Abstract
We demonstrated previously that BK (KCa1.1) channel activity (NPo) increases in response to bisphenol A (BPA). Moreover, BK channels containing regulatory β1 subunits were more sensitive to the stimulatory effect of BPA. How BPA increases BK channel NPo remains mostly unknown. Estradiol activates BK channels by binding to an extracellular site, but neither the existence nor location of a BPA binding site has been demonstrated. We tested the hypothesis that an extracellular binding site is responsible for activation of BK channels by BPA. We synthesized membrane-impermeant BPA-monosulfate (BPA-MS) and used patch clamp electrophysiology to study channels composed of α or α + β1 subunits in cell-attached (C-A), whole-cell (W-C), and inside-out (I-O) patches. In C-A patches, bath application of BPA-MS (100 μM) had no effect on the NPo of BK channels, regardless of their subunit composition. Importantly, however, subsequent addition of membrane-permeant BPA (100 μM) increased the NPo of both α and α + β1 channels in C-A patches. The C-A data indicate that in order to alter BK channel NPo, BPA must interact with the channel itself (or some closely associated partner) and diffusible messengers are not involved. In W-C patches, 100 μM BPA-MS activated current in cells expressing α subunits, whereas cells expressing α + β1 subunits responded similarly to a log-order lower concentration (10 μM). The W-C data suggest that an extracellular activation site exists, but do not eliminate the possibility that an intracellular site may also be present. In I-O patches, where the cytoplasmic face was exposed to the bath, BPA-MS had no effect on the NPo of BK α subunits, but BPA increased it. BPA-MS increased the NPo of α + β1 channels in I-O patches, but not as much as BPA. We conclude that BPA activates BK α via an extracellular site and that BPA-sensitivity is increased by the β1 subunit, which may also constitute part of an intracellular binding site.
Keywords: KCa1.1, KCNMA1, KCNMB1, endocrine disruptor, estrogen, non-genomic
Introduction
Large conductance Ca2+/voltage-activated K+ (BK) channels are expressed in the plasma membranes and intracellular organelles of numerous cell types.1 BK channels are important physiological regulators whose functions are impacted by and/or underlie disease.2,3 These channels are composed of pore-forming α subunits encoded by the KCNMA1 gene.4 The minimal BK channel is an assembly of 4 α subunits around a central axis. Each α subunit has an extracellular N-terminus, 7 transmembrane segments, and an intracellular C-terminus containing numerous regulatory domains and sites.5 α-only tetramers are fully functional K+-selective channels with inherent Ca2+- and voltage-sensitivity. Variations in α subunit properties result from alternative splicing of KCNMA1.6 However, a variety of important post-translational modifications also regulate BK channel properties, including their trafficking and anchoring to the membrane.7,8 Other differences in BK channels can be due to co-assembly with regulatory β subunits, 4 of which have been identified.9 β subunits can dramatically change channel properties including gating kinetics, Ca2+/voltage-sensitivity, and pharmacology.10 Interestingly, it has been demonstrated recently that the N-terminus of intermediate conductance Ca2+-activated K+ channels reduces BK channel NPo by the same open channel block underlying β2- and β3-induced inactivation.11 β subunits can also influence the response of BK channels to pharmacological blockers and openers.12-15
BK channels are activated by estradiol16 and xenoestrogens.13,17,18 Regulation by estrogenic substances, in multiple cases, involves β subunits.13,16,18 We demonstrated previously that BPA increases the open probability (NPo) of BK channels without affecting single channel conductance.19 BPA appears to have less of an effect on BK channels lacking the β1 subunit, as a 10-fold higher concentration of BPA was needed to activate BK α subunits alone.19 Thus, effects of BPA, like those of other xenoestrogens, seem to be modulated by β subunits. However, a particular extracellular or intracellular binding site for the molecule has not been identified; BPA is lipophilic and it would be reasonable to suggest sites on either side of the membrane. It is possible that BPA binds to the α subunit, but this interaction is made much more efficient by addition of the β1 subunit.19 Another possibility may be that 2 different BPA binding sites exist when the β1 subunit is present.19 Whether these sites are intracellular, extracellular, or both remains unclear. In this study, we test the hypothesis that an extracellular binding site is responsible for activation of BK channels by BPA. In order to examine this possibility, we synthesized membrane-impermeant BPA-monosulfate (BPA-MS). This novel reagent allows us to determine whether an intracellular or extracellular binding site exists (under the presumptions that BPA-MS: [1] can bind to the same site(s) as BPA and [2] similarly increase channel activity). W-C, C-A, and I-O recordings were made on cells expressing BK channels composed of α or α + β1 subunits.
