatic steatosis [37]. Importantly, FIP200 mutant mice exhibited defective diet-induced activation of lipogenic pathways. Consistent with these findings, ASMase−/− mice are resistant to HFD-induced expression of Srebp1c, Dgat2, Fas and Acc. Moreover, autophagy disruption by shRNA-mediated suppression of Atg7 failed to cause hepatic steatosis in lean mice [27], while lower hepatic lipid content has been reported in Atg7Δhep mice fed HFD [38]. Although the reasons for the discrepancy between the latter findings [38] and the previous report in Atg7Δhep mice [20] are unknown, it is clear that that role of autophagy in lipid metabolism and hepatic steatosis is complex and may be influenced by a number of factors including experimental conditions, genetic background or age of mice.
Hepatic cholesterol and NASH: contrast between ASMase−/− mice and amitriptyline treatment in wild type mice
An important aspect of the increase in hepatic cholesterol content in the ASMase−/− mice is that the bulk of it is free and sequestered in lysosomes. This phenotype in hepatocytes is in agreement with findings described in macrophages from ASMase−/− mice [39]. The localization of cholesterol in this compartment reflects the high affinity of SM to bind cholesterol [40, 41], which decreases the efflux/trafficking of cholesterol out of lysosomes. This trafficking defect impairs the sterification of cholesterol in the ER by acyl-CoA:cholesterol acyl transferase, as shown in ASMase−/− macrophages, which can further contribute to the increase in liver free cholesterol seen in ASMase−/− mice. Interestingly, enrichment of wild type macrophages with exogenous SM reproduces the increased lysosomal cholesterol seen in macrophages from ASMase−/− mice due to decreased cholesterol efflux [39]. A crucial difference that accounts for this contrast is the residual ASMase activity in both paradigms (0% activity in the ASMase−/− mice vs ~20% activity after amiltriptyline). The lack of ASMase is known to cause the lysosomal storage disease Niemann-Pick disease type A, which is characterized by the accumulation of lipids such as SM, cholesterol and glycosphingolipids in the affected organs, predominantly in brain and peripheral tissues such as liver [23]. The residual ASMase activity left after amitriptyline treatment is sufficient to prevent the increment of hepatic SM levels and subsequent increase in cholesterol seen in ASMase−/− mice with no residual activity. The relation of ASMase activity with cholesterol homeostasis is likely regulated by the SM content. It has been shown that SM depletion by exogenous sphingomyelinase blocks while SM enrichment promotes proteolytic processing of SREBP-2 at site 1, the same site that is regulated by the levels of sterols, to achieve an optimal ratio of SM to cholesterol [42]. Furthermore, as cholesterol is increasingly recognized as an important player in NASH [43], the protection of ASMase−/− mice to diet-induced early-stage NASH seems paradoxical. Perhaps as important as the extent of cholesterol increase is the subcellular location where cholesterol accumulates. In this regard, mitochondrial cholesterol plays a relevant role in NASH as it has been shown to sensitize to TNF/Fas-mediated NASH [22]. Importantly, we show that the increased hepatic cholesterol levels in the ASMase−/− mice are found in lysosomes but not in mitochondria. This outcome determines not only the sparing of mitochondrial GSH, as recently reported [28], but also the resistance of ASMase−/− mice to LMP, which is an important mechanism of lipotoxicity [21]. The causal role of increased lysosomal cholesterol in apoptosis resistance is further established in wild type PMH treated with the amphiphile U18666A and the reversal of apoptosis resistance of ASMase−/− PMH upon cholesterol extraction by the oxysterol 25-hydroxycholesterol. In addition to the regulation of intracellular cholesterol distribution and LMP, ASMase is proapoptotic and mediates death receptor-induced hepatocellular apoptosis [8–10]. In conclusion, ASMase promotes ER stress, autophagy and LMP, which in turn mediate steatosis and lipotoxicity caused by HFD and saturated fatty acids, events that are blunted in ASMase null mice (Figure 4J). Therefore, targeting ASMase may be of benefit in protecting against diet-induced liver injury and NASH.
Supplementary Material
ACKNOWLEDGEMENTS
We want to thank the superb technical assistance of Susana Núñez. We are indebted to Dr. Montserrat Mari for valuable suggestions and critical reading of the manuscript. The study was performed (in part) in the Center Esther Koplowitz.
Financial support: The work was supported by CIBEREHD, Fundació la Marató de TV3 and grants PI11/0325 (META) from the Instituto Salud Carlos III and grants SAF2009-11417, SAF2011-23031, and SAF2012-34831 from Plan Nacional de I+D, Spain; and the center grant P50-AA-11999 (Research Center for Liver and Pancreatic Diseases, NIAAA/NIH).
