Abstract
This study examined dyadic interrelations between episodic memory and depressive symptom trajectories of change in old and advanced old age. We applied dynamic models to 10-year incomplete longitudinal data of initially 1,599 married couples from the Study of Asset and Health Dynamics Among the Oldest Old (AHEAD; Mage = 75 years at T1). We found domain-specific lead-lag associations (time lags of two years) among and between spouses. For memory, better performance among husbands protected against subsequent memory decline among wives with no evidence of a directed effect in the other direction. For depressive symptoms, wives’ scores predicted subsequent depression increase and memory decline among husbands. Possible individual covariates (age, education, functional limitations) and spousal covariates (length of marriage, number of children, and whether or not the couple remained intact over the study period) did not account for differential lead–lag associations. Our findings of antecedent–consequent relations between wives and husbands are consistent with lifespan notions that individual development both influences and is influenced by contextual factors such as close social relationships.
Keywords: dyad, spouses, successful aging, dynamic modeling, growth curve modeling
Memory and Depressive Symptoms are Dynamically Linked among Married Couples: Longitudinal Evidence from the AHEAD Study
Lifespan psychological research has long proposed that individual development both actively influences and is influenced by contextual factors such as significant others (P. B. Baltes & Staudinger, 1996; M. M. Baltes & Carstensen, 1998; Bronfenbrenner, 1979; Cairns, Elder, & Costello, 1996; Zajonc & Markus, 1975). Marriage partners may represent a key contextual factor given that late-life marriages are characterized by considerable closeness and spouses typically share many joint experiences (Antonucci & Akiyama, 1991; Lang, 2001; Meegan & Berg, 2002). The current study explores the dynamic (i.e., time ordered) interplay between age-related changes in two central domains of functioning, cognition and well-being, from a dyadic perspective. This is an important extension of past research because studies on unrelated individuals in old age typically document weak to moderate correlations of levels and changes across the two domains (Carmelli, Swan, La Rue, & Eslinger, 1997; Isaacowitz & Smith, 2003; Luszcz, 1992; Wetherell, Reynolds, Gatz, & Pedersen, 2002), but the mechanisms underlying such associations are not well understood. We propose that explicitly targeting how significant others such as marriage partners may affect individual developmental trajectories will illuminate the dynamic nature of such associations. By applying dynamic models to 10-year longitudinal data of initially 1,599 married couples from the AHEAD study, we simultaneously model longitudinal changes in indicators of cognition and well-being and test hypotheses about their dynamic interrelationships over time, both among wives and husbands as well as between spouses.
In a first step, we review the extant literature on cognition–well-being associations as derived from research on unrelated individuals. In a second step, we illustrate how studying individual development in general, and cognition–well-being associations in particular, can benefit from a dyadic perspective. Finally, we propose that innovative applications of contemporary methodology can be used to study dynamic cognition–well-being relations from a dyadic angle.
Dynamics of Cognition and Well-Being in Old Age
Research focusing on cognitive functioning and well-being in samples of unrelated individuals emphasizes the central role of these two domains of functioning in old age and how they are inherently involved in processes of adaptation and successful aging (Baltes & Baltes, 1990; Rowe & Kahn, 1997; Ryff & Singer, 1998). As to the direction of how aspects of cognition and well-being might be dynamically related, various unidirectional, bidirectional, and third-variable accounts have been advanced. One unidirectional position argues that low cognitive functioning may limit an individual's capacity to manage the routines of everyday life and thereby act as a risk factor for well-being decline (cf. Maier & Smith, 1999; see also Schmeichel, Volokhov, & Demaree, 2008). Another unidirectional position posits that the effect may go in the opposite direction with depressive thoughts occupying mental capacity, thereby resulting in less-than-optimal cognitive performance (e.g., Bäckman, Hill, & Forsell, 1996). A third position proposes a bidirectional relation according to which cognition and well-being constitute separable, yet reciprocally interacting sectors of the “good life” (Lawton, 1983). Finally, cognition-well-being relations may merely reflect the effects of an underlying third variable that relates to both domains (e.g., health constraints: Mroczek & Spiro, 2005; Verhaeghen, Borchelt, & Smith, 2003).
The overall empirical evidence concerning cognition–well-being relationships in individuals is less than conclusive. However, the application of methodological advances that capture processes as they unfold over time has helped disentangle the potentially time-ordered nature of such associations. Specifically, recent evidence suggests that, at least in later adulthood and old age, well-being might predict cognitive decline rather than the other way around. For example, Chow, Hamagami, and Nesselroade (2007) examined moment-to-moment changes in an experimental setting and reported that older adults manifested unidirectional dynamics where higher negative emotions in a given trial predicted lower cognitive performance two trials later. On a vastly different time metric, Gerstorf, Lövdén, Röcke, Smith, and Lindenberger (2007) examined long-term longitudinal changes among 70- to 100-year olds of the Berlin Aging Study and reported that well-being influenced subsequent decline in perceptual speed over time lags of two years with no evidence for a directed effect in the other direction. Based on these findings gathered from an explicit consideration of dynamic associations, we expect a directed effect from depressive symptoms (as a reversed indicator of well-being) onto subsequent long-term longitudinal change in cognitive functioning among the husbands and wives in the present study.
A Dyadic Perspective
In addition to examining individual-level relationships concerning cognition–well-being associations over time, the present study also addresses potential dynamics between developmental trajectories of husbands and wives. We therefore extend past research based on unrelated samples by targeting the degree to which husbands’ functioning in one domain relates to wives’ functioning in the same or another domain, and vice versa. Conceptually, such spousal interrelations represent one important instantiation of contextual influences on developmental trajectories (Baltes & Staudinger, 1996; Hoppmann & Gerstorf, in press). Examining spousal interrelations in elderly couples is especially interesting because older spouses are typically very close and emotionally invested in their relationship (Carstensen et al., 1996; Lang, 2001), and they are often long-term teams and share an extended history of joint experiences (Dixon, 1999; Meegan & Berg, 2002). Based on these lines of reasoning, we expect considerable spousal interrelations in such key domains of functioning as well-being and cognition. These interrelations can take various forms and benefit each individual spouse, but they can also hamper optimal functioning in the respective spouse. For example, age-related declines in cognitive functioning and mental health may require spousal tasks to be re-allocated, the learning of new skills as one spouse takes over tasks from the other spouse, or the adoption of a caregiver role by one spouse. These conceptual arguments map onto empirical reports from elderly couples documenting spousal interrelations in central life domains including activities, cognition, well-being, and health (Carstensen et al., 1995; Dufouil & Alperovitch, 2000; Gerstorf, Hoppmann, Anstey, & Luszcz, in press; Gruber-Baldini, Schaie, & Willis, 1995; Hoppmann, Gerstorf, & Luszcz, 2008; Skarupski et al., 2006; Townsend, Miller, & Guo, 2001).
In our study, the longitudinal couple data set allows us to go beyond past research on spousal interrelations by targeting dynamic lead-lag associations in indicators of two key domains of functioning, cognition (episodic memory) and well-being (depressive symptoms). Previous studies in the cognitive domain indicate that spouses become increasingly similar in old age (Gruber-Baldini et al., 1995) and achieve better outcomes when they collaborate with their spouse as compared to individual performance (Gould, Kurzman, & Dixon, 1994). Interestingly, past research also suggests that spousal interrelations in cognition may be unidirectional. For example, reports from the Seattle Longitudinal Study (Gruber-Baldini et al., 1995) indicate that husbands’ performance on an inductive reasoning task positively influenced their wives’ inductive reasoning performance seven years later, but not vice versa (see also Gerstorf et al., in press; Strawbridge, Wallhagen, Thai, & Shema, in press). We thus acknowledge that spousal interrelations in cognition may not be symmetric across partners, but that one partner may lead subsequent change in the other. We examine such gender-specificity of spousal interrelations in cognition in terms of the direction and size of possible lead-lag associations and determine whether husbands also predict subsequent change in their wives’ cognitive functioning in the present sample.
Conceptual notions about the central role of affective experiences in marriages, particularly in old age (Carstensen et al., 1996; Gottman & Notarius, 2000), as well as empirical reports of spousal similarities (Bookwala & Schulz, 1996; Goodman & Shippy, 2002; Sandberg & Harper, 2000; Tower & Kasl, 1996; Townsend et al., 2001) both suggest spousal interrelations in emotional functioning. Although these may exist for both positive and negative affect qualities, negative emotions appear to be more contagious (Gottman & Notarius, 2000; Larson & Almeida, 1999). Spousal interrelations in well-being and depression may also be gender specific and unidirectional; changes in well-being can originate in one spouse and be transmitted to the other partner. Conceptually, it has been argued that emotional transmission is more likely from husbands to wives rather than vice versa (Larson & Almeida, 1999). Relative to men, women may be more responsive to their partners’ well-being, have more permeable boundaries, and have less power in the relationship. All these proposed factors likely contribute to husbands exerting a bigger influence on wives’ well-being, rather than the other way around. Empirical reports addressing the gender-specificity of spousal interrelations in affective experiences in old age are primarily based on cross-sectional data (Peek, Stimpson, Townsend, & Markides, 2006; Stimpson, Peek, & Markides, 2006). In the current study, we extend these reports by exploring longitudinal gender-differential dynamics between elderly spouses and test if unidirectional predictive effects exist from husbands to wives.
To provide a meaningful interpretation of spousal dynamics in old age, a number of individual and spousal factors need to be taken into account. Specifically, our models will account for individual differences in age, education, and functional limitations to ensure that our findings do not simply reflect well-documented gender differences in (or direct effects of) these additional variables. In a subsequent step, we will also include spousal factors (length of marriage, number of children, and whether or not the couple remained intact over the study period) in order to target the possible role of these marriage characteristics for spousal dynamics in old age. We borrow our terminology from the dyadic literature (Cook & Kenny, 2005) and refer to associations of an individual’s characteristics with his or her own score on a given variable as actor effects (i.e., intra-spouse effects) and with the partner’s score on a given variable as partner effects (i.e., across-spouse effects). Because the two partners in a heterosexual marriage are different from one another and should not be treated as indistinguishable for theoretical reasons, we model gender-specific actor and partner effects and test if similar relationships emerge in husbands and wives.