Results
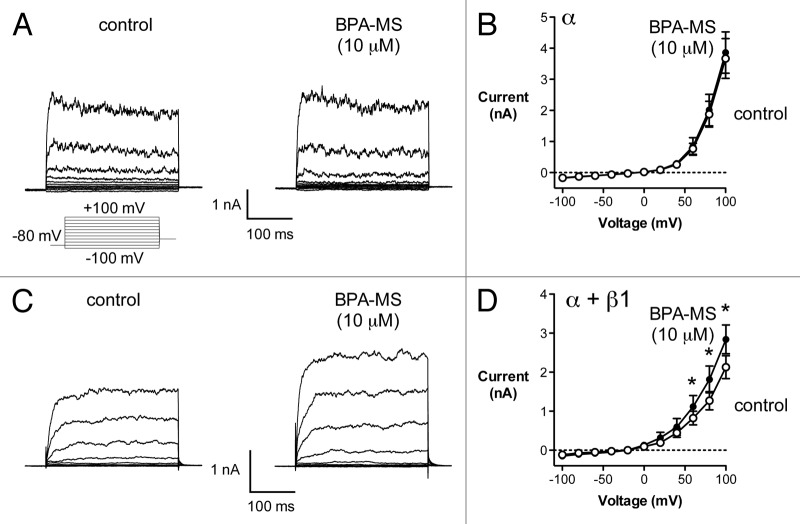
We recorded BK currents in AD 293 cells expressing α or α + β1 subunits (Fig. 1). Our goal was to determine whether BPA-MS, a membrane-impermeant derivative of BPA, increased whole-cell BK current. Further, if BPA-MS were to have an effect, we were interested in determining whether the presence of the β1 subunit influenced it. In cells expressing BK α subunits, whole-cell current was unaffected by 10 μM BPA-MS (Fig. 1A and B). Specifically, in cells expressing α subunits alone, current in the presence of BPA-MS was 106 ± 4% of control (n = 5). There was no BK current in non-transfected cells (data not shown) and the current in transfected cells was identified as BK through its sensitivity to 1 μM penitrem A (93 ± 4% block).12 In cells expressing BK α + β1 subunits, current magnitude was increased by 10 μM BPA-MS (Fig. 1C and D). Specifically, at +100 mV, BPA-MS increased current 34 ± 4% (an increase of 712 ± 103 pA; n = 7) when the β1 subunit was present. These data are very much like what we reported previously for the effect of BPA on whole-cell BK current and the role of the β1 subunit (ref.19); however, these experiments with BPA-MS further indicate that the stimulatory effect of BPA is probably mediated, at least in part, by an extracellular binding site.
Figure 1. BPA-MS (10 μM) activates BK channels in W-C patches in a β1 subunit-dependent manner. (A) Contains families of current traces from a representative cell expressing BK α subunits. The voltage template is shown below; cells were held at -80 mV and stepped from -100 to +100 mV in 20 mV increments. The addition of 10 μM BPA-MS had no effect on current in cells expressing BK α subunits alone. (B) Shows group data (n = 5) illustrating the lack of effect of 10 μM BPA-MS on BK channels composed of α only (P = 0.99 by 2RM-ANOVA). (C) Contains families of current traces from a representative cell expressing BK α + β1 subunits. BPA-MS (10 μM) increased current in cells expressing BK channels composed of α + β1 subunits. (D) Shows group data (n = 7) illustrating the increase in BK α + β1 current elicited by 10 μM BPA-MS (asterisks indicate P < 0.05 by 2RM-ANOVA with Bonferroni post test).
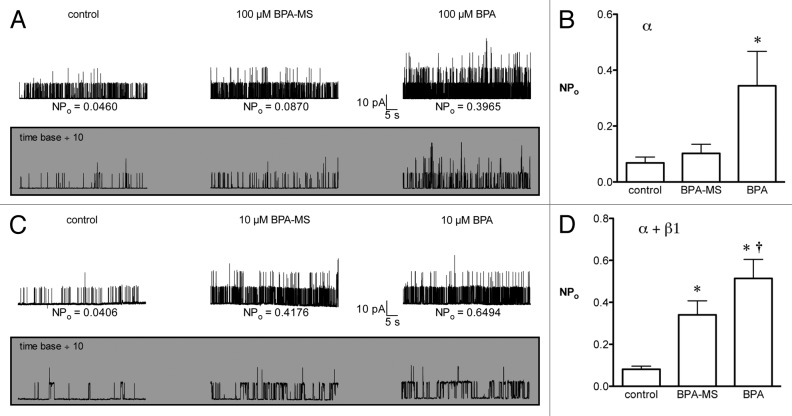
In our previous report, we demonstrated that a 10-fold higher concentration of BPA (100 μM) could activate BK channels composed of α subunits alone.19 Thus, in the present study, we determined whether a log order higher concentration of membrane-impermeant BPA-MS (100 μM) could also activate BK α subunits (Fig. 2). Bath application of 100 μM BPA-MS to W-C patches increased BK α current (Fig. 2A). Specifically, at +100 mV, 100 μM BPA-MS increased BK α current 36 ± 4% (an increase of 1151 ± 187 pA; n = 6). These data support what we demonstrated previously regarding the role of the β1 subunit in determining sensitivity to BPA. Further, these data lead us to suggest that the lower affinity site for activation of BK α by BPA/BPA-MS is extracellular.
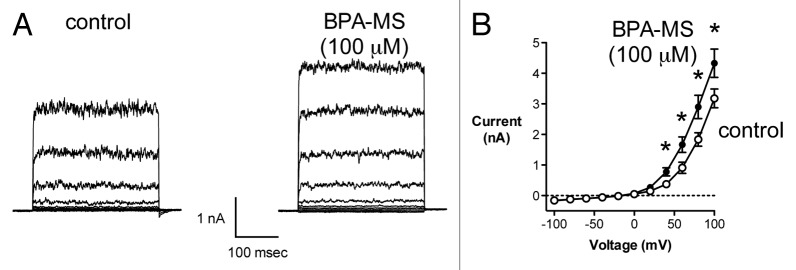
Figure 2. A higher concentration of BPA-MS (100 μM) activates current in cells expressing BK α subunits. (A) Contains families of current traces from a representative cell expressing BK α subunits. BPA-MS (100 μM; note this is 10x higher than Fig. 1) increased current in cells expressing BK channels composed of α subunits only. The voltage template shown in Figure 1 was used here also. (B) shows group data (n = 7) illustrating the increase in BK α current elicited by 100 μM BPA-MS (asterisks indicate P < 0.05 by 2RM-ANOVA with Bonferroni post test).