Abbreviations
- ASMase
acid sphingomyelinase
- ALDLRDKO
ASMase/LDL receptor double knockout
- CHQ
chloroquine
- ER
endoplasmic reticulum
- FFA
free fatty acids
- GTT
glucose tolerance test
- ITT
insulin tolerance test
- IRβ
insulin receptor β subunit
- HCD
high cholesterol diet
- HFD
high fat diet
- LMP
lysosomal membrane permeabilization
- MDC
monodansylcadaverine
- MCD
methionine and choline deficient diet
- MSDH
O-methylserine dodecylamide hydrochloride
- PA
palmitic acid
- PMH
primary mouse hepatocytes
- RAPA
rapamycin
- RD
regular diet
- SubAB
AB5 subtilase cytotoxin
- SMases
sphingomyelinases
- SM
sphingomyelin
- SMS
sphingomyelin synthases
- SPT
serine palmitoyl transferase
- TG
triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2009;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H and Diehl. AM. Citokines in alcoholic and nonalcoholic steatohepatitis. New England J. Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trend. Cell. Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 4.Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 5.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 6.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Ruiz C, Colell A, Mari M, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J. Clin. Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mari M, Colell A, Morales A, Pañeda C, Varela-Nieto I, Garcia-Ruiz C, Fernandez-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J. Clin. Invest. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumitru CA, Gulbins E. TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25:5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- 11.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 12.Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, et al. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J. Biol. Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moles A, Tarrats N, Morales A, Dominguez M, Bataller R, Caballeria, J et al. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am. J. Pathol. 2010;177:1214–1224. doi: 10.2353/ajpath.2010.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moles A, Tarrats N, Fernandez-Checa JC, Mari M. Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology. 2009;49:1297–1307. doi: 10.1002/hep.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moles A, Tarrats N, Fernandez-Checa JC, Mari M. Cathepsin B overexpression due to acid sphingomyelinase ablation promotes liver fibrosis in Niemann-Pick disease. J. Biol. Chem. 2012;287:1178–1788. doi: 10.1074/jbc.M111.272393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deevska GM, Rozenova KA, Giltiay NV, Chambers MA, White J, Boyanovsky BB, et al. Acid Sphingomyelinase Deficiency Prevents Diet-induced Hepatic Triacylglycerol Accumulation and Hyperglycemia in Mice. J. Biol. Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osawa Y, Seki E, Kodama Y, Suetsugu A, Miura K, Adachi M, Ito H, et al. Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through AKT activation and AMP-activated protein kinase suppression. FASEB J. 2011;25:1345–1353. doi: 10.1096/fj.10-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith, R.O, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide, W et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu, M et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A et al. Mitochondrial free cholesterol loading sensitizes to TNF and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Schuchman EH. Acid sphingomyelinase, cell membranes and human disease: lessons from Niemann Pick Disease. FEBS Lett. 2010;584:1895–1900. doi: 10.1016/j.febslet.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 24.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjoh J, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 25.Amir M, Czaja MJ. Autophagy in non-alcoholic stetohepatitis. Exp Rev Gastroenterology Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong S, Jia C, Zhang S, Fan G, Shan P, Sun L et al. The REGg proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 2013;18:380–391. doi: 10.1016/j.cmet.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez A, Matias N, Fucho R, Ribas V, Von Montfort C, Nuño N et al. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J Hepatol. 2013;59:805–813. doi: 10.1016/j.jhep.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appelqvist H, Nilsson C, Garner B, Brown AJ, Kagedal K, Öllinger K. Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am J Pathol. 2011;187:629–639. doi: 10.1016/j.ajpath.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cenedella RJ. Cholesterol inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44:477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 31.Lang PA, Schenck M, Nicolay JP, Kempe DS, Lupescu A, Koka S, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat. Med. 2006;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 32.Prinetti et al. Secondary alterations of sphingolipid metabolism in lysosomal storage disease. Neurochem Res. 2011;36:1654–1668. doi: 10.1007/s11064-010-0380-3. [DOI] [PubMed] [Google Scholar]
- 33.Mallampalli RK, Mathur SN, Warnock LJ, Salome RG, Hunnighake GW. Betamethasone modulation of sphingomyelin hydrolysis up-regulates CTP:cholinephosphate cytidylyltransferase activity in adult rat lung. Biochem J. 1996;318:333–341. doi: 10.1042/bj3180333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos B, Lahti JM, Claro E, Jackowski S. Prevalence of necrosis in C2-ceramide-induced cytotoxicity in NB16 neuroblastoma cells. Mol Pharmacol. 2003;64:502–511. doi: 10.1124/mol.64.2.502. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Xu M, Pitzer AL, Xia M, Boini KM, Li P, Zhang Y. Control of autophagy maturation by acid sphingomyuelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. J Mol Med. 2014;92:473–485. doi: 10.1007/s00109-014-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backar-Wikstrom E, Wikstrom JD, Ariav Y, Tirosh B, Kaiser N, Cerasi E, Leibowitz G. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes. 2013;62:1227–1237. doi: 10.2337/db12-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D, Molusky MM, Song J, Hu CR, Fang F, Rui C, et al. Autophagy deficiency by FIP200 deletion uncouples hepatic steatosis from liver injury in NAFLD. Mol Endocrinol. 2013;27:1643–1654. doi: 10.1210/me.2013-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 39.Leventhal AR, Chen W, Tall AR, Tabas I. Acid sphingomyelinase-deficient macrophages have defective cholesterol trafficking and efflux. J. Biol. Chem. 2001;276 doi: 10.1074/jbc.M106455200. 4496-44983. [DOI] [PubMed] [Google Scholar]
- 40.Slotte JP. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem. Phys. Lipids. 1999;102:13–27. doi: 10.1016/s0009-3084(99)00071-7. [DOI] [PubMed] [Google Scholar]
- 41.Ridway ND. Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochim. Biophys Acta. 2000;1484:129–141. doi: 10.1016/s1388-1981(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 42.Scheek S, Brown MS, Goldstein JL. Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc. Natl. Acad. Sci. USA. 1997;94:11179–11183. doi: 10.1073/pnas.94.21.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–1403. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.