A Dynamic Model of Dyadic Interrelations
From a methodological perspective, examining time-ordered interrelations between partners is analogous to the study of lead-lag associations between domains of functioning. For the purpose of the present study, we use a dynamic modeling tool, the dual change score model (DCSM; McArdle & Hamagami, 2001). This model has successfully been used to evaluate time-ordered developmental hypotheses of associations between variables of fluid and crystallized intelligence (Ferrer & McArdle, 2004; Finkel, Reynolds, McArdle, & Pedersen, 2007; Ghisletta & Lindenberger, 2005), social participation and cognition (Lövdén, Ghisletta, & Lindenberger, 2005), brain structure and cognition (McArdle et al., 2004), and well-being and cognition (Gerstorf et al., 2007). The DCSM thus promises to be a highly valuable tool to investigate temporal dynamics between possible antecedents and outcomes of developmental change (cf. Lövdén, Li, Shing, & Lindenberger, 2007). In the current study, we use a version of a DCSM to examine dynamic associations between two spouses rather than examining dynamic associations between domains of functioning in unrelated individuals (see also Gerstorf et al., in press; Hamagami, McArdle, & Fisher, 2006; McArdle, Hamagami, Kadlec, & Fisher, 2007). Specifically, we treat the couple as the unit of analysis and simultaneously model longitudinal changes in facets of well-being and cognition for both wives and husbands. This allows us to subject specific competing hypotheses about the nature of their dynamic interrelationships over time to empirical testing. For example, the DCSM allows us to empirically examine whether husbands’ cognitive functioning is predictive of subsequent decline in their wives’ cognitive performance. In addition, we can compare this test against models testing alternative hypotheses, for example, that wives’ cognitive performance precedes and predicts cognitive decline in husbands. These competing dynamic hypotheses will be tested at the intra-spouse level (e.g., well-being – cognition among wives) as well as at the across-spouse level, both within domains (e.g., cognition among wives – cognition among husbands) and across domains (e.g., well-being among wives – cognition among husbands).
For our study, we selected a word-recall test of episodic memory as a measure of cognitive functioning because it represents a powerful indicator of cognitive decline in old and advanced old age and has good psychometric properties (McArdle, Fisher, & Kadlec, 2007). To index well-being, we used the widely established Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977). The CES-D scale primarily assesses emotional-affective rather than cognitive-evaluative aspects of well-being, predominantly depressive symptoms, unpleasant affect, and mood.
Aims of the Present Study
This study attempts to examine dynamic interrelations in cognitive and emotional development between spouses in old and advanced old age. Using 10-year longitudinal data of initially married 1,599 couples from the AHEAD study (Mage = 75 years at T1), we ask three sets of questions. In a first step, we examine lead-lag actor or intra-spouse effects over successive two-year intervals by empirically determining whether unidirectional or bidirectional accounts best describe longitudinal memory–depression associations among wives and husbands: Testing unidirectional accounts, we ask whether level of memory predicts subsequent change in depressive symptoms or whether it is level of depressive symptoms that predicts change in memory. We also investigate bidirectional accounts that both lead-lag dynamics exist, be they of equal size or not.
In a second step, we consider lead-lag partner or across-spouse effects by exploring whether level of functioning of one partner in one domain predicts subsequent two-year change of the other partner in the same or a different domain. Drawing from evidence on emotional transmissions in middle-aged families, we expect unidirectional predictive effects of husbands for wives rather than vice versa both within and across the memory and depression domains.
In a final step, we introduce spousal covariates into our model (in addition to individual covariates included in all models) in order to examine if time-lagged relations are due to marriage characteristics. The variables targeted include age, education, and functional limitations as individual difference factors, as well as length of marriage, number of children, and whether or not the couple remained intact over the study period as spousal factors.
Method
A four-variable DCSM was fitted to six waves of 10-year longitudinal data (1993–2004) from initially married couples in the Asset and Health Dynamics Among the Oldest Old study (AHEAD). Descriptions of the larger AHEAD study, its design, participants, variables, and assessment procedures can be found in McArdle et al. (2007), Soldo, Hurd, Rodgers, and Wallace (1997), and Townsend et al. (2001). Select details relevant to the present study are given below.
Participants and Procedure
The total AHEAD sample (N = 8,222) represented a probability sample of households in the contiguous US of non-institutionalized adults aged 70 and older (i.e., born in 1923 or earlier; 74+% response rate). If more than one member of a sampled household was born pre-1924, one of them was randomly selected; and if that individual was married or living with a partner, the spouse or partner was also selected regardless of the age of that person. In our study, we included all married and co-residing couples with valid data on the variables of interest in 1993 (N = 1,599 couples or 3,198 individuals, 90+% White). At baseline assessment, couples were married for an average of 44.82 years (SD = 14.49; range: 1–77 years) and had an average of 2.99 children (SD = 2.07; range: 0–10). Relative to the remaining AHEAD sample, participants in the couple subsample were younger, 74.04 vs. 77.96 years, F (1, 8218) = 698.8; reported fewer depressive symptoms, 1.23 vs. 2.00, F (1, 7378) = 275.7; and performed better on memory, 8.40 vs. 7.15, F (1, 7192) = 186.7, all p’s < .001.
Interviews with participants aged 70 to 79 years were primarily conducted over the phone, whereas those aged 80 and older were generally interviewed in person. Targeted investigations found very few systematic differences between modes of assessment (Herzog & Rodgers, 1988). Over time, the same versions of the tests were administered. We use couple data from six waves of longitudinal assessments spanning 10 years: baseline in 1993 (T1; nwives = 1,599, nhusbands = 1,599); 1995 (T2; nwives = 1,404, nhusbands = 1,328) 1998 (T3; nwives = 1,237, nhusbands = 1,068); 2000 (T4; nwives = 1,097, nhusbands = 879); 2002 (T5; nwives = 949, nhusbands = 674); and 2004 (T6; nwives = 845, nhusbands = 549).1 T2 occurred on average 2.00 years (SD = 0.28), T3 4.24 years (SD = 0.44), T4 6.34 years (SD = 0.48), T5 8.53 years (SD = 0.50), and T6 10.43 years (SD = 0.50), respectively, after T1. By T2, T3, T4, T5, and T6, 6, 16, 26, 36, and 44%, respectively, had deceased; these mortality hazards are lower than in the residual sample (12, 27, 39, 51, and 61%, respectively).
Measures
Episodic memory was assessed using a unit-weight composite of performances on the immediate and delayed free recall tests. The immediate recall test was typically given near the end of the first interview quarter and asked participants to recall as many nouns as possible from a list of 10 nouns selected from four different lists. The delayed recall test was administered about five minutes later asking participants again to recall as many nouns as possible out of the earlier presented 10 nouns. Our measure represents the number of correct words remembered, ranging from 0 to 20. At all occasions, internal consistencies (across immediate and delayed recall sores) were good (wives: averaged α over time = .84, range .83 to .86; husbands: averaged α over time = .82, range .78 to .85). Details of the cognitive measures in the AHEAD study and their measurement properties are published in Brandt, Spencer, and Folstein (1988) and Ofstedal, Fisher, and Herzog (2005).
Depressive symptoms were assessed with eight items selected from the CES-D scale (Radloff, 1977). Items asked participants whether they had experienced the following symptoms “much of the time during the past week” (coded as 1) or not (coded as 0): Feeling depressed, everything was an effort, restless sleep, was (not) happy, felt lonely, (did not) enjoy life, felt sad, and could not get going. Positively valenced items were reverse coded. Our sum measure, computed if a participant answered one or more CES-D items, thus represents the number of depressive symptoms experienced frequently, with a possible range of 0 to 8. At all occasions, internal consistencies were good (wives: averaged α over time = .76, range .73 to .79; husbands: averaged α over time = .74, range .72 to .76). Details of the CES-D items in the AHEAD study and their measurement properties can be found in Townsend et al. (2001) and Turvey, Wallace, and Herzog (1999).
Covariates
To control for potential confounds, some of our models included individual (age, education, initial functional limitations) and spousal factors (length of marriage, number of children, and whether or not a couple remained intact over time) as time-invariant covariates. Education was measured as the number of years in school. We computed a composite sum index of functional limitations at T1 across items that asked whether or not respondents reported any difficulty in carrying out 10 instrumental activities of daily living. These activities included using the phone, managing money, taking medications, shopping groceries, preparing hot meals, walking several blocks, climbing one flight of stairs, lifting or carrying 10 lbs of weight, picking up a dime, and pushing or pulling large objects.
Data preparation
Scores for memory and depressive symptoms were standardized to a T metric (Mean = 50; SD = 10), with the T1 AHEAD couple sample (n = 3,198 individuals) providing the reference. This transformation ensured a common metric, while maintaining the psychometric properties of the scores and the longitudinal changes in means and variances. It also allowed for a direct comparison of scores between wives and husbands. No data imputation procedure was applied. The average longitudinal observation interval was 4.47 years (SD = 3.58) for wives and 3.97 years (SD = 3.46) for husbands. Table 1 presents the age at assessment along with means and standard deviations for memory and depressive symptoms, separately for wives and husbands. Participants were tested, on average, in their mid to late seventies and early eighties with an average three-year age difference between wives and husbands, F (1,1598) = 603.6, p < .001. Wives also reported, on average, more depressive symptoms, F (1,1598) = 34.7, p < .001, and performed better on the memory task than their husbands, F (1, 1598) = 168.0, p < .001, whereas differences in education were minor, F (1, 1598) = 14.1, p < .001. As is also evident, the means for memory increase at some later measurement occasions due to longitudinal attrition, but the magnitude of between-person differences in both variables was not differentially affected.2 Implications of nonrandom attrition for interpreting our results are considered in the Discussion section.
Table 1.
Age at Assessment and Descriptive Statistics for Measures Entered into the Four-Variable Dual Change Score Model: Data from the Study of Asset and Health Dynamics Among the Oldest Old (AHEAD).
| Wives |
Husbands |
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | n | Age | Mean | SD | n | Age | Mean | SD |
| Memory | ||||||||
| T1 | 1,599 | 72.25 | 51.97 | 10.21 | 1,599 | 75.82 | 48.03 | 9.39 |
| T2 | 1,391 | 73.94 | 53.11 | 9.99 | 1,309 | 77.43 | 48.24 | 9.37 |
| T3 | 1,232 | 75.76 | 52.43 | 10.11 | 1,059 | 79.11 | 47.76 | 9.49 |
| T4 | 1,083 | 77.31 | 52.41 | 9.48 | 866 | 80.64 | 47.33 | 8.88 |
| T5 | 940 | 79.03 | 51.16 | 9.18 | 666 | 82.32 | 46.35 | 8.83 |
| T6 | 834 | 80.71 | 49.78 | 9.29 | 542 | 83.89 | 45.72 | 8.06 |
| Depressive symptoms | ||||||||
| T1 | 1,599 | 72.25 | 50.90 | 10.63 | 1,599 | 75.82 | 49.10 | 9.24 |
| T2 | 1,404 | 73.96 | 50.95 | 11.02 | 1,328 | 77.47 | 48.73 | 9.32 |
| T3 | 1,237 | 75.78 | 53.93 | 11.69 | 1,068 | 79.13 | 51.26 | 10.15 |
| T4 | 1,097 | 77.37 | 53.97 | 11.93 | 879 | 80.68 | 51.24 | 10.28 |
| T5 | 949 | 79.06 | 54.10 | 11.81 | 674 | 82.36 | 51.12 | 10.67 |
| T6 | 845 | 80.76 | 53.66 | 11.81 | 549 | 83.94 | 51.68 | 10.68 |
| Education | ||||||||
| T1 | 1,599 | 72.25 | 11.77 | 3.07 | 1,599 | 75.82 | 11.48 | 3.81 |
| Functional limitations | ||||||||
| T1 | 1,587 | 72.25 | 1.22 | 1.63 | 1,597 | 75.84 | 0.97 | 1.56 |
Note. 1Memory and depressive symptoms were standardized to the T metric using the T1 AHEAD couple sample (N = 3,198) as the reference, Mean = 50, SD = 10. At baseline assessment, couples were married for an average of 44.82 years (SD = 14.49; range: 1–77 years) and had an average of 2.99 children (SD = 2.07; range: 0–10).
