Abstract
Membrane contact sites (MCSs) allow the exchange of molecules and information between organelles, even when their membranes cannot fuse directly. In recent years, a number of functions have been attributed to these contacts, highlighting their critical role in cell homeostasis. Although inter-organellar connections typically involve the endoplasmic reticulum (ER), we recently reported the presence of a novel MCSs between melanosomes and mitochondria. Melanosome-mitochondrion contacts appear mediated by fibrillar bridges resembling the protein tethers linking mitochondria and the ER, both for their ultrastructural features and the involvement of Mitofusin 2. The frequency of these connections correlates spatially and timely with melanosome biogenesis, suggesting a functional link between the 2 processes and in general that organelle biogenesis in the secretory pathway requires interorganellar crosstalks at multiple steps. Here, we summarize the different functions attributed to MCSs, and discuss their possible relevance for the newly identified melanosome-mitochondrion liaison.
Keywords: lysosome-related organelles, lipid transfer, calcium signaling, protein-protein interactions, organelle dynamics, ATP supply, cytosolic microdomains
Eukaryotic cells contain numerous membrane-bound organelles, necessary to accomplish and segregate specialized functions. However, this partitioning also raises the problem of how subcellular organelles communicate. One way is by vesicular traffic, typically working within the secretory/endocytic pathway. This kind of transport requires that the membranes of interacting organelles can fuse with each other, either directly or by means of intermediate compartments. For instance, the endoplasmic reticulum (ER) and the plasma membrane (PM) cannot fuse directly, but they are functionally connected through multiple membrane traffic steps, a time-consuming process. Alternatively, organelles may rapidly connect one to another by means of membrane contact sites (MCSs), where their membranes become closely juxtaposed (10–30 nm).1-3 In this way, even organelles belonging to “independent” compartments, such as the ER and mitochondria, can exchange or share molecules, functions, information. In recent years, MCSs have been shown to be required for a variety of functions on many organelles,2 emerging as a widespread mechanism operating in cell physiology and pathology.
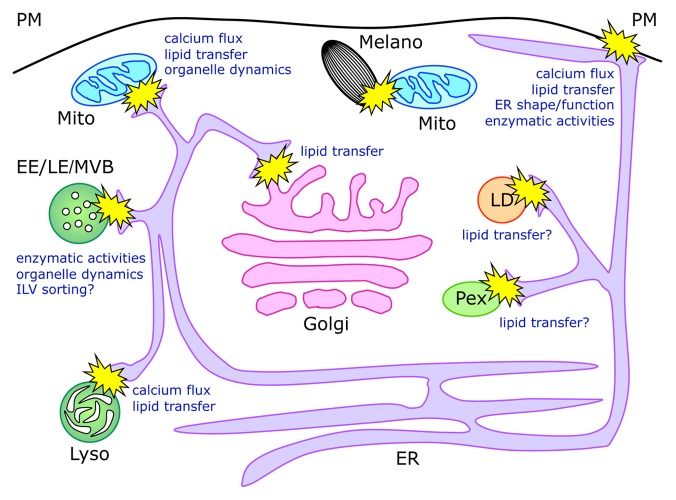
The ER represents the largest membrane-bound compartment, and plays critical roles in protein and lipid synthesis and in the regulation of calcium signaling.4 Therefore, it is not surprising that this organelle, besides being functionally connected to the secretory/endocytic pathway via vesicular transport, is also able to directly interact by means of MCSs with virtually all other subcellular organelles, including Golgi apparatus, endosomes, lysosomes, plasma membrane, lipid droplets, mitochondria, and peroxisomes (Fig. 1). Although the main functions attributed to the interorganellar interactions regard the transfer and metabolism of lipids and the modulation of calcium fluxes and homeostasis, more recent evidence points to several additional roles, including sharing of enzymatic activities or control of organelle dynamics (Fig. 1).
Figure 1. Schematic representation of membrane contact sites and their functions. Organelle dynamics indicates both the shape and motility of involved organelles. For LD and Pex, which are believed to originate from the ER, the role of direct contacts observed with this compartment remains unclear and may be implicated in lipid transfer between the ER and the mature form of the organelles.78,79 ER, endoplasmic reticulum; Pex, peroxisomes; LD, lipid droplets; Mito, mitochondria; Melano, melanosomes; Golgi, Golgi apparatus; EE, early endosomes; LE, late endosomes; MVBs, multivesicular bodies; Lyso, lysosomes; ILV, intraluminal vesicles.
