Abstract
Klotho is a transmembrane protein expressed primarily in kidney, parathyroid gland, and choroid plexus. The extracellular domain could be cleaved off and released into the systemic circulation. Klotho is in part effective as β-glucuronidase regulating protein stability in the cell membrane. Klotho is a major determinant of aging and life span. Overexpression of Klotho increases and Klotho deficiency decreases life span. Klotho deficiency may further result in hearing loss and cardiac arrhythmia. The present study explored whether Klotho modifies activity and protein abundance of KCNQ1/KCNE1, a K+ channel required for proper hearing and cardiac repolarization. To this end, cRNA encoding KCNQ1/KCNE1 was injected in Xenopus oocytes with or without additional injection of cRNA encoding Klotho. KCNQ1/KCNE1 expressing oocytes were treated with human recombinant Klotho protein (30 ng/ml) for 24 h. Moreover, oocytes which express both KCNQ1/KCNE1 and Klotho were treated with 10 µM DSAL (D-saccharic acid-1,4-lactone), a β-glucuronidase inhibitor. The KCNQ1/KCNE1 depolarization-induced current (IKs) was determined utilizing dual electrode voltage clamp, while KCNQ1/KCNE1 protein abundance in the cell membrane was visualized utilizing specific antibody binding and quantified by chemiluminescence. KCNQ1/KCNE1 channel activity and KCNQ1/KCNE1 protein abundance were upregulated by coexpression of Klotho. The effect was mimicked by treatment with human recombinant Klotho protein (30 ng/ml) and inhibited by DSAL (10 µM). In conclusion, Klotho upregulates KCNQ1/KCNE1 channel activity by 'mainly' enhancing channel protein abundance in the plasma cell membrane, an effect at least partially mediated through the β-glucuronidase activity of Klotho protein.
Keywords: Klotho, K+ channels, cardiac action potential, hearing, renal proximal tubule
Introduction
Klotho, a protein expressed in a wide variety of tissues including kidney,1,2 has a profound impact on aging and life span.3,4 The extracellular domain of Klotho may function as protease or hormone.5 Klotho deficiency results in severe growth retardation and accelerated aging eventually leading to early death.3 Klotho overexpression leads to substantial prolongation of life span.3,4 Klotho is required for the inhibitory effect of FGF23 on 1,25(OH)2D3 producing 1α-hydroxylase.2,4,6,7 1,25(OH)2D3 stimulates intestinal and renal Ca2+ and phosphate transport.8,9 In part due to excessive 1,25(OH)2D3 formation, Klotho deficiency increases plasma Ca2+ 10 and phosphate9 concentration, resulting in vascular calcification,11 growth deficit,2 and rapid aging.2,6,7 Klotho insufficiency further leads to hearing loss, cardiac arrhythmia, and sudden cardiac death.1 Moreover, Klotho deficiency enhances glucose tolerance.12
Hearing loss and cardiac arrhythmia may result from genetic defects of the K+ channel subunits KCNE1 or KCNQ1.13-15 Moreover, KCNQ1 polymorphisms have been associated with diabetes.16,17 KCNQ1/KCNE1 is expressed in a variety of tissues including the heart,13,15 skeletal muscle,18 stria vascularis of the inner ear,19 renal proximal tubule,20 gastric parietal cells,21-23 intestinal epithelia,20,22-26 and hepatocytes.27-29 KCNQ1 knockout mice suffer from deafness30,31 and impairment of gastric acid secretion,31,32 as well as intestinal electrolyte and substrate transport.33 KCNQ1 deficiency further impairs cell volume regulation.28,29,34-36 KCNQ1 deficiency further affects cardiac repolarization.37
Besides its impact on 1,25(OH)2D3 formation, Klotho may regulate Na+, phosphate cotransport,38,39 Na+/K+ ATPase,40 Ca2+ channels41 and renal outer medullary K+ channels42 by more direct influence on the channels and transport proteins. The present study thus explored whether Klotho modifies the function of KCNQ1/KCNE1 channels. To this end, voltage-gated current was determined in Xenopus oocytes expressing KCNQ1/KCNE1 with or without coexpression of Klotho, treatment with human recombinant Klotho protein or treatment with DSAL (D-saccharic acid-1,4-lactone), a β-glucuronidase inhibitor. Moreover, the effect of Klotho coexpression on KCNQ1/KCNE1 protein abundance at the cell membrane was quantified by chemiluminescence and visualized by confocal microscopy.