Statistical Procedures
In this study, we use a four-variable extension of the dual change score model (for extensive discussions of this model, see Ferrer & McArdle, 2004; McArdle & Hamagami, 2001). A DCSM combines features of the latent growth curve model (e.g., Meredith & Tisak, 1990) and the cross-lagged regressions approach (e.g., Rogosa, 1980). Similar to the group of latent growth curve models, the DCSM estimates latent intercept and slope factors for a given variable at the population level simultaneously with systematic variance around these means and a separate residual variance term. As an extension of the typical latent growth curve model, a two-variable DCSM additionally allows the estimation of time-lagged relations between states on a given variable (e.g., X at time t) and the reliable, error-free portion of subsequent change on another variable (e.g., change in Y between t and t+1). As an extension of the typical cross-lagged correlations between variables, a two-variable DCSM allows both adjusting for unequal reliabilities of the variables and separating dynamic changes within variables from lead-lag relations across variables.
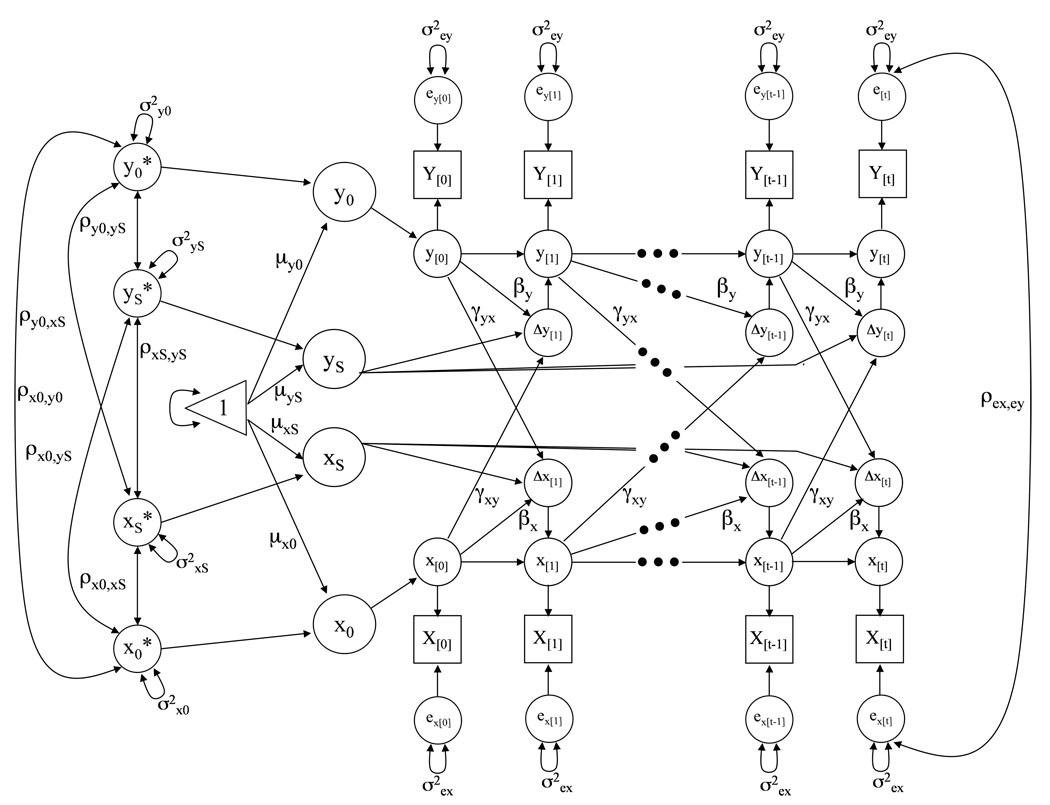
A graphical representation of a DCSM for a system of two variables X and Y is given in Figure 1. The diagram shows manifest variables as squares, latent variables as circles, the required constant as a triangle, fixed model parameters as one-headed arrows, and random parameters as two-headed arrows. Unlabeled paths are fixed to 1. The observed variables X[0], X[1], X[t−1], X[t], and Y[0], Y[1], Y[t−1], Y[t] represent the various assessments of the constructs X and Y, respectively. The scores x[t] (or y[t]) are defined as the unit-weighted sum of the latent score at x[t−1] (or y[t−1]) plus the latent difference score Δx[t] (or Δy[t]), which represent the latent, reliable change score between x[t−1] and x[t] (or y[t−1] and y[t]; McArdle & Hamagami, 2001). The intercept (x0, y0) and slope factors (xs, ys) are supposed to account for the time series of both variables X and Y. Intercepts denote an individual’s score at T1, and the slope factors relate to the difference scores Δx[t] and Δy[t] with a constant loading of 1, thus denoting linear two-year changes in our study. Intercepts and slopes are estimated at the population level (μx0, μxS; μy0, μyS), they are allowed to vary (σx0, σxS; σy0, σyS) and covary (ρx0xS; ρy0yS; ρx0y0; ρxSyS; ρx0ys; ρy0xS). The residual terms (ex; ey) are assumed to be normally distributed with a mean of zero and a time-invariant variance, and are allowed to covary between partners within time but not across time (ρex,ey).
Figure 1.
Graphical representation of a two-variable Dual Change Score Model (DCSM; McArdle & Hamagami, 2001) for a system of two variables X and Y. Observed (manifest) variables are represented by squares, unobserved (latent) variables by circles, regression weights by one-headed arrows, variances and covariances by two-headed arrows, and the triangle represents a constant indicating means and intercepts. All unlabeled paths are set to 1. In the current study, we applied a four-variable extension of a DCSM to examine dynamic associations between wives’ and husbands’ levels and longitudinal changes in episodic memory and depressive symptoms. Please see text for a more detailed description.
As an extension of typical linear growth curve models, the difference scores are defined as the unit-weighted sum of linear change within a given variable plus two additional lagged effects. The first addition is a self-feedback, auto-proportion parameter (βx, βy) indicating the effect of a given variable at time t–1 on change in the same variable between t–1 and t. For example, in our study, this parameter may represent the effect of level of wives’ depressive symptoms on subsequent change in wives’ depressive symptoms. The second addition is an inter-variable, cross-lagged parameter (γxy, γyx) signifying the effect of a given variable (e.g., X) at t–1 on subsequent change in the other variable (e.g., Y) between t–1 and t. For example, in our study, this parameter may represent the effect of level of wives’ depressive symptoms on subsequent change in husbands’ depressive symptoms. For simplicity and model identification, the values of β’s and γ’s are commonly assumed to be constant across time (although this assumption is testable). With β’s and γ’s set to zero, the two-variable DCSM is equivalent to a (two-variable) linear latent growth curve model.
Our major empirical interest in this study is in the cross-lagged coupling γ or dynamics parameters. Specifically, implementing a four-variable DCSM allows for a direct empirical comparison of competing substantive hypotheses about two sets of temporal dynamics: First, we can estimate four actor effects within spouses. Among wives, for example, performance on episodic memory tests may predict subsequent change in depressive symptoms (γW.MEM→W.DEP), or conversely, the number of depressive symptoms may predict subsequent memory change (γW.DEP→W.MEM). An additional two analogous dynamics will be estimated among husbands (γH.MEM→H.DEP, γH.DEP→H.MEM). Second, we can estimate a total of eight partner effects across spouses, four of these within the memory and depression domains and another four across both domains. Within domains, we can test whether husbands’ level of functioning predicts subsequent change in the same domain among their wives (γH.MEM→W.MEM; γH.DEP→W.DEP), or whether the effects are in the opposite direction with wives playing a leading role for change in husbands (γW.MEM→H.MEM; γW.DEP→H.DEP). Across domains, we can finally test and empirically compare competing hypotheses if a given partners’ functioning in one domain precedes and predicts subsequent change for the spouse in the other functional domain (γH.MEM→W.DEP; γW.DEP→H.MEM; γW.MEM→H.DEP; and γH.DEP→W.MEM).
To adjust for potentially inflated covariances among our variables due to their common association with known individual difference factors, all models include both wives’ and husbands’ age, education, and functional limitations as covariates. This also makes sure that our findings do not simply reflect well-documented gender-differences in (or direct effects of) these additional variables. The dynamics parameters reported are thus statistically adjusted for the non-linear change component of the auto-proportion parameters and for age, education, and functional limitations of both partners. Covariates were centered at the mean of the AHEAD couple sample resulting in intercept means, intercept variances, and slope-intercept covariances being estimated at age 75 years as well as average education and functional limitations. In total, our four-variable DCSM estimated 66 model parameters (four times seven parameters within each of the four time series, plus eight cross-variable intercorrelations among both wives and husbands, 16 cross-variable intercorrelations between wives and husbands, two covariances of time-invariant residual terms between wives and husbands for each memory and depressive symptoms3, and 12 dynamics parameters between the time series) as well as a total of 75 parameters for age, education, and functional limitations of both wives and husbands. In subsequent analyses, we additionally include several spousal characteristics (length of marriage, number of children, and whether or not the couple remained intact over the study period) as further covariates. Models were fit to the data using Mplus (Muthén & Muthén, 1998).4 Incomplete data were treated as missing at random (Little & Rubin, 1987) using full-information maximum likelihood (FIML) estimation algorithms to all data points available.
Results
In an initial step, we determined the relative amount of between-person and within-person variance in the data. Using random intercept-only models, we found that between-person variance (i.e., the intra-class correlation) in memory amounted to .49 for wives and .46 for husbands, with the remainder (wives: 51%; husbands: 54%) being within-person variation. Depressive symptoms revealed a similar pattern of within-person variation: 55% among wives and 58% among husbands. The data thus contain both substantial amounts of between-person differences and within-person variation over time. Noting that there were indeed intraindividual variations to model, a four-variable DCSM was used to describe and evaluate how the noted changes in episodic memory and depressive symptoms among wives and husbands were structured over time.
Our findings are organized in four sections. First, we use nested-model comparisons as an omnibus test to empirically examine if memory–depression dynamics indeed exist both at the actor level (intra-spouse effects) and partner level (across-spouse effects). Second, we focus on actor-level effects and closely examine various accounts of associations between memory and depressive symptoms within spouses, and we illustrate their differential direction and size. Third, we target partner-level effects and determine the direction and size of across-spouse, lead-lag associations between wives and husbands. Finally, we explore if and how additionally covarying for spousal factors (length of marriage, number of children, and whether or not the couple remained intact over the study period) had an effect on the dynamic associations found between episodic memory and depressive symptoms at either the actor level or the partner level.