We performed additional W-C experiments to further characterize the concentration-dependence of BPA-MS effects (Fig. 3). In cells expressing BK α + β1 subunits, BPA-MS increased current in a concentration-dependent manner (Fig. 3A). The increase in BK α + β1 current by BPA-MS (Fig. 3B) was qualitatively similar to effects of BPA we reported previously.19 Quantitatively, in cells expressing BK α + β1 subunits, the magnitude of BPA-MS-activated current was less than that elicited by BPA (Fig. 3B). However, the current activated by BPA-MS was greater in cells expressing BK α + β1 subunits than cells expressing BK α alone (Fig. 3B). These data may indicate that effects of BPA-MS are less than BPA because intracellular and extracellular binding sites exist, but bath-applied, membrane-impermeant BPA-MS is restricted to acting on only the extracellular site. Further, the data may indicate that BPA-MS is a more potent and efficacious agonist of BK channels when the β1 subunit is present.
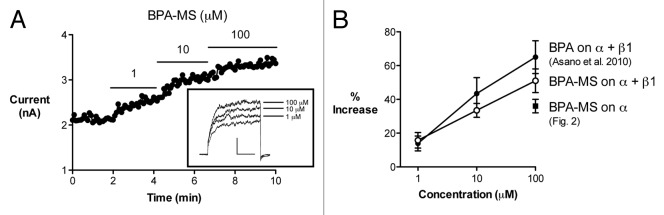
Figure 3. Concentration-dependence of effects of BPA-MS. (A) Contains data from a representative W-C experiment on a cell expressing BK α and α + β1. BPA-MS increased current in a concentration-dependent manner. Inset shows currents under control conditions and in the presence of 1, 10, and 100 μM BPA-MS. (B) Contains group data for the experiment described in (A) (n = 9) and offers a comparison of BPA-MS to results obtained previously with BPA in cells expressing BK α + β1 (ref.19). Further, the results from Figure 2 are replotted here to compare effects of BPA-MS on channels composed of BK α or α + β1.
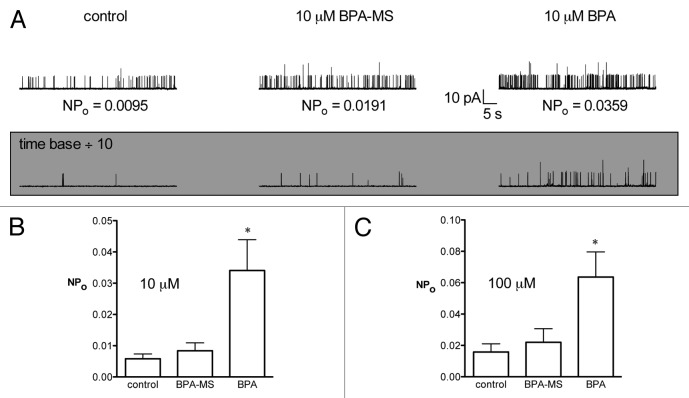
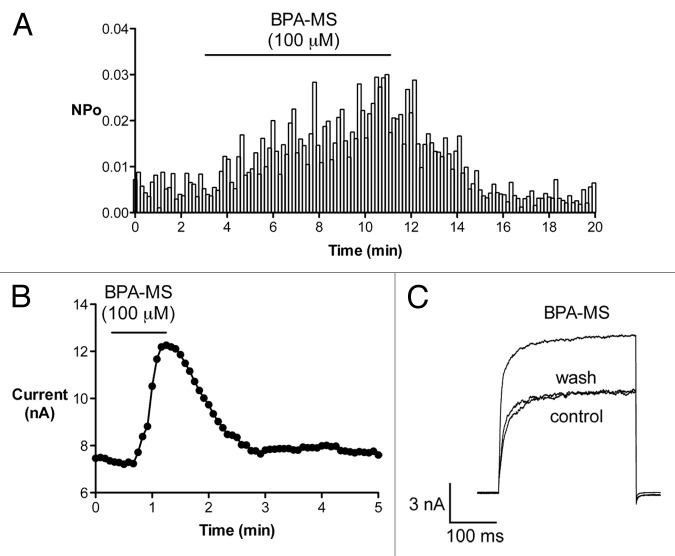
Our next set of experiments was designed to demonstrate that BPA-MS is, indeed, membrane-impermeant. Our rationale was that: (1) the W-C data suggest a low affinity extracellular site for BPA exists; (2) BPA is membrane-permeant and should activate channels in C-A patches through extracellular sites and intracellular sites (if any exist); (3) BPA-MS, if membrane-impermeant, should be unable to activate BK channels (α or α + β1) physically isolated from the test solutions by the pipette glass and plasma membrane. Results from C-A patches are shown in Figure 4. In C-A patches on cells expressing BK α + β1, adding 10 μM BPA-MS to the bath had no effect on NPo (Fig. 4A). In contrast, adding 10 μM BPA to the bath increased NPo 695 ± 325% (n = 8; Fig. 4A and B). We performed additional experiments with a higher concentration of BPA-MS (100 μM) to more rigorously test whether it might cross the membrane and activate channels. In C-A patches on cells expressing BK α + β1, adding 100 μM BPA-MS to the bath had no effect on channel activity (NPo was 132 ± 9% of control; n = 4). In contrast, adding 100 μM BPA to bath solution in these experiments increased NPo 521 ± 154% (P < 0.05). These data demonstrating differential effects of BPA and BPA-MS on NPo reassure us that the latter is likely membrane-impermeant (and that our preparation of BPA-MS is not contaminated with significant amounts of BPA). Further, the data also lead us to suggest that AD 293 cells do not metabolize BPA-MS by desulfation to produce BPA, as can be observed in cells expressing estrone sulfatase activity.20
Figure 4. BPA-MS is membrane-impermeant as shown by results in C-A patches. (A) Contains current traces from a representative cell expressing BK α + β1 subunits. Currents were recorded in C-A mode in symmetrical K+ (pCa 7) at a patch potential of +40 mV. BPA-MS had no effect on NPo, but BPA increased NPo. (B) Shows group data (n = 8) illustrating the effect of BPA, but not BPA-MS, to increase NPo in C-A patches of BK α + β1. Asterisk indicates P < 0.05 by 1RM-ANOVA with Bonferroni post test. (C) Indicates that similar results were obtained using a 10-fold higher concentration of BPA and BPA-MS (n = 5).