Results
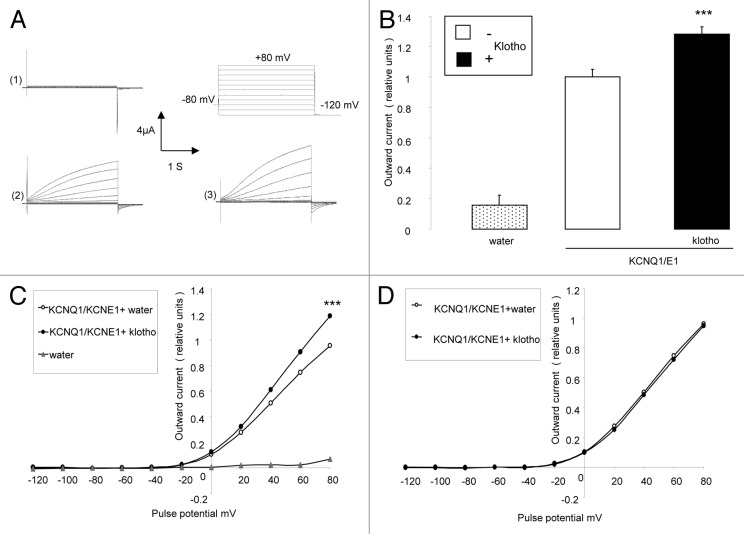
The present study explored, whether Klotho modifies KCNQ1/KCNE1 channels. To this end, cRNA encoding KCNQ1/KCNE1 was injected into Xenopus oocytes without or with cRNA encoding Klotho. In KCNQ1/KCNE1 expressing, but not in water-injected Xenopus oocytes, outward currents (IKs) were observed following depolarizing pulses (up to +80 mV), applied from a holding potential of -80 mV (Fig. 1A). Additional expression of Klotho in KCNQ1/KCNE1 expressing oocytes was followed by a significant increase in the amplitude of the peak outward current (IKs) at +80 mV (Fig. 1B and C). Plotting the amplitude of the peak outward current (IKs) against the corresponding pulse potential revealed the typical slow-delayed activation of KCNQ1/KCNE1 rectifier in the presence and absence of Klotho coexpression (Fig. 1C). Normalization of the peak outward current (IKs) to the maximum peak outward current of each respective group dissipated the differences between oocytes coexpressing KCNQ1/KCNE1 with klotho and oocytes expressing KCNQ1/KCNE1 alone (Fig. 1C). Coexpression of Klotho did not significantly modify the KCNQ1/KCNE1 activation threshold. The potential needed to reach the half-maximal peak outward current was similar in Xenopus oocytes expressing KCNQ1/KCNE1 alone and in Xenopus oocytes coexpressing both KCNQ1/KCNE1 and Klotho (Fig. 1D).
Figure 1. Effect of Klotho coexpression on current in KCNQ1/KCNE1 expressing Xenopus oocytes. (A) Original tracings demonstrating outward K+ currents activated by depolarization from -120 to +80 mV in 20 mV steps from a holding potential of -80 mV in Xenopus oocytes injected with water (1), injected with cRNA encoding KCNQ1/KCNE1 (2) and in Xenopus oocytes injected with cRNA encoding KCNQ1/KCNE1 and Klotho (3). (B) Arithmetic means ± SEM (n = 16–57) of the normalized depolarization-induced K+ current at +80 mV in Xenopus oocytes injected with water (dotted bar), with cRNA encoding KCNQ1/KCNE1(white bar) or with cRNA encoding KCNQ1/KCNE1 and Klotho (black bar). *** indicates statistically significant (P < 0.001) difference of KCNQ1/KCNE1 and Klotho expressing Xenopus oocytes from Xenopus oocytes expressing KCNQ1/KCNE1 alone. (C) Arithmetic means ± SEM (n = 16–57) of the normalized depolarization-induced K+ current as a function of voltage in Xenopus oocytes injected with water (gray triangles), with cRNA encoding KCNQ1/KCNE1 (white circles) or with cRNA encoding KCNQ1/KCNE1 and Klotho (black circles). *** indicates statistically significant (P < 0.001) difference of KCNQ1/KCNE1 and Klotho expressing Xenopus oocytes from Xenopus oocytes expressing KCNQ1/KCNE1 alone. (D) Arithmetic means ± SEM (n = 56–57) of the normalized depolarization-induced K+ current to the maximum peak current of each respective group as a function of voltage in Xenopus oocytes injected with cRNA encoding KCNQ1/KCNE1 (white circles) or with cRNA encoding KCNQ1/KCNE1 and Klotho (black circles).
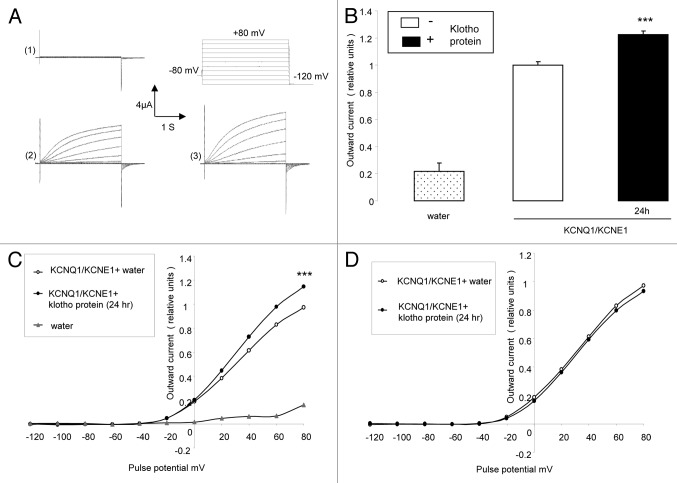
Further experiments explored, whether the effect of Klotho coexpression could be mimicked by treatment of KCNQ1/KCNE1 expressing Xenopus oocytes with recombinant human Klotho protein. As shown in Figure 2, treatment of KCNQ1/KCNE1 expressing Xenopus oocytes with recombinant human Klotho protein (30 ng/ml) for 24 h was followed by a significant increase of the KCNQ1/KCNE1 peak outward current (Fig. 2B and C). Similar to what has been observed following coexpressing Klotho in KCNQ1/KCNE1 expressing Xenopus oocytes, the treatment of KCNQ1/KCNE1 expressing Xenopus oocytes with Klotho protein increased the peak outward current values (Fig. 2B and C) but did not significantly modify the KCNQ1/KCNE1 activation threshold (Fig. 2D).