Statistically Nested-Model Comparisons and Average Trends
To directly test if intra-spouse and across-spouse dynamics exist in the AHEAD study, we compare the goodness-of-fit indices of two statistically nested models. Specifically, a less parsimonious Full Dynamics model that freely estimated the four intra-spouse dynamics parameters along with the eight across-spouse dynamics parameters was compared to a more parsimonious model that set all 12 dynamics parameters to zero. Relative to the Full Dynamics model (χ2 = 544.2, df = 354), the No Dynamics model resulted in a highly significant loss of model fit (χ2 = 673.9, df = 366; Δχ2 = 129.7, df= 12, p < .001). This suggests that we can reject the null hypothesis that dynamics do not exist between memory and depressive symptoms at both actor and partner levels. Two other fit indices revealed a similar pattern of results – a lower Root Mean Square Error of Approximation (RMSEA; .018 vs. .023) and a higher Comparative Fit Index (CFI; .981 vs. .970) – indicating that the Full Dynamics model provided relatively better fit than the No Dynamics model.
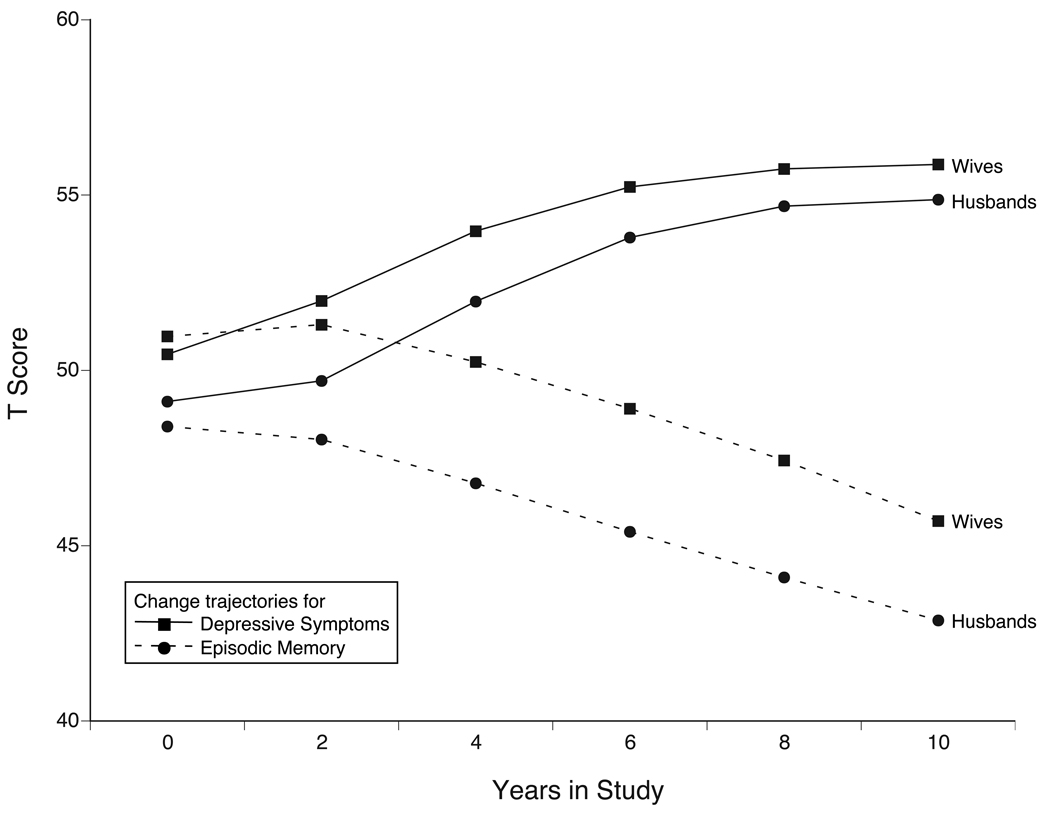
To provide the background for subsequent steps, Table 2 presents parameter estimates from the Full Dynamics model and Figure 2 graphically illustrates the conjoint product of the various change components modeled by our four-variable DCSM (i.e., linear changes, auto-proportion parameters as presented in Table 2 and the dynamics parameters as presented in Table 3). Specifically, the figure shows model-implied mean longitudinal change trajectories from the Full Dynamics model for wives’ episodic memory (X) and depressive symptoms (Y) and for husbands’ episodic memory (Z) and depressive symptoms (V), as produced by the following formulas: x[t] = 1*xs + (1+βx)*x[t−1] + γyx*y[t−1] + γzx*z[t−1] + γvx*v[t−1]; y[t] = 1*ys + (1+βy)*y[t−1] + γxy*x[t−1] + γzy*z[t−1] + γvy*v[t−1]; z[t] = l*zs + (1+βz)*z[t−1] + γxz*x[t−1] + γyz*y[t−1] + γvz*v[t−1]; and v[t] = 1*vs + (l+βv)*v[t−1] + γxv*x[t−1] + γyv*y[t−1] + γzv*z[t−1], when applied to a person aged 75 years, and with average education and functional limitations. For x[0], y[0], z[0] and v[0], the mean equals x0, y0, z0, and v0, respectively.5 Figure 2 shows that model-implied average changes for both spouses are sizeable across the 10-year study period (episodic memory: 0.5+ SD decline, and depressive symptoms: 0.5+ SD increase). These model-implied prototypical changes over time are somewhat different from the pattern of observed means displayed in Table 1. This discrepancy illustrates that the model produces estimates of average within-person change in the overall sample whether or not an individual (or couple) stayed in the sample over time (i.e., MAR assumption; cf. Gerstorf et al., 2007). Acknowledging these average trends over time allows us to better understand the direction and size of the dynamics parameters from the Full Dynamics model that will be presented in more detail in the following.
Table 2.
Results from a Four-Variable Dual Change Score Model (Full Dynamics) for Episodic Memory and Depressive Symptoms, Using the 10-Year AHEAD Couple Sample: Parameter Estimates for Memory and Depressive Symptoms.
| Wives |
Husbands |
|||
|---|---|---|---|---|
| Parameter | Episodic memory |
Depressive Symptoms |
Episodic memory |
Depressive Symptoms |
| Fixed effects | ||||
| Initial mean µ0 | 50.97* | 50.46* | 48.40* | 49.11* |
| (0.27) | (0.29) | (0.23) | (0.24) | |
| Slope mean µs | −4.16* | 2.11 | −0.48 | −0.33 |
| (1.95) | (1.39) | (1.33) | (0.22) | |
| Proportion β | −0.86* | 0.59 | 0.46 | −1.59* |
| (0.26) | (0.44) | (0.27) | (0.57) | |
| Random effects | ||||
| Initial variance σ20 | 41.49* | 35.16* | 29.63* | 19.43* |
| (2.99) | (2.62) | (1.84) | (2.44) | |
| Slope variance σ2s | 11.24 | 3.13 | 2.54 | 13.11 |
| (5.80) | (3.39) | (2.07) | (8.46) | |
| Error variance σ2e | 41.13* | 64.49* | 40.31* | 48.94* |
| (1.06) | (1.40) | (0.93) | (1.31) | |
| Correlations actor level | ||||
| Initial <-->slope | .20* | −.33 | −.29 | .38* |
| Memory initial <-->initial | – | −.14* | – | −.09* |
| Memory initial <-->slope | – | −.01 | – | .01 |
| Memory slope <-->initial | – | .25 | – | −.27 |
| Memory slope <-->slope | – | −.52 | – | −.75 |
| Correlations partner level | ||||
| Memory initial <-->initial | .11* | −.04 | – | −.05 |
| Memory initial <-->slope | −.49* | .04 | – | −.04 |
| Memory slope <-->initial | .12 | .45* | – | −.21* |
| Memory slope <-->slope | .75 | −.70 | – | −.52 |
| Depression initial <-->initial | – | .09 | – | – |
| Depression initial <-->slope | – | .51* | – | – |
| Depression slope <-->initial | – | −.49* | – | – |
| Depression slope <-->slope | – | .78 | – | – |
| Memory residual <-->residual | .02* | – | – | – |
| Depression residual <-->residual | – | .08* | – | – |
Note. 1, 599 couples at T1. Episodic memory and depressive symptoms were standardized to the T metric using the T1 AHEAD couple sample (N = 3,198) as the reference, Mean = 50, SD = 10. All estimates, except correlations, are unstandardized. Slope parameters divided by 10. The significance tests assigned to the correlations refer to the corresponding covariances. Estimates are residualized for age, education, and functional limitations of both partners. Model fit statistics: χ2 (df) = 544.2 (354); Comparative Fit Index, CFI = 0.981; and root mean square error of approximation, RMSEA = 0.018.
p < . 05 or below.
Figure 2.
Model-implied mean longitudinal change trajectories over time for wives’ and husbands’ episodic memory (dashed lines) and depressive symptoms (solid lines), as revealed from a four-variable Dual Change Score Model (Full Dynamics) for wives and husbands of sample average age (75 years). Estimated average changes for both wives and husbands were sizeable over the 10-year observation period (memory: 0.5+ SD decline, and depressive symptoms: 0.5+ SD increase).
Table 3.
Parameter Estimates from a Four-Variable Dual Change Score Model (Full Dynamics) of Memory (MEM) and Depressive Symptoms (DEP), Using the 10-Year AHEAD Sample: Dynamics Parameters.
| Parameter | Estimate | SE |
|---|---|---|
| Actor effects | ||
| Dynamics γH.MEM→H.DEP | −0.28 | 0.50 |
| Dynamics γH.DEP→H.MEM | 0.60 | 0.82 |
| Dynamics γW.MEM→W.DEP | 0.09 | 0.24 |
| Dynamics γW.DEP→W.MEM | −0.73* | 0.34 |
| Partner effects: Within-domain associations | ||
| Dynamics γH.DEP→W.DEP | −0.90 | 0.46 |
| Dynamics γW.DEP→H.DEP | 1.58* | 0.49 |
| Dynamics γH.MEM→W.MEM | 1.54* | 0.41 |
| Dynamics γW.MEM→H.MEM | −0.31 | 0.17 |
| Partner effects: Cross-domain associations | ||
| Dynamics γH.MEM→W.DEP | −0.19 | 0.38 |
| Dynamics γW.DEP→H.MEM | −0.60* | 0.27 |
| Dynamics γW.MEM→H.DEP | 0.31 | 0.32 |
| Dynamics γH.DEP→W.MEM | 0.97* | 0.37 |
Note. 1,599 couples at T1. Episodic memory (MEM) and depressive symptoms (DEP) were standardized to the T metric using the T1 AHEAD couple sample (N = 3,198) as the reference, Mean = 50, SD = 10. Unstandardized estimates and standard errors are presented. Estimates are residualized for age, education, and functional limitations of both partners.
Model fit statistics: χ2 (df) = 544.2 (354); Comparative Fit Index, CFI = 0.981; and root mean square error of approximation, RMSEA = 0.018.
p < .05 or below.
Dynamics of Cognition and Well-Being in Old Age
Having established, at an overall level, that time-ordered dynamics should not be disregarded, we move to the second step and specifically explore which memory–depression dynamics may exist at the actor or intra-spouse level. The dynamics parameters for the actor effects show (Table 3, rows 2 thru 6) that the cross-lagged effects from episodic memory onto depressive symptoms were not statistically significant (among wives: γ = 0.09, SE = 0.24; among husbands: γ = −0.28, SE = 0.50, both p’s > .10). The same was true for the predictive effect of depressive symptoms for change in episodic memory among husbands (γ = 0.50, SE = 0.28, p > .05). In contrast, the dynamics parameter of wives’ depressive symptoms onto subsequent changes in wives’ memory performance was negative and reliably different from zero (γ = −0.73, SE = 0.34, p < .05). Figure 2 displays the average trends over time to demonstrate how this dynamics parameter is embedded in other components of change and to interpret its direction. Specifically, the negative dynamics parameter indicates that more depressive symptoms precede and predict two-year negative deviations from linear decline in episodic memory (i.e., stronger than average decline).