We excised I-O patches from cells expressing BK channels composed of α or α + β1 subunits and determined the effect of bath-applied BPA-MS (Fig. 5). Our goal was to determine whether BPA-MS increased BK channel NPo at the intracellular face of the membrane. Further, if BPA-MS were to activate BK channels, we were interested in determining whether the presence of the β1 subunit influenced this effect. In I-O patches from cells expressing BK α alone, adding 100 μM BPA-MS to the bath had no significant effect on NPo (156 ± 15% of control, n = 8; Fig. 5A and B). However, adding 100 μM BPA to the bath increased NPo 462 ± 147% (Fig. 5A and B). The response to intracellular BPA-MS was much different when BK channels contained the β1 subunit (Fig. 5C). In I-O patches from cells expressing BK α + β1 subunits, adding 10 μM BPA-MS to the bath increased NPo 403 ± 97% (n = 15; Fig. 5C and D). Replacing BPA-MS with BPA increased NPo 910 ± 247% (Fig. 5C and D). Thus, BPA-MS can activate BK channels from the cytoplasmic face of the membrane only if the β1 subunit is present. These data lead us to suggest that the β1 subunit may comprise or complete an intracellular binding site for BPA.
Figure 5. When BK channels contain β1 subunits, BPA-MS can activate from the cytoplasmic face of the membrane. (A) Shows a family current traces at +40 mV in a representative I-O patch with BK α (symmetrical 140 mM K+; pCa 6.3). The shaded inset shows a portion of the same current traces on an expanded time scale. (B) Contains group data (n = 8) and shows that 100 μM BPA-MS does not significantly increase the NPo of BK α. In contrast, 100 μM BPA significantly increases NPo. Asterisk indicates P < 0.05 vs. control by 1RM-ANOVA with Bonferroni post test. (C) Shows a family of current traces at +40 mV from a representative I-O patch of BK α + β1 (symmetrical 140 mM K+; pCa 7). The shaded inset shows a portion of the same traces on an expanded time scale. (D) Contains group data (n = 15) showing a significant increase in NPo elicited by BPA-MS and BPA. Asterisk indicates P < 0.05 vs. control by 1RM-ANOVA with Bonferroni post test. Dagger indicates P < 0.05 vs. control and BPA-MS by 1RM-ANOVA with Bonferroni post test.
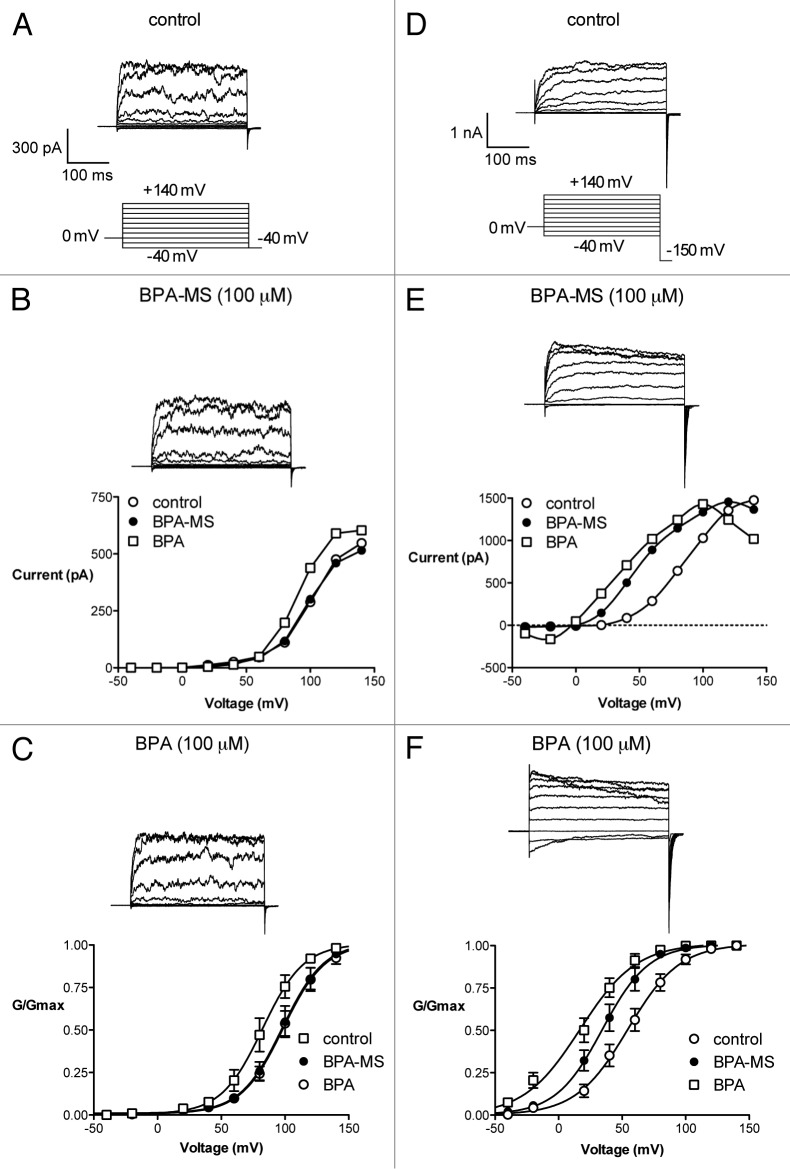
Until this point, all of our single channel experiments had been performed at only 1 voltage, +40 mV on the membrane. We were interested in determining the effect of BPA-MS on BK channel activity at a variety of voltages. Rather than repeat the experiments outlined above at constant holding potentials, we opted to construct activation curves for channels in I-O patches (Fig. 6). Thus, I-O patches were excised from cells expressing BK α or α + β1 and stepped to voltages between -40 and +140 mV in symmetrical 140 mM K+ with 500 nM Ca2+. Currents were recorded under control conditions and with bath application of 100 μM BPA-MS or 100 μM BPA. Conductance (G) was calculated by dividing current by voltage; G was then normalized to the maximum for each patch (Gmax). When BK channels were composed of α subunits alone, BPA-MS had no effect on the voltage at which 50% of the channels were activated (V½ = 98 ± 2 and 98 ± 2 mV, n = 8; Fig. 6A-C). However, bath application of membrane-permeant BPA did increase channel activity at lower voltages (V½ = 82 ± 2 mV). The BPA-induced hyperpolarizing shift was parallel, as the slope factor (k) was not significantly altered (16 ± 2 for all 3 curves). When BK channels contained