We now identified a novel ER-independent interorganellar connection, involving mitochondria and melanosomes, lysosome-related organelles (LROs) of pigment cells devoted to the synthesis, transport, and transfer of melanin pigments.5 Indeed, quantitative ultrastructural analysis and tomographic reconstruction showed that a significant fraction of melanosomes is located in direct contact with mitochondria, that these interorganellar connections are mediated by fibrillar bridges, and that they are labeled by and require Mitofusin (Mfn) 2, similarly to the ER-mitochondria juxtaposition. Moreover, melanosome-mitochondrion contacts were associated to the melanogenesis process, since they were more abundant where and when active melanosome biogenesis takes place, while they were reduced in conditions of abnormal melanosome biogenesis.5 These findings reveal the presence of a physical and functional connection between mitochondria and the secretory/endocytic pathway that for the first time does not involve the ER and is implicated in physiological and pathological organelle biogenesis.
Nevertheless, the molecular players and physiological function of melanosome-mitochondrion connections remain to be uncovered, and can be hypothesized based on the role of other better-characterized MCSs.
MCSs regulate calcium fluxes and signaling
Calcium concentrations in the millimolar range are found in the extracellular space and in the lumen of the ER, the Golgi apparatus, and acidic organelles, whereas the cytosolic concentration of the ion is kept low (typically 100 nM).6 MCSs, in particular between the ER and PM, or the ER and mitochondria, have been implicated in the regulation of direct calcium transfer between organelles. This mechanism has the advantage of maximizing the efficiency of calcium signaling and interorganellar exchange, while avoiding excessive or prolonged changes in the overall concentration of cytosolic calcium, which in turn could be detrimental for the cell.2,7
In all cells, a store-operated calcium entry pathway (SOCE) functions at ER-PM MCSs to efficiently refill ER stores upon calcium release, typically caused by opening of inositol-1,4,5-triphosphate receptors (Ins(1,4,5)P3Rs). The transmembrane ER proteins STIM sense the depletion of calcium in the ER and oligomerize, translocating to ER-PM contact sites and activating the CRAC/Orai1 Ca2+ channels on the PM.8-10 In this manner, calcium depletion in the ER recalls the ion available in the extracellular space, which enters the cytosol and can reload the ER stores via the Sarcoplasmic/Endoplasmic Reticulum Ca2+ ATPases. In muscle cells an additional ER-PM MCSs is found that comprises voltage-gated calcium channels on the PM, functionally connected with Ryanodine Receptors (RyRs) on the ER, so that opening of the former also results in activation of the latter to maximize cytosolic calcium influx during excitation-contraction coupling.7,11
On the other hand, MCSs between the ER and mitochondria, also known as mitochondria-associated-membranes (MAMs)12 are mainly implicated in spatially restricting and buffering the calcium fluxes triggered by opening of Ins(1,4,5)P3Rs or RyRs on the ER membrane. Indeed, close juxtaposition between the 2 compartments generates cytosolic microdomains, where high calcium concentrations are achieved,13-15 and is required for the ability of mitochondria to efficiently uptake the calcium released from the ER by the low affinity mitochondrial calcium uniporter (MCU).16,17 The correct functioning of this system and the interorganellar distance are crucial for mitochondrial calcium homeostasis and appear necessary both to regulate ATP production, which depends on several Ca2+ dependent metabolic enzymes in the mitochondrial matrix, and to avoid Ca2+ overload in the mitochondria, which instead promotes apoptosis.18,19
A role in the modulation of calcium fluxes and/or signaling has also been postulated for MCSs between ER and late endosomes/lysosomes (LE/LYS). Indeed, both lysosomes and LROs are acidic calcium stores and lysosomes have been shown to release calcium upon different stimuli, by means of Ca2+ permeable channels, including 2 pore channels (TPC), transient receptor potential mucolipin (TRPML) channels, and Ins(1,4,5)P3Rs/RyRs.6,20-22 This in turn can evoke and/or amplify ER-dependent Ca2+ release, with implications for calcium oscillations in different systems.23,24 Moreover, defective lysosomal calcium homeostasis has been associated to endocytic and lysosomal dysfunction, abnormalities in membrane traffic, and lysosomal storage diseases.25,26 Among LROs, melanosomes contain high calcium concentrations and are thought to participate in calcium homeostasis and/or signaling, since melanin is able to bind and buffer the ion, likely functioning as an intracellular calcium reservoir.27-29 The melanosome-mitochondrion juxtaposition could be involved in buffering and/or sensing of calcium possibly released by melanosomes during maturation, controlling the local concentration of the ion and evoking further signals between the 2 organelles, required for proper melanosome biogenesis.