Figure 2. Effect of treatment with recombinant Klotho protein on current in KCNQ1/KCNE1 expressing Xenopus oocytes. (A) Original tracings demonstrating outward K+ currents activated by depolarization from -120 to +80 mV in 20 mV steps from a holding potential of -80 mV in Xenopus oocytes injected with water (1),or injected with cRNA encoding KCNQ1/KCNE1 without (2) or with (3) a 24 h pretreatment with recombinant Klotho protein (30 ng/ml). (B) Arithmetic means ± SEM (n = 4–17) of the normalized depolarization-induced K+ current at +80 mV in Xenopus oocytes injected with water (dotted bar), or with cRNA encoding KCNQ1/KCNE1 without (white bar) or with (black bar) a 24 h pretreatment with recombinant Klotho protein (30 ng/ml). *** indicates statistically significant (P < 0.001) difference of Klotho treated KCNQ1/KCNE1 expressing Xenopus oocytes from untreated KCNQ1/KCNE1 expressing oocytes. (C) Arithmetic means ± SEM (n = 4–17) of the normalized depolarization-induced K+ current as a function of voltage in Xenopus oocytes injected with water (gray triangles), or with cRNA encoding KCNQ1/KCNE1 without (white circles) or with (black circles) a 24 h pretreatment with recombinant Klotho protein (30 ng/ml). *** indicates statistically significant (P < 0.001) difference of Klotho treated KCNQ1/KCNE1 expressing Xenopus oocytes from untreated KCNQ1/KCNE1 expressing Xenopus oocytes. (D) Arithmetic means ± SEM (n = 15–17) of the depolarization-induced K+ current (normalized to the maximum peak current of each respective group) as a function of voltage in Xenopus oocytes injected with cRNA encoding KCNQ1/KCNE1 without (white circles) or with (black circles) a 24 h pretreatment with recombinant Klotho protein (30 ng/ml).
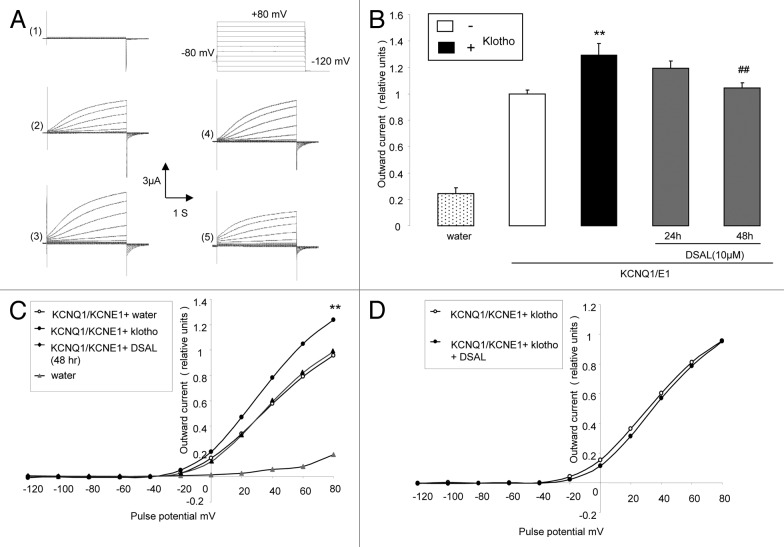
Additional experiments were performed to test, whether the effect of Klotho coexpression could be reversed by DSAL (D-saccharic acid-1,4-lactone), a β-glucuronidase inhibitor. As illustrated in Figure 3, treatment of Xenopus oocytes expressing both KCNQ1/KCNE1 and Klotho with the DSAL (10 µM) for 48 h significantly blunted the effect of Klotho coexpression on the peak outward current (Fig. 3B and C).
Figure 3. Effect of β-glucuronidase inhibitor (DSAL) on current in KCNQ1/KCNE1 and Klotho expressing Xenopus oocytes. (A) Original tracings demonstrating outward K+ currents activated by depolarization from -120 to +80 mV in 20 mV steps from a holding potential of -80 in Xenopus oocytes injected with water (1), injected with cRNA encoding KCNQ1/KCNE1 alone (2) and in Xenopus oocytes injected with cRNA encoding both KCNE1/KCNQ1 and Klotho in the absence of β-glucuronidase inhibitor (3), or in the presence of DSAL for 24 h (4) or 48 h (5). (B) Arithmetic means ± SEM (n = 9–23) of the normalized depolarization-induced K+ current at +80 mV in Xenopus oocytes injected with water (dotted bar), injected with cRNA encoding KCNQ1/KCNE1 alone (white bar) or injected with cRNA encoding both KCNQ1/KCNE1 and Klotho in the absence of β-glucuronidase inhibitor DSAL (black bar), or in the presence of DSAL for 24 h (first gray bar) or 48 h (second gray bar). ** indicates statistically significant (P < 0.01) difference of Klotho and KCNQ1/KCNE1 expressing Xenopus oocytes from Xenopus oocytes expressing KCNQ1/KCNE1 alone. ## indicates statistically significant (P < 0.01) difference of DSAL-treated from untreated KCNQ1/KCNE1 and Klotho expressing Xenopus oocytes. (C) Arithmetic means ± SEM (n = 9–23) of the normalized depolarization-induced K+ current as a function of voltage in Xenopus oocytes injected with water (gray triangles), injected with cRNA encoding KCNQ1/KCNE1 alone (white circles) and in Xenopus oocytes injected with cRNA encoding both KCNQ1/KCNE1 and klotho in the absence of β-glucuronidase inhibitor DSAL (black circles), or in the presence of DSAL for 48 h (black triangles). ** indicates statistically significant (P < 0.01) difference of Klotho and KCNQ1/KCNE1 expressing Xenopus oocytes from Xenopus oocytes expressing KCNQ1/KCNE1 alone. (D) Arithmetic means ± SEM (n = 18–23) of the depolarization-induced K+ current (normalized to the maximum peak current of each group) as a function of voltage in Xenopus oocytes injected with cRNA encoding KCNQ1/KCNE1 and Klotho without treatment (white circles) or treated for 48 h with DSAL (black circles).