the β1 subunit, BPA-MS hyperpolarized the V½ (from 54 ± 2 to 34 ± 2 mV, n = 12; Fig. 6D-F). Bath application of membrane-permeant BPA increased channel activity further (V½ = 17 ± 3 mV). The BPA-induced hyperpolarizing shift was parallel, as k was not significantly altered (20 ± 2, 18 ± 2, and 22 ± 3 for control, BPA-MS, and BPA, respectively). Data from these activation curves support the single channel experiments in that: (a) BPA, but not BPA-MS, activates BK α in I-O patches; (b) in I-O patches, BPA-MS activates BK channels containing the β1 subunit; (c) membrane-permeant BPA activates BK α + β1 more than BPA-MS. Further, these data indicate that BPA and BPA-MS activate BK channels over a much wider range of voltages than we had examined in the previous experiments.
Figure 6. BPA-MS activates BK channels containing the β1 subunit over a wide range of voltages. (A) Shows families of current traces from a representative I-O patch taken from a cell expressing BK α (symmetrical 140 mM K+; pCa 6.3). The voltage template is shown below. (B) Contains the I-V relationship for this single patch. BPA (100 μM), but not BPA-MS (100 μM), appears to affect current. (C) Contains group data (n = 8) showing that BPA-MS does not shift the midpoint (V½) of the activation curve. (D) Shows families of current traces from a representative I-O patch taken from a cell expressing BK α + β1 (symmetrical 140 mM K+; pCa 6.3). A slightly different voltage template is shown below. (E) Contains the I-V relationship for this single patch. Both BPA-MS (100 μM) and BPA (100 μM) appear to affect current. (F) Contains group data (n = 12) showing that BPA-MS and BPA both hyperpolarize the V½, with BPA being more effective. See text for statistical analyses of V½ and k for each set of experiments.
Effects of BPA-MS on NPo are reversible (Fig. 7). The results in Figure 7A are from a single I-O patch pulled from a cell expressing BK α + β1. NPo at +40 mV is plotted vs. time. BPA-MS increased channel activity, but channel activity returned to baseline upon washout. Thus, effects of BPA-MS mediated at the intracellular face of the membrane are reversible. Data from a representative W-C experiment are shown in Figure 7B and C. These data indicate that effect of BPA-MS to increase BK current from extracellular face of the membrane is also reversible. We reported previously that BPA had no effect on BK channel unitary conductance.19 It appears that BPA-MS, at least from the cytoplasmic side, does not alter unitary conductance either (see Fig. 5; also a lack of effect can be inferred from Fig. 6, where G is unaffected). However, to determine whether BPA-MS affected unitary conductance from the extracellular side of the membrane, we performed experiments in outside-out patches. Extracelllular BPA-MS had no effect on BK channel amplitude, as channels demonstrated conductances of 240 ± 10 and 241 ± 10 pS before and during exposure to 100 μM BPA-MS, respectively (n = 4).
Figure 7. The effect of BPA-MS on NPo is rapid and reversible. (A) NPo vs. time is plotted for a single I-O patch pulled from a cell expressing BK α + β1 (symmetrical 140 mM K+; pCa 7; patch potential +40 mV). Cytoplasmic BPA-MS significantly activates BK α + β1. Washing out BPA-MS rapidly returns NPo toward baseline. (B) Shows current vs. time for a representative W-C experiment. Bath-applied BPA-MS increases current in a reversible manner. (C) Contains current traces from experiment plotted in (B).