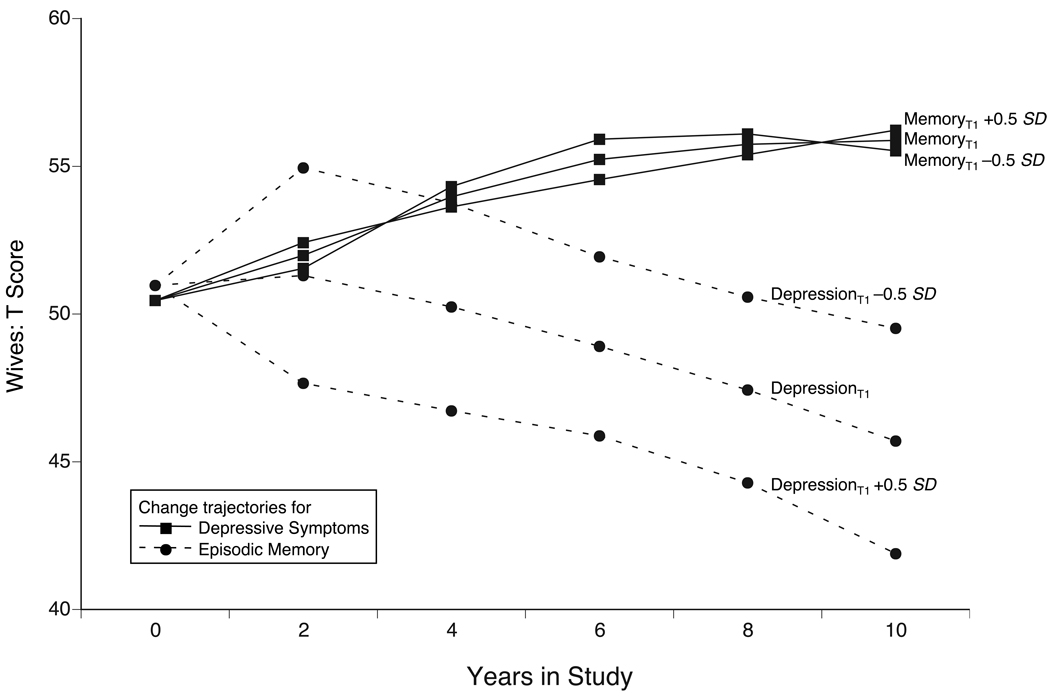
To illustrate the size and direction of these effects, Figure 3 uses a hypothetical scenario. Specifically, wives are assumed to vary in their initial sample means for depressive symptoms by half a standard deviation (i.e., 5 T-score units) while we keep constant the initial sample means for episodic memory (cf. Lövdén et al., 2005). In such a hypothetical case where all wives showed similar episodic memory performance at T1, but differed in their initial levels of depressive symptoms, it can be seen that the model-implied mean changes over time differ considerably from one another (dashed lines): Wives who reported more depressive symptoms initially (i.e., +0.5 SD) would subsequently show a relatively steep memory decline, whereas low initial depressive symptoms (i.e., −0.5 SD) would result in shallower memory decline over time. Conversely, if we graph changes in depressive symptoms as a function of different initial levels of memory (solid lines) we only see minor differentiating effects. In sum, our data suggest that depressive symptoms lead changes in episodic memory among wives, but not among husbands. No evidence was found for hypotheses proposing a leading role of episodic memory for change in depressive symptoms.
Figure 3.
Graphical illustration of the differential magnitude of dynamic actor effects between episodic memory and depressive symptoms in wives and their effects over time. Dashed lines represent model-implied sample means on wives’ episodic memory from a four-variable Dual Change Score Model (Full Dynamics) for the hypothetical case that the initial sample means for wives’ depressive symptoms were varied by half a standard deviation (i.e., 5 T-score units). Under the assumption of comparable wives’ episodic memory at T1, wives with few initial depressive symptoms (depressionT1 −0.5 SD) showed relatively shallow memory decline, whereas those with more depressive symptoms initially (depressionT1 +0.5 SD) showed relatively steep memory decline subsequently. In contrast, the solid lines indicate that wives’ depressive symptoms trajectories of change over time were minimally changed as a function of different initial levels of episodic memory at T1.
A Dyadic Perspective
In a third step, we consider lead-lag relations between wives and husbands by targeting the eight partner-level or across-spouse dynamics parameters, as presented in Table 3 (rows 7 through 16). We found that the direction and size of lead-lag associations between spouses varies by psychological domain and also crosses domains. In the depression domain, we found that the dynamics parameter from depressive symptoms of wives to subsequent change in depressive symptoms of husbands was positive and reliably different from zero (γ = 1.58, SE = 0.49, p < .001). Taking the average increase in depression over time into account (see Figure 2), the positive dynamics parameter suggests that more depressive symptoms among wives were predictive of stronger than average subsequent increases in depressive symptoms among husbands. In the memory domain, in contrast, the opposite unidirectional pattern between wives and husbands appeared to prevail: It was the memory performance of husbands that predicted subsequent change in memory among wives (γ = 1.54, SE = 0.41, p < .001) rather than the reverse (γ = −0.31, SE = 0.17, p > .10). The positive sign of the significant dynamics parameter in conjunction with the average memory decline over time (see Figure 2) suggests that better cognitive performance among husbands predicted shallower than average subsequent memory decline among wives. In addition, two across-domain partner dynamics effects also emerged. First is the predictive effect of depressive symptoms among wives for subsequent memory change among husbands (γ = −0.63, SE = 0.27, p < .05). This pattern indicates that more depressive symptoms among wives were related to stronger subsequent memory decline among husbands. Second is a predictive effect of husbands’ depression for subsequent memory change among wives (γ = 0.97, SE = 0.37, p < .01). The direction of this spousal dynamic appears to suggest some “buffering” effect according to which husbands’ depressive symptoms predicted better memory functioning over time for wives.
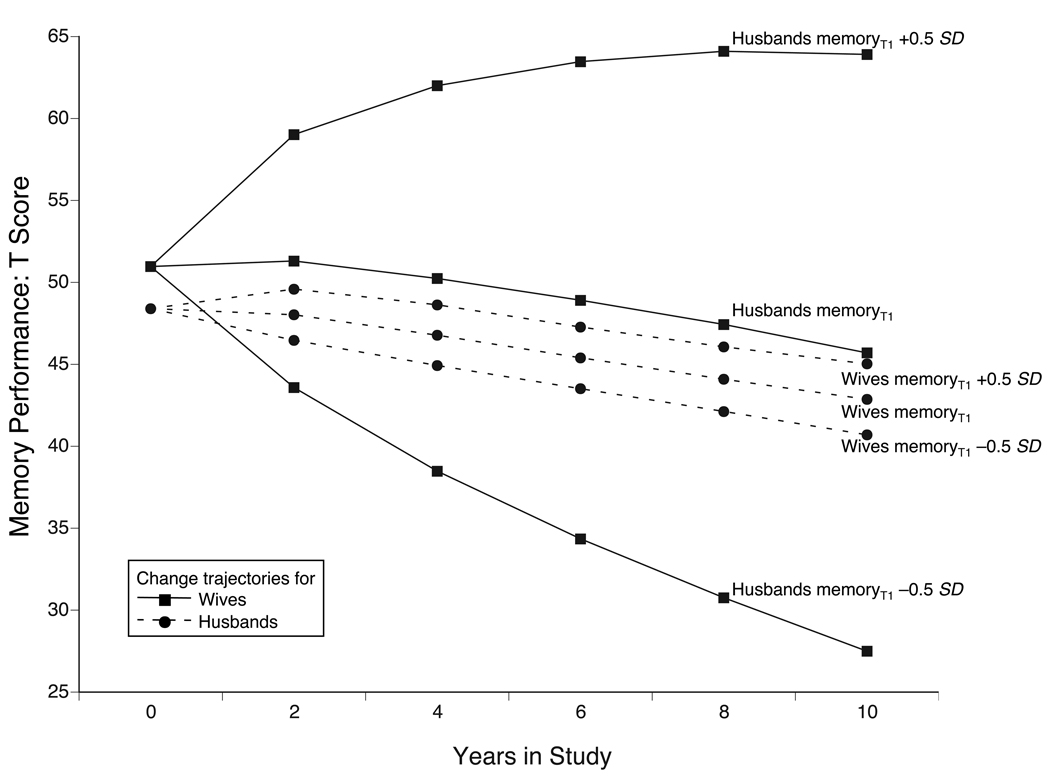
To illustrate the size and direction of one of these dynamic partner effects, we again use a hypothetical scenario (see Figure 4) in which all wives are hypothetically assumed to have identical initial memory performance at baseline with the only difference being that initial memory performance of husbands differs by ± 0.5 SD. Over time then, wives with cognitively fit husbands (+0.5 SD) would show much shallower subsequent memory decline, whereas wives with cognitively less fit husbands (−0.5 SD) would show much steeper subsequent memory decline (solid lines). In contrast, little evidence was found for a directed effect in the other direction: The dashed lines indicate that husbands’ episodic memory trajectories of change over time were minimally altered as a function of different initial levels of wives’ memory at T1. Figure 4 thus exemplifies what effects the differential magnitude of the spousal dynamics may have on the model-implied mean longitudinal change trajectories over time. In sum, parameter estimates from a Full Dynamics Model that covaried for individual differences in age, education, and functional limitations suggest that lead-lag relations over time lags of two years exist between married partners’ late-life developmental trajectories on memory and depressive symptoms.
Figure 4.
Graphical illustration of the differential magnitude of dynamic partner effects between wives’ and husbands’ episodic memory and their effects over time. Solid lines represent model-implied sample means on wives’ episodic memory from a four-variable Dual Change Score Model (Full Dynamics) for the hypothetical case that the initial sample means for husbands’ episodic memory were varied by half a standard deviation (i.e., 5 T-score units). Under the assumption of comparable wives’ episodic memory at T1, wives with cognitively fit husbands (husbands’ memoryT1 +0.5 SD) showed relatively shallow memory decline, whereas those with cognitively less fit husbands (husbands’ memoryT1 −0.5 SD) showed relatively steep memory decline. In contrast, the dashed lines indicate that husbands’ episodic memory trajectories of change over time were minimally changed as a function of different initial levels of wives’ episodic memory at T1.
The Role of Spousal Covariates
In a final step, we explored whether several spousal covariates had an effect on the dynamic associations found between episodic memory and depressive symptoms at either the actor level or the partner level. To start with, we note that the dynamics parameters, and all other model parameters, reported above were residualized for several individual covariates (ages, years of education, and functional limitations of both wives and husbands). This suggests that age, education, and functional limitations did not play a major role in accounting for differential dynamic associations between wives’ and husbands’ episodic memory and depressive symptoms.