In a final series of experiments chemiluminescence and confocal microscopy were employed to test, whether Klotho coexpression influenced the KCNQ1/KCNE1 protein abundance in the cell membrane. As illustrated in Figure 4B, coexpression of both Klotho and KCNQ1-Flag/KCNE1 was followed by a significant increase of KCNQ1-Flag/KCNE1 abundance in the plasma membrane as determined by chemiluminescence. The same effect was also visualized by confocal microscopy (Fig. 4A). The coexpression of Klotho thus increased KCNQ1/KCNE1 channel protein abundance in the plasma membrane.
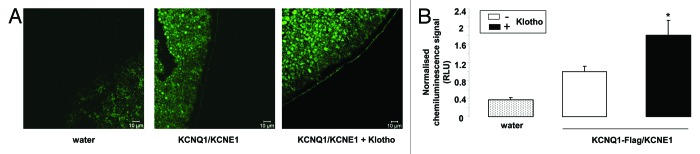
Figure 4. Effect of Klotho coexpression on KCNQ1/KCNE1 protein abundance in the cell membrane of Xenopus oocytes. (A) Confocal microscopy of the KCNQ1/KCNE1 protein abundance in Xenopus oocytes injected with water (left), injected with cRNA encoding KCNQ1/KCNE1 alone (middle) or expressing KCNQ1/KCNE1 together with Klotho (right). The images are representative for 3 independent experiments. (B) Arithmetic means ± SEM (n = 55–76) of the chemiluminescence of KCNQ1-Flag/KCNE1 protein abundance in Xenopus oocytes injected with water (dotted bar), injected with cRNA encoding KCNQ1-Flag/KCNE1 alone (white bar), or expressing KCNQ1-Flag/KCNE1 with Klotho (black bar). * (P < 0.05) indicates statistically significant difference from the protein abundance in Xenopus oocytes expressing KCNQ1-Flag/KCNE1 alone.
The effect of Klotho coexpression could again be mimicked by treatment of KCNQ1/KCNE1 expressing Xenopus oocytes with recombinant human Klotho protein. As shown in Figure 5, treatment of KCNQ1/KCNE1 expressing Xenopus oocytes with recombinant human Klotho protein (30 ng/ml) for 24 h was followed by a significant increase of the KCNQ1/KCNE1 KCNQ1-Flag/KCNE1 abundance in the plasma membrane).
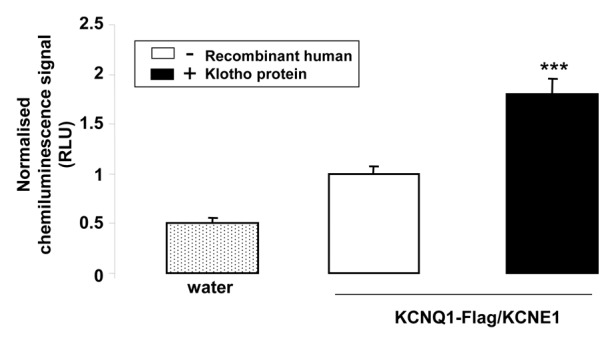
Figure 5. Effect of treatment with recombinant Klotho protein on KCNQ1/KCNE1 protein abundance in the cell membrane of Xenopus oocytes. Arithmetic means ± SEM (n = 70–72) of the chemiluminescence of KCNQ1-Flag/KCNE1 protein abundance in Xenopus oocytes injected without (dotted bar) or with cRNA encoding KCNQ1-Flag/KCNE1 without (white bar) or with (black bar) a 24 h pretreatment with recombinant Klotho protein (30 ng/ml). *** (P < 0.001) indicates statistically significant difference from the protein abundance in Xenopus oocytes expressing KCNQ1-Flag/KCNE1 alone.
Discussion
The present study uncovers a novel function of Klotho, i.e., the upregulation of the slowly activating outward current generated by the heterotetrameric K+ channel KCNQ1/KCNE1. Coexpression of Klotho or treatment with the Klotho protein increased the abundance of channel protein and the respective K+ conductance. The effect may be due to direct influence of Klotho on the channel protein or due to influence of other oocyte molecules indirectly modifying KCNQ1/KCNE1 protein abundance in the cell membrane. Klotho may affect primarily the KCNE1 subunit, the KCNQ1 subunit or both ion channel subunits in parallel.