Discussion
BPA, a component of polycarbonate plastic used in food and beverage containers, is an estrogenic endocrine disruptor found in the urine of > 95% of Americans.21 We and others have demonstrated that a wide variety of ion channels (GABA receptors, nicotinic receptors, voltage-gated Na+ channels, voltage-gated Ca2+ channels, and BK channels) are targets of BPA.19,22-27 Specifically, we have shown previously that BPA activates BK channels; however, channels containing regulatory β1 subunits were approximately 1 log-order more sensitive to BPA.19 In the present study, we tested the hypothesis that an extracellular binding site is responsible for activation of BK channels by BPA. This hypothesis was based on previous studies indicating that membrane-impermeant estrogen and estrogen receptor modulators activate BK channels by binding to an extracellular site.16,17 In order to test the hypothesis, we synthesized membrane-impermeant BPA-MS and used patch clamp electrophysiology to study BK channels composed of α or α + β1 subunits in C-A, W-C, and I-O patches. Our 4 major findings included: (1) BK α was activated by extracellular 100 μM BPA-MS (Fig. 2); (2) the β1 subunit made BK channels 10x more sensitive to extracellular BPA-MS (Fig. 1); (3) BK channels containing the β1 subunit were activated by intracellular BPA-MS, whereas α only BK channels were not (Figs. 5 and 6); and (4) BPA was a more efficacious activator of BK α + β1 than was BPA-MS (Figs. 3, 5, and 6). These data lead us to conclude that the hypothesis is supported. That is, an extracellular binding site appears to be responsible, at least in part, for the activation of BK channels by BPA (and BPA-MS). Importantly, however, we did not predict that BPA-MS would activate BK α + β1 channels from the cytoplasmic face of the membrane or that a difference would exist in the magnitude of NPo responses to intracellular BPA and BPA-MS. These surprising findings lead us to suggest that the β1 subunit may also constitute at least a part of an intracellular binding site.
The idea that that the β1 subunit may contribute to an intracellular binding site gains support from a substantial and integrative body of work from Dopico and colleagues on lithocholate, a cholane-derived steroid.14,28-31 Lithocholate activates smooth muscle BK channels from wild type, but not β1 subunit knockout mice.14 The second transmembrane domain of β1 is critical for conferring sensitivity to lithocholate, especially residues T169, L172, and L173.29-31 Membrane topology and computational modeling place these 3 residues in an intracellular-facing region where hydrogen bonding and hydrophobic interactions form a binding site. Whether this pocket is responsible for our observed effects of intracellular BPA on BK channels remains to be determined. However, this is our prediction as this site also mediates responses to the nonsteroidal BK channel opener HENA (3-hydroxyolean-12-en-30-oate).28
There is a clear case for wanting to understand the effects of lithocholate on BK channels, as bile acids can be found in the circulation in high micromolar concentrations, dilate arterioles (including ileal vessels, which facilitates adsorption of fat), and reduce blood pressure.32 There is no such thing as a “physiological” level of BPA, so the need to understand the effects of BPA on ion channels comes from a toxicology point of view. BPA, with an annual production over six billion pounds, is one of the highest volume chemicals worldwide.33 BPA is used to manufacture polycarbonate plastics, including those for food and beverage containers, which represent the major sources of exposure for most people. In fact, the body fluids of > 95% of Americans test positive for BPA.21 This may be cause for concern, because BPA has been identified as an estrogenic endocrine disruptor. Insight into the biologically relevant mechanisms of BPA began in 1993 with the observation that it was released from polycarbonate plastics during autoclaving and interfered with estrogen binding proteins in yeast.34 The structure of BPA allows it to bind receptors in the human body normally occupied by steroids to exert its anti-androgenic and estrogenic effects. BPA interacts with nuclear estrogen receptors (ER) α and β, albeit with low affinity.35 By doing so, BPA exerts its nuclear (i.e., genomic) effects. BPA also possesses the ability to alter estrogen signaling through 2 less conventional pathways.36 Specifically, BPA can bind to membrane-bound ERα and a G protein-coupled ER (GPER).37 Both membrane-bound ERα and GPER couple to non-genomic signaling pathways that have multiple effects, most of which have only recently become appreciated.38 Ion channels are also targets for BPA effects.19,22-27 Whether these ion channel effects contribute to the association of BPA exposure with disease remains to be determined.39
The first identified ion channel target for BPA was recombinant GABAA receptors in 2001.22 Complex effects were demonstrated, as low concentrations of BPA potentiated responses to low concentrations of GABA, but high concentrations of BPA inhibited GABA responses. Later, BPA was shown to elicit postsynaptic current in CA3 pyramidal neurons, likely mediated by GABAA channels.23 The activation of GABAA channels by BPA may be interestingly related to mechanisms responsible for BK channel activation by BPA, as both channels are also potentiated by ethanol.40 Effects of BPA on the other identified ion channel targets share a common feature, as BPA uniformly inhibits a variety of voltage-gated Na+ channels (tetrodotoxin-sensitive and -resistant) and voltage-gated Ca2+ channels (L-, N-, P/Q-, and T-type).24,26,27 In contrast, we show that BPA activates BK channels, which, interestingly, share some structural and regulatory features of voltage-gated Na+ and Ca2+ channels. Whether these non-genomic effects of BPA on BK channels (as well as other ion channels) are relevant to human disease remains to be determined. Genomic effects of BPA on ion channel expression are also possible and merit investigation. The present study, however, focused on non-genomic mechanisms and we have shown that 100 μM BPA-MS activated current in cells expressing α subunits, whereas cells expressing α + β1 subunits responded equally to 10 μM BPA-MS. The W-C data suggest that an extracellular site exists for BPA on the α subunit. Our data show that in I-O patches, where the cytoplasmic face was exposed to the bath, BPA-MS had no effect on the NPo of channels containing α alone; however, BPA-MS increased the NPo of BK channels composed of α + β1 subunits. These I-O data suggest that the β1 subunit completes or comprises an intracellular binding site for BPA. Together, the data lead us to conclude that BPA activates BK channels via an extracellular binding site and via an intracellular binding site that depends on the presence of the β1 subunit. We predict that the intracellular binding site may be the same one identified for interactions with lithocholate and HENA.