Adding the length of marriage and the number of children as time-invariant spousal covariates of the latent factors of intercept and change did not substantially alter the dynamic structure reported earlier. Specifically, the No Dynamics model setting all 12 dynamic associations at the actor and partner level to zero described the structure of our data less precisely than the Full Dynamics model (χ2 = 580.3, df= 386 vs. χ2 = 707.5, df= 398; Δχ2 = 127.2, df = 12, p < .001). Consistent with the above results, we found that the actor dynamics effect of wives’ depressive symptoms predicting stronger decline in wives’ memory performance over time remained (γ = −0.70, SE = 0.32, p < .05). Similarly, the four partner dynamics effects identified above when we only controlled for the effects of individual covariates remained when we additionally controlled for spousal covariates: Within domains, we found that wives’ depressive symptoms predict a stronger increase in depressive symptoms among husbands (γ = 1.51, SE = 0.46, p < .001) and that husbands’ memory predicts less pronounced subsequent memory decline for wives (γ = 1.51, SE = 0.40, p <.001). Across domains, our results again indicated that wives’ depressive symptoms precede steeper memory decline among husbands (γ = −0.61, SE = 0.26, p <.01) and that husbands’ depressive symptoms predicted better memory functioning over time for wives (γ = 0.92, SE = 0.35, p <.01).6 Inclusion of individual and spousal covariates did not substantively alter the spousal dynamics reported, but contributed significant proportions of overall explained variance to the model parameters (e.g., wives’ level of episodic memory: R2 = .34 relative to R2 = .16 for a model that only included the ages of both partners as covariates; wives’ change in episodic memory: R2 = .14 relative to R2 = .05 for a model that only included the ages of both partners as covariates).
In a concluding series of follow-up analyses, we also found a substantively identical pattern as reported above when we restricted the sample to those couples who remained intact over time (i.e., both partners remained in the same household and provided data for all four variables of interest at each occasion: 1,199 couples at T2; 885 couples at T3; 670 couples at T4; 480 couples at T5; and 359 couples at T6; Full Dynamics model: χ2 = 481.5, df= 354 vs. No Dynamics model: χ2 = 575.3, df = 366; Δχ2 = 93.8, df = 12, p <.001). In a similar vein, dynamics of memory and depressive symptoms at the actor and partner level were present when we targeted very old spouses only (ncouples = 631 with an average age of wives and husbands at T1 of 75 years or more; Full Dynamics model: χ2 = 498.3, df = 354 vs. No Dynamics model: χ2 = 558.2, df = 366; Δχ2 = 59.9, df = 12, p < .001).
In sum, the covaried individual (education, functional limitations) and spousal factors (length of marriage, number of children) did not account for our major finding that wives and husbands appear to be dynamically active in the spousal system of level and change in episodic memory and depressive symptoms.
Discussion
The present study directly examined spousal interrelations in old age in a dynamic modeling framework and specifically examined indicators of cognitive fitness (episodic memory) and well-being (depressive symptoms) as time-varying entities in the dynamic system. We applied a four-variable Dual Change Score Model (McArdle & Hamagami, 2001) to 10-year incomplete longitudinal data of initially 1,599 married couples from the AHEAD study (Mage = 75 years at T1). Gender-specific lead-lag associations over two-year time lags were found both within and across spouses with the direction and size of these effects varying by psychological domain and also going across domains. Within spouses, depressive symptoms were associated with subsequent declines in memory in wives only. Across spouses, wives’ depressive symptoms were found to predict subsequent depression increase as well as memory decline among husbands. For memory, in contrast, better performance among husbands revealed protective effects against subsequent decline among wives with no evidence for a directed effect in the other direction. Possible individual covariates (age, education, functional limitations) and spousal covariates (length of marriage, number of children, and whether or not the couple remained intact over the study period) did not account for these differential lead–lag associations. We note that our study represents a first descriptive step and does not allow for causal inferences. Our findings of antecedent–consequent relations between wives and husbands are consistent with lifespan psychological notions that individual development both actively influences and is influenced by contextual factors such as close social relationships. Our discussion starts with an interpretation of the observed actor-level or intra-spouse effects. In a next step, we discuss findings concerning spousal interrelations at the partner level. Finally, we highlight important conceptual implications and methodological considerations as well as further steps necessary to substantiate our results.
Dynamics of Cognition and Well-Being in Old Age
In a first step, we provide an interpretation of actor-level findings concerning dynamic associations between cognition and well-being in our study. Both episodic memory and depressive symptoms showed sizeable changes over time, and between-person variability in these longitudinal changes was dynamically linked at the actor level among wives, but not among husbands. Findings for wives provide evidence for a unidirectional account of the dynamics: More depressive symptoms precede and predict two-year negative deviations from linear decline in episodic memory (i.e., stronger decline). These results are consistent with recent reports indicating that (decline in) aspects of cognitive functioning may not precede change in aspects of well-being, with such reports being derived from vastly different time metrics ranging from moment-to-moment variation in an experimental setting (Chow et al., 2007), to longitudinal change across time lags of two years over a total of 12 years (Gerstorf et al., 2007), and linking 60-year lifetime changes in cognitive functioning to well-being in old age (Gow et al., 2005). Our findings should not be taken to suggest that aspects of well-being represent a key factor underlying cognitive decline, but that components of well-being might moderate (e.g., slow down or accelerate) decline in indicators of cognitive functioning in later life (cf. Gerstorf et al., 2007). It is in this sense that our findings add to the growing body of research showing that aspects of well-being are not only a consequence of, but also a source for successful aging (Danner, Snowdon, & Friesen, 2001; Levy et al., 2002; Lyubomirsky, King, & Diener, 2005).
It is unclear though whether well-being directly or indirectly relates to the maintenance and decline of cognitive functioning in old age. One position argues that emotions exert physiological effects on cardiovascular and immune functioning that may have long-term effects on the brain and in turn influence level and change in cognitive performance (Danner et al., 2001; Kiecolt-Glaser et al., 2002). Another position refers to the resources necessary for proper cognitive functioning (see MacDonald et al., 2006; West et al., 2002); depressed feelings may occupy and distract substantial portions of these cognitive resources (e.g., from executive control), thereby resulting in less-than-optimal cognitive performance.
It remains an open conceptual question as to why we did not find similar associations among the husbands of this study. We would like to offer one potential reason, however. Specifically, results of the present study are in line with past research showing that women report higher overall levels of depressive symptoms than men (Anstey et al., in press; Barefoot et al., 2001). It could be that depressive symptoms only exert their negative effects on cognitive performance and change after surpassing a certain threshold, and such a scenario may have simply been true for more wives than husbands in the present sample. Future work may want to look at subgroups based on clinically defined depression scores to address such potential qualitative differences in the influence of subclinical and clinically relevant forms of depressive symptoms on cognitive performance and change. On a related note, we would like to highlight that the present study is in the all-too-rare position of having tested dynamic memory–depression associations using data from a large-scale sample with an equal number of men and women. Past research testing such associations in samples of unrelated individuals are often based on smaller samples with, relatively speaking, much more data provided by women than men (McArdle et al., 2007). This is certainly valid because old age is a phase in life that more women than men experience, but it may also have implicitly led to more insights into cognition–well-being relationships in women as compared to men. Large-scale data sets like ours may be particularly suited to explicitly investigate potential gender-specific associations.
A Dyadic Perspective
In line with the central notion of our study, most of our findings point to spousal interrelations in cognition–well-being relationships in elderly couples. These results can be referred to as partner effects illustrating that aging-related change in aspects of cognition and well-being is intrinsically linked between spouses. This patterning alone is interesting because it points to the utility of studying more than one focal person when examining trajectories of change in key domains of functioning across old age. Specifically, we found unidirectional predictive effects from depressive symptoms in wives for subsequent increase in depressive symptoms in husbands. This finding counters past research on affective transmissions in everyday life among midlife couples suggesting a greater susceptibility to spousal influences for wives than for husbands (Larson & Almeida, 1999). One possible explanation for our directed effect in the opposite direction may be related to post-retirement changes in the power structure within marriages. Perhaps spouses of the older adult cohorts examined here had to re-negotiate their roles when husbands left the labor force so that wives end up being more powerful in later life compared to earlier life phases. Another possible explanation relates to gender differences in dealing with emotionally challenging circumstances in late-life marriages. Carstensen and colleagues (1995), for example, showed that in long-term married couples, wives were much more likely to express their emotions, both positive and negative, whereas husbands exerted more emotional withdrawal and defensiveness. It is reasonable to assume that the emotionally expressive person is much more likely to transmit depressive symptoms to his or her spouse than the emotionally withdrawn person. Both lines of reasoning are at least partially consistent with reports from the EPESE study that long-term longitudinal associations over three to six years between spouses in old age go in both directions (Tower & Kasl, 1996).
Interestingly, wives’ depressive symptoms were not only related to their own but also to their husbands’ decline in episodic memory performance. We can only speculate as to the underlying mechanisms. One possible explanation is an important third variable that originates in wives and affects both partners: Social activity engagement. More specifically, past research on social activities indicates that wives take a more active role in initiating and performing social activities than husbands (Antonucci, 1994, 2001; Hoppmann et al., 2008). We also know from the literature that social activities place high demands on cognitive skills, thereby potentially protecting against cognitive decline (Fratiglioni, Paillard-Borg, & Winblad, 2004; Lövdén et al., 2005). Bringing these two lines of evidence together, spousal involvement in social activities by wives may represent one mechanism that buffers cognitive decline in themselves and their husbands as well.
Results from the present study also indicate that better cognitive performance in husbands related to subsequently less pronounced decline among wives. Our findings are consistent with reports from married couples in several large-scale longitudinal studies, which have also demonstrated that cognitive functioning among husbands was predictive of subsequent cognitive change among wives, but not vice versa (Gerstorf et al., 2008; Gruber-Baldini et al., 1995; Strawbridge et al., in press). Conjointly, these results suggest that late-life cognitive development is not solely a product of intraindividual resources, but appears to be closely intertwined between elderly spouses. One interpretation of the unidirectional nature of this cognitive partner effect is that a cognitively fit husband allows his wife to lead an active life, for example by engaging in social activities that challenge her cognitive abilities, whereas a cognitively impaired husband may require his wife to stay home more often and place her resources into the provision of care. To better understand such potential dynamics, future research must specifically target whether preserved cognitive fitness among husbands serves as a protective and buffering factor against wives’ decline in episodic memory, whether impaired cognition among husbands may act as a risk factor for cognitive decline among wives, or whether both mechanisms are operating in tandem.
A final partner effect was counter-intuitive and suggested a buffering effect of husbands’ depressive symptoms onto subsequent memory functioning for wives. From a conceptual perspective, this finding may be better understood if one pictures the potential consequences of husbands’ depressive symptoms within the spousal relationship system. For example, maybe wives with depressed husbands have to be alert and consequently outperform their female counterparts whose husbands are not emotionally challenged. We nevertheless think that this finding should be interpreted with caution; it will need to be replicated before it becomes an established phenomenon.