MCSs mediate lipid transfer and metabolism
The synthesis of membrane lipids takes place primarily in the ER; however, specific biosynthetic reactions are performed on other organelles, such as the Golgi apparatus and the mitochondria.30,31 Thus, either vesicular or non-vesicular transport is necessary to appropriately distribute lipids to the different subcellular compartments. MCSs play an important role in the regulation of lipid homeostasis by their ability to mediate non-vesicular lipid transfer among distinct compartments through the action of lipid-transport proteins (LTPs).1,2 A number of LTPs and their modes of action have being identified, including oxysterol-binding protein (OSBP) and OSBP-related proteins (ORP),32 operating at the ER-Golgi and ER-PM interface, and implicated in the exchange and metabolism of sterols and phosphoinositides;11,30,33 the ceramide-transfer protein CERT34,35 and the glucosylceramide-transfer protein FAPP2,36,37 operating non-vesicular transport at the ER-Golgi and intra-Golgi (cis-trans) contact sites, respectively; the extended synaptotagmins (known as tricalbins in yeast), functioning as ER–PM tethers and most likely implicated in (glycerophospho) lipid transfer;38,39 and possibly in yeast the ER-mitochondria encounter structure (ERMES) complex, an ER–mitochondrial tether, comprising several subunits containing lipid-binding domains.2,40
Sterol-binding proteins have been identified on LE/LYS as well, however at this location they appear to function mostly as lipid sensors and scaffold proteins, rather than lipid transfer effectors (see below). Nevertheless, melanosome-mitochondrion contacts may serve to control the quality and quantity of lipids on maturing melanosomes, regulating physical properties and abundance of their membranes, and the formation of specialized domains necessary for membrane traffic processes, such as the intralumenal sorting and processing of the structural protein Pmel17.41,42 Along the same line, mitochondria have been reported to supply membranes to forming autophagosomes,43 which could originate from the ER at MAMs.44 Melanosome biogenesis might share molecular mechanisms with autophagy, since genes involved in the autophagic process were identified in a screening for novel pathways involved in melanogenesis.45 Thus, by means of MCSs, mitochondria could provide melanosomes with membranes or other components required for the shape and size changes occurring during their maturation. Interestingly, the small GTPase Rab32, which localizes to both the ER and mitochondria, and regulates the properties of MAMs,46,47 is also involved in the formation of autophagosomes,48 and in melanosome biogenesis and transport,49,50 thus representing a potential candidate regulator of this crosstalk.
MCSs promote protein-protein interactions and cell signaling
It is becoming evident that at least some MCSs function as signaling hubs, by facilitating the scaffolding of signaling protein complexes and allowing the carry out of catalytic reactions, either in cis or in trans, with enzyme and substrate located on the same or on juxtaposed organelles, respectively. For instance, some ORPs have been shown to act as protein scaffolds, coordinating lipid sensing and metabolism with cell signaling and membrane traffic events,51 and the protein kinase mTORC2 and the lipid/protein phosphatase PTEN localize to the ER and operate in cis at MAMs.52,53 Moreover, internalized EGFR on endosomes, and in particular on the limiting membrane of multivesicular bodies (MVB), becomes dephosphorylated in trans, by means of the protein tyrosine phosphatase 1B (PTP1B) located on ER membranes at MCSs between the 2 compartments.54 Close juxtaposition between the ER and MVBs allows enzyme and substrate to interact and might also be implicated in the subsequent fate of the receptor, such as sequestration in intra-luminal vesicles (ILV) and lysosomal degradation.54,55 Likewise, the ER-PM juxtaposition may allow the regulation of phosphatidylinositol 4-phosphate (PI4P) levels at the cell surface, by means of the ER-localized PIP phosphatase Sac1, although recent evidence supports an alternative mechanism.11,33,56
Similarly, the melanosome-mitochondrion interaction might play a role in the assembly and modulation of signaling pathways and membrane traffic events necessary for melanogenesis. Of note, melanosomal membranes carry a peculiar type of intracellular G-protein-coupled receptor (GPCR), named OA1, which is involved in melanosome biogenesis and transport,57-59 and appears functionally associated to melanosome-mitochondrion contacts.5 Thus, the OA1 GPCR might promote the formation of MCSs between the 2 organelles, either directly acting as a tether or, most likely, indirectly by means of its signaling cascade or by its ability to stimulate the melanogenic process.