In the heart, stimulation of KCNQ1 is expected to accelerate repolarization, whereas KCNQ1 inhibition delays cardiac repolarization.43 KCNE1 determines the activation time course of the heterotetrameric channel.44 KCNE1/KCNQ1 channel activity is thus decisive for cardiac function.13-15 At least in theory, decreased stimulation of KCNQ1 in the heart could contribute to the occurrence of cardiac arrhythmia in Klotho hypomorphic mice.1
K+ channel activity is further a determinant of tubular transport. In the proximal renal tubule K+ channels provide the driving force for Na+-coupled transport of glucose and other substrates across the apical membrane and at the same time decreases electrogenic HCO3- exit across the basolateral cell membrane, thus influencing cytosolic pH and apical Na+/H+ exchanger.45 Accordingly, proximal renal tubular transport is compromised in animals lacking KCNQ1.20
KCNQ1 is further expressed in liver,27-29 skeletal muscle,18 and several epithelia.21-26,33 In the liver, for instance, KCNQ1 governs cell volume and thus cell volume-sensitive functions including glucose uptake.46 Beyond that KCNQ1 is important for a variety of functions including hearing,30,31 gastric acid secretion,31,32 as well as intestinal and renal transport.33
K+ channels are decisive for cell volume regulation.47,48 K+ channels further influence cellular K+ loss during apoptosis and thus participate in the machinery of suicidal cell death.49-53 By influencing HCO3- exit K+ channel activity influences cytosolic pH, which in turn influences caspase activation54 and glycolysis.55
In conclusion, the present observations point to a novel effect of Klotho, i.e., the upregulation of the slowly activating heterotetrameric K+ channel KCNQ1/KCNE1. At least in theory, loss of this effect may contribute to the consequences of Klotho deficiency.
Materials and Methods
Xenopus Oocytes were explanted from adult Xenopus laevis (NASCO). Xenopus laevis frogs were anesthesized by a 0.1% Tricain solution. After confirmation of anesthesia and disinfection of the skin, a small abdominal incision was made and oocytes were removed, followed by closure of the skin by sutures. All animal experiments were conducted in accordance with the Helsinki Declaration of 1975 and according to the German law for the welfare of animals and the surgical procedures on the adult Xenous laevis were reviewed and approved by the respective government authority of the state Baden-Württemberg (Regierungspräsidium) prior to the start of the study (Anzeige für Organentnahme nach §6).
Constructs
For generation of cRNA, constructs were used encoding wild-type human KCNQ1/KCNE156 wild-type human KCNQ1-Flag carrying an extracellular Flag tag epitope57 and wild-type mouse Klotho.38 The constructs were used for the generation of cRNA as described previously.58,59
Voltage clamp in Xenopus oocytes
Xenopus oocytes were prepared as previously described.60,61 cRNA encoding KCNQ1 (3.5 ng) and 1.5 ng cRNA encoding KCNE1 were injected with or without 10 ng of cRNA encoding Klotho62 on the next day of preparation of the Xenopus oocytes. All experiments were performed at room temperature 3 d after injection.63,64 The oocytes were maintained at 17 °C in ND96 solution containing: 88.5 mM NaCl, 2 mM KCl, 1 mM MgC12, 1.8 mM CaC12, 5 mM HEPES, Tretracycline (Sigma, 0.11 mM), Ciprofloxacin (Sigma, 4 μM), Gentamycin (Refobacin, 0.2 mM) and Theophylin (Euphylong, 0.5 mM) as well as Sodium Pyruvate (Sigma, 5 mM) were added to the ND96, pH was adjusted to 7.5 by addition of NaOH. The control superfusate (ND96) contained 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2,1 mM MgCl2 and 5 mM HEPES, pH was adjusted to 7.4 by addition of NaOH. Where indicated, recombinant human β-Klotho protein (10, 30 or 50 ng/ml, R&D Systems) and D-saccharic acid 1,4-lactone monohydrate (DSAL, 10 µM, Sigma) were added. In 2-electrode voltage-clamp experiments KCNQ1/KCNE1 channel currents were elicited every 10 s with 3 s depolarizing pulses up to +80 mV applied from a holding potential of -80 mV. Pulses were applied in 20 mV increments. The data were filtered at 2 kHz and recorded with a Digidata 1322A A/D-D/A converter and ClampexV 0.9.2 software for data acquisition (Axon Instruments).65,66 The analysis of the data was performed with Clampfit 9.2 (Axon Instruments) software.