Methods
Cell culture and transfection
The techniques used here were similar to those we have used previously.19 AD 293 cells (Agilent Technologies) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Culture flasks were incubated in a 5% CO2 incubator, humidified, and kept at 37 °C. Plasmids encoding hSlo α and hSlo α + β1 were kindly provided by Dr Jonathan Lippiat (University of Leeds).41 Cells were transiently transfected with pIRES-hSloα or pIRES-hSloαβ1 and pmaxGFP (AMAXA) using Lipofectamine LTX with PLUS reagent (Invitrogen). Cells at 50–70% confluence in 35-mm dishes were transfected with 0.5–2.5 μg of DNA. Transfected cells were selected in DMEM supplemented with 0.5 mg/ml G418, 1% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Currents were recorded from cells expressing green fluorescent protein (GFP) 1–3 d later.
Electrophysiology
BK channel currents were recorded at room temperature from inside-out (I-O), cell-attached (C-A), and whole-cell (W-C) patches as described previously.12,19 The bath flowed at a rate of approximately 2–3 ml/min into a chamber with a volume of approximately 0.2–0.3 ml throughout the recordings. For W-C recordings, bath solution contained (mM) 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, and 5 Tris; pH 7.4. Chemicals were purchased from Fisher Scientific or Sigma-Aldrich. For W-C recordings, pipette solution contained (mM) 140 KCl, 1 MgCl2, 1 EGTA, 0.281 CaCl2, 1 Mg-ATP, 0.1 Na-GTP, 10 HEPES, and 5 Tris; pH 7.1; pCa 7. I-O and C-A recordings were made in symmetrical (mM) 140 KCl, 1 MgCl2, 1 EGTA, 10 HEPES, and 5 Tris; pH 7.1. CaCl2 was added to these solutions to achieve free Ca2+ concentrations of 100 nM (pCa 7; calculated using Maxchelator; http://www.stanford.edu/∼cpatton/maxc.html) or 500 nM (pCa 6.3; calculated in ref. 42) as indicated for individual experiments. Because Cl- was symmetrical in all recording conditions, no adjustments were made for the negligible junction potentials. W-C and single channel pipettes were fashioned from borosilicate glass and had tip resistances of 2–3 and 5–10 MΩ, respectively. In W-C recordings, cell capacitance and series resistance were compensated as completely as possible by circuitry of the amplifier (Axopatch 200B; Molecular Devices). Currents were low pass filtered at 1 kHz and digitized at 5 kHz. pClamp 9 software was used for data acquisition and analysis (Molecular Devices).
Synthesis of BPA-MS
BPA-MS was synthesized from BPA and SO3-pyridinium complex, as described previously.20 BPA and 1.1 equivalents of SO3 were stirred in dry pyridine under Ar for 36 h. Solvent was removed under reduced pressure and the crude reaction mixture was purified by column chromatography (10% methanol in CH2Cl2). The pyridinium salt was dissolved in water and passed down a Dowex 50W X-8 cation exchange column, sodium form. 1H-NMR (CD3OD, 300 MHz): δ7.18 (s, 4H), 7.02 (2H, d, J = 8.8 Hz), 6.67 (2H, d, J = 8.8 Hz), 1.6 (6H, s), 13C-NMR (CD3OD, 101 MHz): δ 156.2, 151.5, 149.2, 142.7, 126.7, 128.4, 121.9, 115.6, 42.8, 31.5. ESIMS (negative ion mode) 291 (BPA-MS monoanion).
Statistics
Data are presented as the mean ± standard error of n number of patches. Current-voltage relationships were analyzed by 2-way repeated measures analysis of variance (2RM-ANOVA). NPo values under control conditions and with BPA-MS and BPA stimulation (i.e., 3 values) in C-A and I-O patches were compared by 1-way repeated measures analysis of variance (1RM-ANOVA). Bonferroni post hoc tests followed 1RM-ANOVA and 2RM-ANOVA when appropriate to determine where differences existed. When only 2 values were compared, a paired Student t test was used. In all tests, P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I.S.F. and S.A. were supported by NIH T-32 training grant HL090610. A Research Funds Development Grant from West Virginia University, the WV-INBRE program, and NIGMS P20 grant GM103434 supported this work.