MCSs control organelle dynamics and distribution
ER-mitochondria MCSs have been shown to play a role in mitochondrial fusion-fission and overall motility.60 These processes are crucial for mitochondrial biogenesis, distribution, and function, and their alteration results in inherited or age-related neurodegenerative diseases.61 Mitochondria continuously fuse and divide, by means of pro-fusion (Mfn 1 and 2, and OPA1, on the mitochondrial outer and inner membrane, respectively) and pro-fission (DRP1) proteins.61 They are also highly dynamic and move bidirectionally along microtubules, by exploiting the mitochondrial GTP-ase MIRO and its effector MILTON to recruit kinesin 1 and determine the prevalence of peripheral vs. centripetal organelle transport.62 The ER-mitochondria juxtaposition appears intertwined with these processes, since the pro-fission machinery is recruited and operates at sites where ER tubules contact mitochondria,63 and components of the pro-fusion machinery, namely Mfn 1 or 2 on the mitochondrial side and Mfn 2 on the ER, have been implicated in the interorganellar tethering.64 Moreover, Mfn 2 appears directly required for transport of axonal mitochondria by interacting with the MIRO/MILTON complex and affecting both kinesin and dynein-based transport.65
Despite both mitochondria and the ER are highly motile organelles, they remain linked even as they move along microtubules.66 The maintenance of interorganellar contacts during microtubule-based motility is also a feature of endosomes, which mature and move while they remain bound to the ER.66,67 In the latter case, LTPs appear implicated in orchestrating membrane lipid content with organelle motility and connection with other compartments. On LE, the Rab7 effector ORPL1 is required for dynein activity upon recruitment by the RILP/dynactin complex.68 ORPL1 is also a cholesterol sensor, and in low cholesterol conditions it undergoes a conformational change that induces ER-LE MCSs, displacing the dynein/dynactin complex from RILP and leading to peripheral LE distribution.69 Similarly, the endosomal cholesterol-transfer proteins STARD3 and STARD3NL generate MCSs between LEs and the ER and appear implicated in endosome morphology and dynamics, independently on ORPL1 and PTP1B.70 In either case, it is not known whether sterol exchange occurs at the interorganellar junctions.
As mitochondria, the ER, and endosomes, melanosomes are highly motile organelles, traveling along microtubules and actin filaments.71 Mitochondria and melanosomes might cope with their interactions during movement by means of dynamic, transient contacts, in a “stop-and-go” or in a “kiss-and-run” fashion. Alternatively, the organelles might be joint by more persistent and stable contacts, allowing coordinated motility and distribution. The latter possibility would be more compatible with the generation of cytosolic microdomains, allowing the localized exchange of small molecules between the 2 organelles, as in the case of calcium at the ER-mitochondria juxtaposition. In addition to the regulation of calcium fluxes, another main function of mitochondria is to produce ATP and both their abundance and distribution in tissues and cells correlates with energetic needs. Moreover, mitochondria are able to redistribute at sites of high-energy requirement, such as neuronal,72 and immunological synapses,73,74 regulating their motility in response to intrinsic and extrinsic calcium concentrations.75,76 Melanosome biogenesis and transport certainly represent other physiological processes requiring energy. Thus, mitochondria juxtaposition might be required to timely and locally supply melanosomes with the ATP needed either for their movement along microtubule and actin tracks, or for melanin synthesis, or for controlling the melanosomal pH and membrane composition.