Chemiluminescence
For detection of KCNQ1-Flag cell surface expression, the oocytes were first incubated with primary monoclonal mouse anti-Flag antibody (1:200, Sigma Aldrich) and subsequently with secondary, HRP-conjugated anti-mouse IgG antibody (1:2500, GE Healthcare Life Sciences). Individual oocytes were placed in 96 well plates with 20 µl of SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce) and chemiluminescence of single oocytes was quantified in a luminometer (Walter Wallac 2 plate reader, Perkin Elmer) by integrating the signal over a period of 1 s. Results display normalized relative light units. Integrity of the measured oocytes was assessed by visual control after the measurement to avoid unspecific light signals from the cytosol.64,67
Immunocytochemistry
To visualize KCNQ1 cell surface expression the oocytes were fixed in 4% paraformaldehyde for 2 h at room temperature. After washing with PBS, the oocytes were cryoprotected in 30% sucrose, frozen in mounting medium and placed on cryostat. Sections were collected at a thickness of 8 µm on coated slides and stored at -20 °C. For immunostaining, sections were dried at room temperature, fixed in aceton/methanol (1:1), washed in PBS and blocked for 1 h in 5% bovine serum albumin in PBS. The primary antibody (rabbit polyclonal directed to the KCNQ1-Carboxyterminal end, 1:250, Abcam) was incubated overnight at 4 °C. Binding of primary antibody was visualized with a goat anti-rabbit-FITC conjugated IgG antibody (1:1000, Invitrogen, Molecular Probes). Then, oocytes were analyzed by a fluorescence laser scanning microscope (LSM 510, Carl Zeiss MicroImaging GmbH) with A-Plan 40x/0.25.68 Brightness and contrast settings were kept constant during imaging of all oocytes in each injection series.
Statistical analysis
Data are provided as means ± SEM, n represents the number of experiments. All oocyte experiments were repeated with at least 3 batches of oocytes; in all repetitions qualitatively similar data were obtained. Data were tested for significance using ANOVA 1-way, and results with P < 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge the technical assistance of E Faber. The manuscript was meticulously prepared by L Subasic, Sari Rübe and Ali Soleimanpour
This study was supported by the Deutsche Forschungsgemeinschaft (GK 1302) and by a IZKF-Nachwuchsgruppe of the Medical Faculty of the University of Tübingen (No. 1889–0-0). The authors of this manuscript declare that they have neither financial nor any other conflicts of interest.
References
- 1.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–82. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 2.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 3.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 4.Kuro-o M, M K-o Klotho. Pflugers Arch. 2010;459:333–43. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 5.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 6.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–2. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology. 2002;143:683–9. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- 8.Ramasamy I. Recent advances in physiological calcium homeostasis. Clin Chem Lab Med. 2006;44:237–73. doi: 10.1515/CCLM.2006.046. [DOI] [PubMed] [Google Scholar]
- 9.Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–79. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 10.Kuro-o M, M K-o Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–41. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 11.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–72. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anour R, Andrukhova O, Ritter E, Zeitz U, Erben RG. Klotho lacks a vitamin D independent physiological role in glucose homeostasis, bone turnover, and steady-state PTH secretion in vivo. PLoS One. 2012;7:e31376. doi: 10.1371/journal.pone.0031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 14.Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Fauré S, Gary F, Coumel P, Petit C, et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15:186–9. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 15.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 16.Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, Ng DP, Holmkvist J, Borch-Johnsen K, Jørgensen T, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. 2008;40:1098–102. doi: 10.1038/ng.208. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008;40:1092–7. doi: 10.1038/ng.207. [DOI] [PubMed] [Google Scholar]
- 18.Finsterer J, Stöllberger C. Skeletal muscle involvement in congenital long QT syndrome. Neurol Sci. 2004;25:238–40. doi: 10.1007/s10072-004-0329-x. [DOI] [PubMed] [Google Scholar]
- 19.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallon V, Grahammer F, Richter K, Bleich M, Lang F, Barhanin J, Völkl H, Warth R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J Am Soc Nephrol. 2001;12:2003–11. doi: 10.1681/ASN.V12102003. [DOI] [PubMed] [Google Scholar]