The Role of Individual and Spousal Covariates
In an attempt to address third-variable accounts for time-lagged associations between spouses’ episodic memory and depressive symptoms, all our models covaried for individual differences in age, education, and functional limitations. This approach ensured that our results do not simply reflect the role of these candidate factors that are known to differ between genders and to relate to either of the two domains of interest (Gottman & Notarius, 2000; Gruber-Baldini et al., 1995). We found no evidence that these individual difference factors or marriage characteristics (length of marriage, number of children, and whether a couple remained intact over time) that were included in subsequent steps played a major role in accounting for the spousal interrelations observed. Future research, however, would benefit from addressing these and other factors in more detail as potential sources for differential dynamics. It is conceivable that different trends for lead-lag relations may emerge when subgroups of spouses are specifically targeted. That is, spousal dynamics may be different or show change over time when resources in a given domain for one partner are taxed (e.g., Strawbridge, Wallhagen, & Shema, 2007). For example, when one partner drops below certain thresholds of functioning and experiences serious cognitive decline, questions arise as to how this affects the respective spouse’s well-being who all of a sudden sees himself or herself transitioning from the role of a spouse into the role of a caregiver (see Lyons, Zarit, Sayer, & Whitlatch, 2002). Future research may benefit from analyses aiming at theoretically defined subgroups and thereby shed light on qualitatively different processes of what is going on in a relationship at a specific point in time. Such endeavors are essential steps toward better understanding dynamic lead-lag relations between aspects of cognitive functioning and well-being in old and very old age. These limitations aside, we take our results to provide initial indications that spousal dynamics have sizeable effects on age-related change trajectories in key domains of functioning for both partners, and that these associations cannot be reduced to the set of antecedent conditions examined here.
Methodological Considerations: A Dynamic Model of Dyadic Interrelations
By employing contemporary methodologies, the current study was able to capture dynamic associations between spouses’ developmental change trajectories. In contrast to considering patterns of growth (e.g., static intercept–overall change correlations) in the variables under study as revealed in typical latent growth curve models, inspection of dynamic cross-lagged γ parameters in the four-variable DCSM informed us about lead-lag patterns in the data between wives and husbands (see Ferrer & McArdle, 2004). We have used this technique for a formal empirical comparison among different competing substantive hypotheses about if and how wives’ and husbands’ episodic memory and depressive symptoms show lagged developmental sequences. In doing so, the DCSM overcomes some of the limitations of related dynamic methods such as cross-lagged correlations (see also Rogosa, 1980). Specifically, the DCSM accounts for differential reliabilities and stabilities of the variables examined, and it separates intra-construct changes from inter-construct dynamics (cf. Ghisletta & Lindenberger, 2005; Lövdén et al., 2005). We also note that our study is one of the first to have extended the examination of dynamic antecedent-consequent associations beyond the two-variable case to higher-order multivariate models (for another recent example, see Ghisletta & Lindenberger, 2005). Such endeavors are important in comprehending how age-related changes in a given domain are embedded in complex systems of influence that are not fully described by a two-variable model. However, we acknowledge that this is a highly complex enterprise and caution against strong conclusions from our primarily descriptive analyses. We specifically point to the following methodological constraints.
To begin, the validity of our findings is conditional upon a number of statistical assumptions that include ergodocity, sample homogeneity, and data missing-at-random (cf. Lövdén et al., 2007). For example, the estimation of model parameters is based upon all longitudinal data segments, thereby giving implicitly more weight to couples who remained in the study. Because those couples were likely to be higher functioning, our results may underestimate the true extent of age-related change (i.e., decline in memory and increase in depressive symptoms) in the population. In addition, we acknowledge that for reasons of model identification we had to specify all dynamics parameters as time-invariant population parameters with no variance, which reflects the strict assumption that these dynamics are invariant across individuals (see Gerstorf et al., 2007). It may also be possible that the directional lead-lad associations found here among old-age spouses over two-year time lags do not generalize to other time metrics (e.g., from day to day) or different periods of the lifespan (e.g., mid-adulthood). Finally, we opted for modeling longitudinal change over time in study rather than combining the ages of wives and husbands into some arbitrary age composite that may then have served as the metric for the examination of change. As a consequence, it was not possible to determine if and how potential retest effects for episodic memory may have contributed to our findings. The existence of cognitive retest effects may not be a major concern regarding our findings of dynamic associations unless wives and husbands were to profit differentially from repeated test exposure. Given that previous examinations of retest effects in the AHEAD study indicated that such retest effects exist but were not different between women and men (Rodgers, Ofstedal, & Herzog, 2003), it appears permissible to not examine retest effects.
Limitations and Outlook
We have found longitudinal evidence for antecedent–consequent relations between aspects of married spouses’ cognitive and emotional functioning in that wives and husbands are both dynamically and differentially active in the couple system. We would like to highlight, however, that we are just beginning to illuminate the complex interplay of how contextual factors, like significant others, actively shape and are shaped by developmental changes in key domains of functioning across the adult lifespan and old age (Baltes & Staudinger, 1996; Cairns et al., 1996). The present study sheds some initial light on the developmental ordering and spousal interrelations in two psychological domains in old and advanced old age. We think that these initial efforts deserve further replication and extension. One of the major open questions relates to the direction and size of dynamic associations between spouses. It could be that the conundrum of equivocal research reports mirrors the fact that the nature and functional implications of dyadic associations depend upon the domain considered (e.g., cognition vs. emotion), the time metric examined (e.g., daily stress vs. long-term longitudinal change over several years), and the age groups under study (e.g., middle-aged vs. old age). Much work remains to be done to resolve these and other questions, and we believe that the current study represents one step along the way toward that greater goal.
Acknowledgments
Work on this project began while Denis Gerstorf was at the Department of Psychology, University of Virginia and while Christiane A. Hoppmann was at the School of Psychology at the Georgia Institute of Technology, GA, USA, both supported by separate Research Fellowships awarded by the German Research Foundation (DFG). This research was also supported by a MERIT award from the National Institute on Aging (AG-007137) to John J. McArdle.
The Study of Asset and Health Dynamics Among the Oldest Old study (AHEAD) was supported by a cooperative agreement (Grant No. UO1 AG09740) between the National Institute on Aging (NIA) and the University of Michigan. We thank Nilam Ram for very helpful comments on the article and Frank Infurna for his help in compiling the data set.
Footnotes
The number of intact couples in which both partners provided data for all four variables of interest at each occasion were as follows: 1,199 couples at T2; 885 couples at T3; 670 couples at T4; 480 couples at T5; and 359 couples at T6.
Both memory and depressive symptoms not only displayed significant change over time and variability in this change, but also showed comparable stability over time at the level of interindividual differences. Specifically, averaged autocorrelation coefficients were r = .47 (p < .001, range from r = .35 to r = .56) for memory of wives; r = .46 (p < .001, range from r = .34 to r = .54) for memory of husbands; r = .45 (p < .001, range from r = .35 to r = .54) for well-being of wives; and r = .41 (p < .001, range from r = .32 to r = .52) for well-being of husbands. At a given measurement occasion, the average within-person correlations between the measures of memory and depression were comparable across spouses with r = −.14 (p < .001, range from r = −.11 to r = −.23) for wives and r = −.21 (p < .001, range from r = −.17 to r = −.27) for husbands. Also, spousal similarities at a given occasion tended to be somewhat stronger for depression (average correlation at a given occasion was r = .29, p < .001, range from r = .25 to r = .35) as compared with memory (average correlation at a given occasion was r = .17, p < .001, range from r = .09 to r = .24).
Allowing the measurement-specific variance to be correlated between persons who take the measure provided superior fit (χ2 = 544.2, df = 354) over a more parsimonious model that set these covariances to zero (χ2 = 600.00, df = 356; Δχ2 = 55.8, Δdf = 2, p < .001).
The MPLUS scripts for all analyses are available from the senior author upon request.
These formulas calculate the implied mean longitudinal trajectories from the four-variable DCSM reported in Table 2 and Table 3 for each variable. Specifically, results from the model are translated into change equations that represent biyearly change on a given variable as a function of itself and all other components in the model. More details can be found in Ferrer & McArdle (2004), Ghisletta and Lindenberger (2005), and Lövdén et al. (2005, 2007).
Targeting the significance of the dynamics parameters in a series of nested model comparisons revealed the same substantive pattern of results. For example, not allowing for a lagged across-spouse or partner effect from memory of husbands for subsequent changes in memory of wives resulted in highly significant losses in fit relative to the Full Dynamics model (γH.MEM→W.MEM = 0: Δχ2 = 15.3, df = 1, p < .001).
Contributor Information
Denis Gerstorf, Email: gerstorf@psu.edu.
Christiane A. Hoppmann, Email: ch295@mail.gatech.edu.
Kelly M. Kadlec, Email: kadlec@usc.edu.
John J. McArdle, Email: jmcardle@usc.edu.