The compartmentalization of biochemical functions requires the reciprocal crosstalk between organelles to maintain the cellular homeostasis and to guarantee an efficient and coordinated response to environmental changes. Interorganellar MCSs allow virtually any combination of membrane-bound compartments to establish a communication. The newly identified contacts between melanosomes and mitochondria demonstrate that the 2 compartments not only are coordinated at the transcriptional level,77 but also interact at the physical level.5 Given the characteristics of melanosomes as models of secretory organelles, it is possible that secretory granules in neuroendocrine cells or other LROs in hematopoietic cells connect with mitochondria during their biogenesis and transport. Understanding the structural and functional features of these interactions is critical not only by a biological point of view, but also for the possibility that organelle biogenesis could be pharmacologically modulated by exploiting and targeting MCSs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by Telethon-Italy (GGP08156 to M.V.S.) and the Vision of Children Foundation-USA (to M.V.S.). Work by T.D. was supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC), and the Fondazione San Paolo, Italy.
References
- 1.Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol. 2011;23:458–63. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833:2526–41. doi: 10.1016/j.bbamcr.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Elbaz Y, Schuldiner M. Staying in touch: the molecular era of organelle contact sites. Trends Biochem Sci. 2011;36:616–23. doi: 10.1016/j.tibs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Novick P, Ferro-Novick S. ER structure and function. Curr Opin Cell Biol. 2013;25:428–33. doi: 10.1016/j.ceb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniele T, Hurbain I, Vago R, Casari G, Raposo G, Tacchetti C, Schiaffino MV. Mitochondria and melanosomes establish physical contacts modulated by Mfn2 and involved in organelle biogenesis. Curr Biol. 2014;24:393–403. doi: 10.1016/j.cub.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–86. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, et al. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–51. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan CJ, Manford AG, Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Curr Opin Cell Biol. 2013;25:434–42. doi: 10.1016/j.ceb.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–8. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–7. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 14.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–90. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–32. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–40. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–5. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csordás G, Renken C, Várnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29:2715–23. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J Biol Chem. 2010;285:35039–46. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg²⁺, NAADP, PI(3,5)P₂ and multiple protein kinases. EMBO J. 2014;33:501–11. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett. 2010;584:2013–21. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci. 2013;126:60–6. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan AJ, Davis LC, Wagner SK, Lewis AM, Parrington J, Churchill GC, Galione A. Bidirectional Ca²⁺ signaling occurs between the endoplasmic reticulum and acidic organelles. J Cell Biol. 2013;200:789–805. doi: 10.1083/jcb.201204078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans. 2007;35:1088–91. doi: 10.1042/BST0351088. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Evans E, Waller-Evans H, Peterneva K, Platt FM. Endolysosomal calcium regulation and disease. Biochem Soc Trans. 2010;38:1458–64. doi: 10.1042/BST0381458. [DOI] [PubMed] [Google Scholar]
- 27.Hoogduijn MJ, Smit NP, van der Laarse A, van Nieuwpoort AF, Wood JM, Thody AJ. Melanin has a role in Ca2+ homeostasis in human melanocytes. Pigment Cell Res. 2003;16:127–32. doi: 10.1034/j.1600-0749.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 28.Salceda R, Sánchez-Chávez G. Calcium uptake, release and ryanodine binding in melanosomes from retinal pigment epithelium. Cell Calcium. 2000;27:223–9. doi: 10.1054/ceca.2000.0111. [DOI] [PubMed] [Google Scholar]
- 29.Bush WD, Simon JD. Quantification of Ca(2+) binding to melanin supports the hypothesis that melanosomes serve a functional role in regulating calcium homeostasis. Pigment Cell Res. 2007;20:134–9. doi: 10.1111/j.1600-0749.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 30.Drin G. Topological regulation of lipid balance in cells. Annu Rev Biochem. 2014;83:51–77. doi: 10.1146/annurev-biochem-060713-035307. [DOI] [PubMed] [Google Scholar]
- 31.Tatsuta T, Scharwey M, Langer T. Mitochondrial lipid trafficking. Trends Cell Biol. 2014;24:44–52. doi: 10.1016/j.tcb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Olkkonen VM, Li S. Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog Lipid Res. 2013;52:529–38. doi: 10.1016/j.plipres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–43. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 34.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–9. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 35.Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–88. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- 36.D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–7. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 37.D’Angelo G, Uemura T, Chuang CC, Polishchuk E, Santoro M, Ohvo-Rekilä H, Sato T, Di Tullio G, Varriale A, D’Auria S, et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature. 2013;501:116–20. doi: 10.1038/nature12423. [DOI] [PubMed] [Google Scholar]