- 21.Grahammer F, Herling AW, Lang HJ, Schmitt-Gräff A, Wittekindt OH, Nitschke R, Bleich M, Barhanin J, Warth R. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120:1363–71. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- 22.Heitzmann D, Grahammer F, von Hahn T, Schmitt-Gräff A, Romeo E, Nitschke R, Gerlach U, Lang HJ, Verrey F, Barhanin J, et al. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J Physiol. 2004;561:547–57. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 2001;442:896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 24.Nicolas M, Demêmes D, Martin A, Kupershmidt S, Barhanin J. KCNQ1/KCNE1 potassium channels in mammalian vestibular dark cells. Hear Res. 2001;153:132–45. doi: 10.1016/S0378-5955(00)00268-9. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto T, Tanabe Y, Shigemoto R, Iwai M, Takumi T, Ohkubo H, Nakanishi S. Immunohistochemical study of a rat membrane protein which induces a selective potassium permeation: its localization in the apical membrane portion of epithelial cells. J Membr Biol. 1990;113:39–47. doi: 10.1007/BF01869604. [DOI] [PubMed] [Google Scholar]
- 27.Demolombe S, Franco D, de Boer P, Kuperschmidt S, Roden D, Pereon Y, Jarry A, Moorman AF, Escande D. Differential expression of KvLQT1 and its regulator IsK in mouse epithelia. Am J Physiol Cell Physiol. 2001;280:C359–72. doi: 10.1152/ajpcell.2001.280.2.C359. [DOI] [PubMed] [Google Scholar]
- 28.Lan WZ, Abbas H, Lemay AM, Briggs MM, Hill CE. Electrophysiological and molecular identification of hepatocellular volume-activated K+ channels. Biochim Biophys Acta 2005; 1668:223-33. [DOI] [PubMed]
- 29.Lan WZ, Wang PY, Hill CE. Modulation of hepatocellular swelling-activated K+ currents by phosphoinositide pathway-dependent protein kinase C. Am J Physiol Cell Physiol. 2006;291:C93–103. doi: 10.1152/ajpcell.00602.2005. [DOI] [PubMed] [Google Scholar]
- 30.Casimiro MC, Knollmann BC, Ebert SN, Vary JC, Jr., Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A. 2001;98:2526–31. doi: 10.1073/pnas.041398998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–55. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarff KL, Judd LM, Toh BH, Gleeson PA, Van Driel IR, Gastric H. Gastric H(+),K(+)-adenosine triphosphatase beta subunit is required for normal function, development, and membrane structure of mouse parietal cells. Gastroenterology. 1999;117:605–18. doi: 10.1016/S0016-5085(99)70453-1. [DOI] [PubMed] [Google Scholar]
- 33.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102:17864–9. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann O, Heinzmann A, Mack A, Manns MP, Seidler U. Mechanisms of secretion-associated shrinkage and volume recovery in cultured rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G711–7. doi: 10.1152/ajpgi.00416.2006. [DOI] [PubMed] [Google Scholar]
- 35.Grunnet M, Jespersen T, MacAulay N, Jørgensen NK, Schmitt N, Pongs O, Olesen SP, Klaerke DA. KCNQ1 channels sense small changes in cell volume. J Physiol. 2003;549:419–27. doi: 10.1113/jphysiol.2003.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.vanTol BL, Missan S, Crack J, Moser S, Baldridge WH, Linsdell P, Cowley EA. Contribution of KCNQ1 to the regulatory volume decrease in the human mammary epithelial cell line MCF-7. Am J Physiol Cell Physiol. 2007;293:C1010–9. doi: 10.1152/ajpcell.00071.2007. [DOI] [PubMed] [Google Scholar]
- 37.Knollmann BC, Casimiro MC, Katchman AN, Sirenko SG, Schober T, Rong Q, Pfeifer K, Ebert SN. Isoproterenol exacerbates a long QT phenotype in Kcnq1-deficient neonatal mice: possible roles for human-like Kcnq1 isoform 1 and slow delayed rectifier K+ current. J Pharmacol Exp Ther. 2004;310:311–8. doi: 10.1124/jpet.103.063743. [DOI] [PubMed] [Google Scholar]
- 38.Dërmaku-Sopjani M, Sopjani M, Saxena A, Shojaiefard M, Bogatikov E, Alesutan I, Eichenmüller M, Lang F. Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of Klotho. Cell Physiol Biochem. 2011;28:251–8. doi: 10.1159/000331737. [DOI] [PubMed] [Google Scholar]
- 39.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–50. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sopjani M, Alesutan I, Dërmaku-Sopjani M, Gu S, Zelenak C, Munoz C, Velic A, Föller M, Rosenblatt KP, Kuro-o M, et al. Regulation of the Na+/K+ ATPase by Klotho. FEBS Lett. 2011;585:1759–64. doi: 10.1016/j.febslet.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Boros S, Bindels RJ, Hoenderop JG. Active Ca(2+) reabsorption in the connecting tubule. Pflugers Arch. 2009;458:99–109. doi: 10.1007/s00424-008-0602-6. [DOI] [PubMed] [Google Scholar]
- 42.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peroz D, Rodriguez N, Choveau F, Baró I, Mérot J, Loussouarn G. Kv7.1 (KCNQ1) properties and channelopathies. J Physiol. 2008;586:1785–9. doi: 10.1113/jphysiol.2007.148254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruscic KJ, Miceli F, Villalba-Galea CA, Dai H, Mishina Y, Bezanilla F, Goldstein SA. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc Natl Acad Sci U S A. 2013;110:E559–66. doi: 10.1073/pnas.1222616110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang F, Rehwald W. Potassium channels in renal epithelial transport regulation. Physiol Rev. 1992;72:1–32. doi: 10.1152/physrev.1992.72.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Boini KM, Graf D, Hennige AM, Koka S, Kempe DS, Wang K, Ackermann TF, Föller M, Vallon V, Pfeifer K, et al. Enhanced insulin sensitivity of gene-targeted mice lacking functional KCNQ1. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1695–701. doi: 10.1152/ajpregu.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang F, Messner G, Rehwald W. Electrophysiology of sodium-coupled transport in proximal renal tubules. Am J Physiol. 1986;250:F953–62. doi: 10.1152/ajprenal.1986.250.6.F953. [DOI] [PubMed] [Google Scholar]