References
- 1.Singh H, Stefani E, Toro L, Intracellular BK. Intracellular BK(Ca) (iBK(Ca)) channels. J Physiol. 2012;590:5937–47. doi: 10.1113/jphysiol.2011.215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–8. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 3.McGahon MK, Zhang X, Scholfield CN, Curtis TM, McGeown JG. Selective downregulation of the BKbeta1 subunit in diabetic arteriolar myocytes. Channels (Austin) 2007;1:141–3. doi: 10.4161/chan.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–4. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 5.Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010;584:2033–42. doi: 10.1016/j.febslet.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soom M, Gessner G, Heuer H, Hoshi T, Heinemann SH. A mutually exclusive alternative exon of slo1 codes for a neuronal BK channel with altered function. Channels (Austin) 2008;2:278–82. doi: 10.4161/chan.2.4.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle BD, Hurst S, Swayze RD, Sheng J, Braun AP. Specific phosphorylation sites underlie the stimulation of a large conductance, Ca(2+)-activated K(+) channel by cGMP-dependent protein kinase. FASEB J. 2013;27:2027–38. doi: 10.1096/fj.12-223669. [DOI] [PubMed] [Google Scholar]
- 8.Tian L, McClafferty H, Knaus HG, Ruth P, Shipston MJ. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels. J Biol Chem. 2012;287:14718–25. doi: 10.1074/jbc.M111.335547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Zaydman MA, Cui J. Regulation of Voltage-Activated K(+) Channel Gating by Transmembrane β Subunits. Front Pharmacol. 2012;3:63. doi: 10.3389/fphar.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweet TB, Cox DH. Measuring the influence of the BKCa beta1 subunit on Ca2+ binding to the BKCa channel. J Gen Physiol. 2009;133:139–50. doi: 10.1085/jgp.200810129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson J, Begenisich T. Mechanistic details of BK channel inhibition by the intermediate conductance, Ca2+-activated K channel. Channels (Austin) 2009;3:194–204. doi: 10.4161/chan.3.3.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asano S, Bratz IN, Berwick ZC, Fancher IS, Tune JD, Dick GM. Penitrem A as a tool for understanding the role of large conductance Ca(2+)/voltage-sensitive K(+) channels in vascular function. J Pharmacol Exp Ther. 2012;342:453–60. doi: 10.1124/jpet.111.191072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. J Biol Chem. 2001;276:34594–9. doi: 10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- 14.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol. 2007;72:359–69. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- 15.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–7. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–31. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 17.Dick GM, Hunter AC, Sanders KM. Ethylbromide tamoxifen, a membrane-impermeant antiestrogen, activates smooth muscle calcium-activated large-conductance potassium channels from the extracellular side. Mol Pharmacol. 2002;61:1105–13. doi: 10.1124/mol.61.5.1105. [DOI] [PubMed] [Google Scholar]
- 18.Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory beta1 subunit: a study of beta1 knockout mice. J Biol Chem. 2001;276:44835–40. doi: 10.1074/jbc.M106851200. [DOI] [PubMed] [Google Scholar]
- 19.Asano S, Tune JD, Dick GM. Bisphenol A activates Maxi-K (K(Ca)1.1) channels in coronary smooth muscle. Br J Pharmacol. 2010;160:160–70. doi: 10.1111/j.1476-5381.2010.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stowell CL, Barvian KK, Young PC, Bigsby RM, Verdugo DE, Bertozzi CR, Widlanski TS. A role for sulfation-desulfation in the uptake of bisphenol a into breast tumor cells. Chem Biol. 2006;13:891–7. doi: 10.1016/j.chembiol.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoshima H, Hossain SJ, Imamura H, Shingai R. Effects of bisphenol A and its derivatives on the response of GABA(A) receptors expressed in Xenopus oocytes. Biosci Biotechnol Biochem. 2001;65:2070–7. doi: 10.1271/bbb.65.2070. [DOI] [PubMed] [Google Scholar]
- 23.Choi IS, Cho JH, Park EJ, Park JW, Kim SH, Lee MG, Choi BJ, Jang IS. Multiple effects of bisphenol A, an endocrine disrupter, on GABA(A) receptors in acutely dissociated rat CA3 pyramidal neurons. Neurosci Res. 2007;59:8–17. doi: 10.1016/j.neures.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Deutschmann A, Hans M, Meyer R, Häberlein H, Swandulla D. Bisphenol A inhibits voltage-activated Ca(2+) channels in vitro: mechanisms and structural requirements. Mol Pharmacol. 2013;83:501–11. doi: 10.1124/mol.112.081372. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa K, Ohno Y. Modulation by estrogens and xenoestrogens of recombinant human neuronal nicotinic receptors. Eur J Pharmacol. 2001;430:175–83. doi: 10.1016/S0014-2999(01)01389-9. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly AO, Eberhardt E, Weidner C, Alzheimer C, Wallace BA, Lampert A. Bisphenol A binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One. 2012;7:e41667. doi: 10.1371/journal.pone.0041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Cao J, Zhu Q, Luan C, Chen X, Yi X, Ding H, Chen J, Cheng J, Xiao H. Inhibition of voltage-gated sodium channels by bisphenol A in mouse dorsal root ganglion neurons. Brain Res. 2011;1378:1–8. doi: 10.1016/j.brainres.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, Parrill AL, Dopico AM. Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Mol Pharmacol. 2013;83:1030–44. doi: 10.1124/mol.112.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukiya AN, Singh AK, Parrill AL, Dopico AM. The steroid interaction site in transmembrane domain 2 of the large conductance, voltage- and calcium-gated potassium (BK) channel accessory β1 subunit. Proc Natl Acad Sci U S A. 2011;108:20207–12. doi: 10.1073/pnas.1112901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel beta(1) subunit is a lithocholate sensor. FEBS Lett. 2008;582:673–8. doi: 10.1016/j.febslet.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. Channel beta2-4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun. 2009;390:995–1000. doi: 10.1016/j.bbrc.2009.10.091. [DOI] [PubMed] [Google Scholar]
- 32.Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995;49:581–9. doi: 10.1016/0006-2952(94)00428-O. [DOI] [PubMed] [Google Scholar]
- 33.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–86. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 35.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol. 2001;14:149–57. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 36.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A. 2000;97:11603–8. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouskine A, Nebout M, Brücker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 2009;117:1053–8. doi: 10.1289/ehp.0800367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72:124–34. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–10. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Bukiya AN, Kuntamallappanavar G, Singh AK, Dopico AM. Distinct sensitivity of slo1 channel proteins to ethanol. Mol Pharmacol. 2013;83:235–44. doi: 10.1124/mol.112.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippiat JD, Standen NB, Harrow ID, Phillips SC, Davies NW. Properties of BK(Ca) channels formed by bicistronic expression of hSloalpha and beta1-4 subunits in HEK293 cells. J Membr Biol. 2003;192:141–8. doi: 10.1007/s00232-002-1070-0. [DOI] [PubMed] [Google Scholar]
- 42.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982;242:C404–8. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]