References
- Anstey KJ, van Sanden C, Cox KS, Luszcz MA. Prevalence and risk factors for depression in a longitudinal, population-based study including individuals in the community and residential care. Australian Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e31802e21d8. (in press) [DOI] [PubMed] [Google Scholar]
- Antonucci TC, Akiyama H. Social relationships and aging well. Generations. 1991;15:39–44. [Google Scholar]
- Antonucci TC. A life-span view of women's social relations. In: Turner BF, Troll L, editors. Women growing older: Psychological perspectives. Thousand Oaks, CA: Sage; 1994. pp. 239–269. [Google Scholar]
- Antonucci TC. Social relations: An examination of social networks, social support, and sense of control. In: Birren JE, editor. Handbook of the psychology of aging. San Diego, CA: Academic Press; 2001. pp. 427–453. [Google Scholar]
- Bäckman L, Hill RD, Forsell Y. The influence of depressive symptomatology on episodic memory functioning among clinically nondepressed older adults. Journal of Abnormal Psychology. 1996;105:97–105. doi: 10.1037//0021-843x.105.1.97. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM, editors. Successful aging: Perspectives from the behavioral sciences. New York, NY: Cambridge University Press; 1990. [Google Scholar]
- Baltes PB, Staudinger UM, editors. Interactive minds: Life-span perspectives on the social foundation of cognition. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- Baltes MM, Carstensen LL. Social-psychological theories and their applications to aging: From individual to collective. In: Bengtson VL, Schaie KW, editors. Handbook of theories of aging. New York, NY: Springer; 1999. pp. 209–226. [Google Scholar]
- Barefoot JC, Mortensen EL, Helms MJ, Avlund K, Schroll M. A longitudinal study of gender differences in depressive symptoms from age 50 to 80. Psychology and Aging. 2001;16:342–345. doi: 10.1037//0882-7974.16.2.342. [DOI] [PubMed] [Google Scholar]
- Berg CA, Upchurch R. A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychological Bulletin. 2007;133:920–954. doi: 10.1037/0033-2909.133.6.920. [DOI] [PubMed] [Google Scholar]
- Bookwala J, Schulz R. Spousal similarity in subjective well-being: The cardiovascular health study. Psychology and Aging. 1996;11:582–590. doi: 10.1037//0882-7974.11.4.582. [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Cambridge. MA: Harvard University Press; 1979. [Google Scholar]
- Cairns RB, Elder GH, Costello EJ. Developmental science. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- Carmelli D, Swan GE, Larue A, Eslinger PJ. Correlates of change in cognitive function in survivors from the Western Collaborative Group Study. Neuroepidemiology. 1997;16:285–295. doi: 10.1159/000109699. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Gottman JM, Levenson RW. Emotional behavior in long-term marriage. Psychology and Aging. 1995;10:140–149. doi: 10.1037//0882-7974.10.1.140. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Graff J, Levenson RW, Gottman JM. Affect in intimate relationships: The developmental course of marriage. In: Magai C, McFadden SH, editors. Handbook of Emotion, Adult Development, and Aging. San Diego, CA: Academic Press; 1996. pp. 227–247. [Google Scholar]
- Chow S-M, Hamagami F, Nesselroade JR. Age differences in dynamical emotion-cognition linkages. Psychology and Aging. 2007;22:765–780. doi: 10.1037/0882-7974.22.4.765. [DOI] [PubMed] [Google Scholar]
- Cook WL, Kenny DA. The actor-partner interdependence model: A model of bidirectional effects in developmental studies. International Journal of Behavioral Development. 2005;29:101–109. [Google Scholar]
- Danner DD, Snowdon DA, Friesen WV. Positive emotions in early life and longevity: Findings from the Nun Study. Journal of Personality and Social Psychology. 2001;80:804–813. [PubMed] [Google Scholar]
- Dixon RA. Exploring cognition in interactive situations: The aging of N+1 minds. In: Hess TM, Blanchard-Fields F, editors. Social Cognition and Aging. San Diego, CA: Academic Press; 1999. pp. 267–290. [Google Scholar]
- Dufouil C, Alperovitch A. Couple similarities for cognitive functions and psychological health. Journal of Clinical Epidemiology. 2000;53:589–593. doi: 10.1016/s0895-4356(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Developmental Psychology. 2004;40:935–952. doi: 10.1037/0012-1649.40.6.935. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Lövdén M, Röcke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology. 2007;43:705–718. doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Hoppmann CA, Anstey KJ, Luszcz M. Dynamic links of cognitive functioning among married couples: Longitudinal evidence from the Australian Longitudinal Study of Ageing. Psychology and Aging. doi: 10.1037/a0015069. (in press) [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Exploring structural dynamics within and between sensory and intellectual functioning in old and very old age: Longitudinal evidence from the Berlin Aging Study. Intelligence. 2005;33:555–587. [Google Scholar]
- Goodman CR, Shippy RA. Is it contagious? Affect similarity among spouses. Aging and Mental Health. 2002;6:266–274. doi: 10.1080/13607860220142431. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Notarius CI. Decade review: Observing marital interaction. Journal of Marriage and the Family. 2000;62:927–947. [Google Scholar]
- Gould O, Kurzman D, Dixon RA. Communication during prose recall conversations by young and old dyads. Discourse Processes. 1994;17:149–165. [Google Scholar]
- Gow AJ, Whiteman MC, Pattie A, Whalley L, Starr J, Deary IJ. Lifetime intellectual function and satisfaction with life in old age: Longitudinal cohort study. British Medical Journal. 2005;331:141–142. doi: 10.1136/bmj.38531.675660.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Schaie KW, Willis SL. Similarity in married couples: A longitudinal study of mental abilities and rigidity-flexibility. Journal of Personality and Social Psychology. 1995;69:191–203. doi: 10.1037//0022-3514.69.1.191. [DOI] [PubMed] [Google Scholar]
- Hamagami F, McArdle JJ, Fisher G. A dynamic structural analysis of depression-cognition cycles in the Health and Retirement Study data. Department of Psychology, University of Southern California; 2006. Unpublished Manuscript. [Google Scholar]
- Herzog RA, Rodgers WL. Interviewing older adults: Mode comparison using data from a face-to-face survey and a telephone survey. Public Opinion Quarterly. 1988;52:84–99. [Google Scholar]
- Hoppmann CA, Gerstorf D. Spousal interrelations in old age. Gerontology. doi: 10.1159/000211948. (in press) [DOI] [PubMed] [Google Scholar]
- Hoppmann CA, Gerstorf D, Luszcz M. On the interplay between spousal social activity trajectories in the Australian Longitudinal Study on Aging in the context of cognitive, physical, and emotional resources. Journals of Gerontology Series B: Psychological Sciences. 2008;63B:P41–P50. doi: 10.1093/geronb/63.1.p41. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Smith J. Positive and negative affect in very old age. Journals of Gerontology Series B: Psychological Sciences. 2003;58B:P143–P152. doi: 10.1093/geronb/58.3.p143. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Lang FR. Regulation of social relationships in later adulthood. Journals of Gerontology Series B: Psychological Sciences. 2001;56B:P321–P326. doi: 10.1093/geronb/56.6.p321. [DOI] [PubMed] [Google Scholar]
- Larson RW, Almeida DM. Emotional transmission in the daily lives of families: A new paradigm for studying family process. Journal of Marriage and the Family. 1999;61:5–20. [Google Scholar]
- Lawton MP. Environment and other determinants of well-being in older people. Gerontologist. 1983;23:349–357. doi: 10.1093/geront/23.4.349. [DOI] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Kunkel SR, Kasl SV. Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology. 2002;83:261–270. doi: 10.1037//0022-3514.83.2.261. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Li SC, Shing YL, Lindenberger U. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and advanced old age: Longitudinal data from the Berlin Aging Study. Neuropsychologia. 2007;45:2827–2838. doi: 10.1016/j.neuropsychologia.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Luszcz MA. Predictors of memory in young-old and old-old adults. International Journal of Behavioral Development. 1992;15:147–166. [Google Scholar]
- Lyons KS, Zarit SH, Sayer AG, Whitlatch CJ. Caregiving as a dyadic process: Perspectives from caregiver and receiver. Journals of Gerontology: Psychological Sciences and Social Sciences. 2002;57B:P195–P204. doi: 10.1093/geronb/57.3.p195. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: Does happiness lead to success? Psychological Bulletin. 2005;131:803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Maier H, Smith J. Psychological predictors of mortality in old age. Journals of Gerontology Series B: Psychological Sciences. 1999;54B:P44–P54. doi: 10.1093/geronb/54b.1.p44. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Hultsch DF, Bunce D. Intraindividual variability in vigilance performance: Does degrading visual stimuli mimic age-related neural noise? Journal of Clinical and Experimental Neuropsychology. 2006;28:655–675. doi: 10.1080/13803390590954245. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 137–176. [Google Scholar]
- McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992 – 2004. Psychology and Aging. 2007;22:525–545. doi: 10.1037/0882-7974.22.3.525. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F, Jones K, Jolesz F, Kikinis R, Spiro A, et al. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the Normative Aging Study. Journals of Gerontology Series B: Psychological Sciences. 2004;59B:P294–P304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F, Kadlec K, Fisher G. A dynamic structural analysis of dyadic cycles of depression in the Health and Retirement Study data. Department of Psychology, University of Southern California; 2007. Unpublished Manuscript. [Google Scholar]
- Meegan SP, Berg CA. Contexts, functions, forms, and processes of collaborative everyday problem solving in older adulthood. International Journal of Behavioral Development. 2002;26:6–15. [Google Scholar]
- Meredith W, Tisak J. Latent curve analysis. Psychometrica. 1990;55:107–122. [Google Scholar]
- Mroczek DK, Spiro A., III Change in life satisfaction during adulthood: Findings from the Veterans Affairs Normative Aging Study. Journal of Personality and Social Psychology. 2005;88:189–202. doi: 10.1037/0022-3514.88.1.189. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus, the comprehensive modeling program for applied researchers user’s guide. Los Angeles, CA: Author; 1998. [Google Scholar]
- Ofstedal MB, Fisher GG, Herzog AR. Documentation of cognitive functioning measures in the Health and Retirement Study. Ann Arbor: University of Michigan; 2005. (HRS/AHEAD Documentation Report DR-006) [Google Scholar]
- Peek MK, Stimpson JP, Townsend AL, Markides KS. Well-being in older Mexican American spouses. The Gerontologist. 2006;46:258–265. doi: 10.1093/geront/46.2.258. [DOI] [PubMed] [Google Scholar]
- Radloff LW. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rodgers WL, Ofstedal MB, Herzog RA. Trends in scores on tests of cognitive ability in the elderly U.S. population, 1993–2000. Journals of Gerontology: Psychological Sciences and Social Sciences. 2003;58B:338–346. doi: 10.1093/geronb/58.6.s338. [DOI] [PubMed] [Google Scholar]
- Rogosa D. A critique of cross-lagged correlation. Psychological Bulletin. 1980;88:245–258. [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9:1–28. [Google Scholar]
- Sandberg JG, Harper JM. In search of a marital distress model of depression in older marriages. Aging and Mental Health. 2000;4:210–222. [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology. 2008;95:1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Skarupski KA, Mendes De Leon CF, McCann JJ, Bienias JL, Wilson RS, Evans DA. Is lower cognitive function in one spouse associated with depressive symptoms in the other spouse? Aging and Mental Health. 2006;10:621–630. doi: 10.1080/13607860600844184. [DOI] [PubMed] [Google Scholar]
- Soldo B, Hurd M, Rodgers W, Wallace R. Asset and Health Dynamics Among the Oldest Old: An overview of the AHEAD study. Journals of Gerontology: Psychological Sciences and Social Sciences. 1997;52B:1–20. doi: 10.1093/geronb/52b.special_issue.1. [DOI] [PubMed] [Google Scholar]
- Stimpson JP, Peek MK, Markides KS. Depression and mental health among older Mexican American spouses. Aging and Mental Health. 2006;10:386–393. doi: 10.1080/13607860500410060. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Shema SJ. Impact of spouse vision impairment on partner health and well-being: a longitudinal analysis of couples. Journal of Gerontology: Social Sciences. 2007;62:315–322. doi: 10.1093/geronb/62.5.s315. [DOI] [PubMed] [Google Scholar]
- Strawbridge W, Wallhagen M, Thai J, Shema S. The influence of spouse lower cognitive function on partner health and well-being among community-dwelling older couples: Moderating roles of gender and marital problems. Aging and Mental Health. doi: 10.1080/13607860802607330. (in press) [DOI] [PubMed] [Google Scholar]
- Tower RB, Kasl SV. Depressive symptoms across older spouses: Longitudinal influences. Psychology and Aging. 1996;11:683–697. doi: 10.1037//0882-7974.11.4.683. [DOI] [PubMed] [Google Scholar]
- Townsend AL, Miller B, Guo S. Depressive symptomatology in middle-aged and older married couples: A dyadic analysis. Journals of Gerontology: Psychological Sciences and Social Sciences. 2001;56B:S352–S364. doi: 10.1093/geronb/56.6.s352. [DOI] [PubMed] [Google Scholar]
- Turvey C, Wallace R, Herzog R. Depression: A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychology. 2003;22:559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FIM, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. Journals of Gerontology Series B: Psychological Sciences. 2002;57B:P246–P255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- Zajonc RB, Marcus GB. Birth order and intellectual development. Psychological Review. 1975;82:74–88. [Google Scholar]