- 38.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014 doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–81. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurbain I, Geerts WJ, Boudier T, Marco S, Verkleij AJ, Marks MS, Raposo G. Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc Natl Acad Sci U S A. 2008;105:19726–31. doi: 10.1073/pnas.0803488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10:343–54. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–67. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–93. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 45.Ganesan AK, Ho H, Bodemann B, Petersen S, Aruri J, Koshy S, Richardson Z, Le LQ, Krasieva T, Roth MG, et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–68. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bui M, Gilady SY, Fitzsimmons RE, Benson MD, Lynes EM, Gesson K, Alto NM, Strack S, Scott JD, Simmen T. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirota Y, Tanaka Y. A small GTPase, human Rab32, is required for the formation of autophagic vacuoles under basal conditions. Cell Mol Life Sci. 2009;66:2913–32. doi: 10.1007/s00018-009-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–81. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park M, Serpinskaya AS, Papalopulu N, Gelfand VI. Rab32 regulates melanosome transport in Xenopus melanophores by protein kinase a recruitment. Curr Biol. 2007;17:2030–4. doi: 10.1016/j.cub.2007.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber-Boyvat M, Zhong W, Yan D, Olkkonen VM. Oxysterol-binding proteins: functions in cell regulation beyond lipid metabolism. Biochem Pharmacol. 2013;86:89–95. doi: 10.1016/j.bcp.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A. 2013;110:12526–34. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P. Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ. 2013;20:1631–43. doi: 10.1038/cdd.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–72. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- 55.Eden ER, Burgoyne T, Edgar JR, Sorkin A, Futter CE. The relationship between ER-multivesicular body membrane contacts and the ESCRT machinery. Biochem Soc Trans. 2012;40:464–8. doi: 10.1042/BST20110774. [DOI] [PubMed] [Google Scholar]
- 56.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Palmisano I, Bagnato P, Palmigiano A, Innamorati G, Rotondo G, Altimare D, Venturi C, Sviderskaya EV, Piccirillo R, Coppola M, et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet. 2008;17:3487–501. doi: 10.1093/hmg/ddn241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giordano F, Bonetti C, Surace EM, Marigo V, Raposo G. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum Mol Genet. 2009;18:4530–45. doi: 10.1093/hmg/ddp415. [DOI] [PubMed] [Google Scholar]
- 59.Schiaffino MV. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol. 2010;42:1094–104. doi: 10.1016/j.biocel.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–25. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–84. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 62.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–57. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–62. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 65.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–40. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190:363–75. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman JR, Dibenedetto JR, West M, Rowland AA, Voeltz GK. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol Biol Cell. 2013;24:1030–40. doi: 10.1091/mbc.E12-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–71. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–25. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alpy F, Rousseau A, Schwab Y, Legueux F, Stoll I, Wendling C, Spiegelhalter C, Kessler P, Mathelin C, Rio MC, et al. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J Cell Sci. 2013;126:5500–12. doi: 10.1242/jcs.139295. [DOI] [PubMed] [Google Scholar]
- 71.Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans. 2011;39:1191–6. doi: 10.1042/BST0391191. [DOI] [PubMed] [Google Scholar]
- 72.MacAskill AF, Atkin TA, Kittler JT. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci. 2010;32:231–40. doi: 10.1111/j.1460-9568.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- 73.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–23. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abarca-Rojano E, Muñiz-Hernández S, Moreno-Altamirano MM, Mondragón-Flores R, Enriquez-Rincón F, Sánchez-García FJ. Re-organization of mitochondria at the NK cell immune synapse. Immunol Lett. 2009;122:18–25. doi: 10.1016/j.imlet.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105:20728–33. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang KT, Niescier RF, Min KT. Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc Natl Acad Sci U S A. 2011;108:15456–61. doi: 10.1073/pnas.1106862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A, Koulisis N, Farrel C, Amos CI, Wei Q, et al. PGC-1 coactivators regulate MITF and the tanning response. Mol Cell. 2013;49:145–57. doi: 10.1016/j.molcel.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilfling F, Haas JT, Walther TC, Jr RV. Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29C:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hettema EH, Erdmann R, van der Klei I, Veenhuis M. Evolving models for peroxisome biogenesis. Curr Opin Cell Biol. 2014;29C:25–30. doi: 10.1016/j.ceb.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]