- 48.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 49.Becker S, Reinehr R, Graf D, vom Dahl S, Häussinger D. Hydrophobic bile salts induce hepatocyte shrinkage via NADPH oxidase activation. Cell Physiol Biochem. 2007;19:89–98. doi: 10.1159/000099197. [DOI] [PubMed] [Google Scholar]
- 50.Bortner CD, Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 2004;448:313–8. doi: 10.1007/s00424-004-1266-5. [DOI] [PubMed] [Google Scholar]
- 51.Föller M, Kasinathan RS, Duranton C, Wieder T, Huber SM, Lang F. PGE2-induced apoptotic cell death in K562 human leukaemia cells. Cell Physiol Biochem. 2006;17:201–10. doi: 10.1159/000094125. [DOI] [PubMed] [Google Scholar]
- 52.Schneider J, Nicolay JP, Foller M, Wieder T, Lang F. Suicidal erythrocyte death following cellular K+ loss. Cell Physiol Biochem. 2007;20:35–44. doi: 10.1159/000104151. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu T, Wehner F, Okada Y. Inhibition of hypertonicity-induced cation channels sensitizes HeLa cells to shrinkage-induced apoptosis. Cell Physiol Biochem. 2006;18:295–302. doi: 10.1159/000097607. [DOI] [PubMed] [Google Scholar]
- 54.Lupescu A, Geiger C, Zahir N, Aberle S, Lang PA, Kramer S, Wesselborg S, Kandolf R, Foller M, Lang F, et al. Inhibition of Na+/H+ exchanger activity by parvovirus B19 protein NS1. Cell Physiol Biochem. 2009;23:211–20. doi: 10.1159/000204110. [DOI] [PubMed] [Google Scholar]
- 55.Boiteux A, Hess B. Design of glycolysis. Philos Trans R Soc Lond B Biol Sci. 1981;293:5–22. doi: 10.1098/rstb.1981.0056. [DOI] [PubMed] [Google Scholar]
- 56.Seebohm G, Strutz-Seebohm N, Ureche ON, Henrion U, Baltaev R, Mack AF, Korniychuk G, Steinke K, Tapken D, Pfeufer A, et al. Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels. Circ Res. 2008;103:1451–7. doi: 10.1161/CIRCRESAHA.108.177360. [DOI] [PubMed] [Google Scholar]
- 57.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, et al. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res. 2007;100:686–92. doi: 10.1161/01.RES.0000260250.83824.8f. [DOI] [PubMed] [Google Scholar]
- 58.Strutz-Seebohm N, Pusch M, Wolf S, Stoll R, Tapken D, Gerwert K, Attali B, Seebohm G. Structural basis of slow activation gating in the cardiac I Ks channel complex. Cell Physiol Biochem. 2011;27:443–52. doi: 10.1159/000329965. [DOI] [PubMed] [Google Scholar]
- 59.Alesutan I, Sopjani M, Dërmaku-Sopjani M, Munoz C, Voelkl J, Lang F. Upregulation of Na-coupled glucose transporter SGLT1 by Tau tubulin kinase 2. Cell Physiol Biochem. 2012;30:458–65. doi: 10.1159/000339039. [DOI] [PubMed] [Google Scholar]
- 60.Bröer S, Bröer A, Hamprecht B. Expression of Na+-independent isoleucine transport activity from rat brain in Xenopus laevis oocytes. Biochim Biophys Acta. 1994;1192:95–100. doi: 10.1016/0005-2736(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 61.Pathare G, Föller M, Daryadel A, Mutig K, Bogatikov E, Fajol A, Almilaji A, Michael D, Stange G, Voelkl J, et al. OSR1-sensitive renal tubular phosphate reabsorption. Kidney Blood Press Res. 2012;36:149–61. doi: 10.1159/000343405. [DOI] [PubMed] [Google Scholar]
- 62.Hosseinzadeh Z, Bhavsar SK, Sopjani M, Alesutan I, Saxena A, Dërmaku-Sopjani M, Lang F. Regulation of the glutamate transporters by JAK2. Cell Physiol Biochem. 2011;28:693–702. doi: 10.1159/000335763. [DOI] [PubMed] [Google Scholar]
- 63.Hosseinzadeh Z, Bhavsar SK, Lang F. Down-regulation of the myoinositol transporter SMIT by JAK2. Cell Physiol Biochem. 2012;30:1473–80. doi: 10.1159/000343335. [DOI] [PubMed] [Google Scholar]
- 64.Hosseinzadeh Z, Bhavsar SK, Lang F. Downregulation of ClC-2 by JAK2. Cell Physiol Biochem. 2012;29:737–42. doi: 10.1159/000178560. [DOI] [PubMed] [Google Scholar]
- 65.Bogatikov E, Munoz C, Pakladok T, Alesutan I, Shojaiefard M, Seebohm G, Föller M, Palmada M, Böhmer C, Bröer S, et al. Up-regulation of amino acid transporter SLC6A19 activity and surface protein abundance by PKB/Akt and PIKfyve. Cell Physiol Biochem. 2012;30:1538–46. doi: 10.1159/000343341. [DOI] [PubMed] [Google Scholar]
- 66.Hosseinzadeh Z, Dong L, Bhavsar SK, Warsi J, Almilaji A, Lang F. Upregulation of peptide transporters PEPT1 and PEPT2 by Janus kinase JAK2. Cell Physiol Biochem. 2013;31:673–82. doi: 10.1159/000350086. [DOI] [PubMed] [Google Scholar]
- 67.Mia S, Munoz C, Pakladok T, Siraskar G, Voelkl J, Alesutan I, Lang F. Downregulation of Kv1.5 K channels by the AMP-activated protein kinase. Cell Physiol Biochem. 2012;30:1039–50. doi: 10.1159/000341480. [DOI] [PubMed] [Google Scholar]
- 68.Pakladok T, Almilaji A, Munoz C, Alesutan I, Lang F. PIKfyve sensitivity of hERG channels. Cell Physiol Biochem. 2013;31:785–94. doi: 10.1159/000350096. [DOI] [PubMed] [Google Scholar]