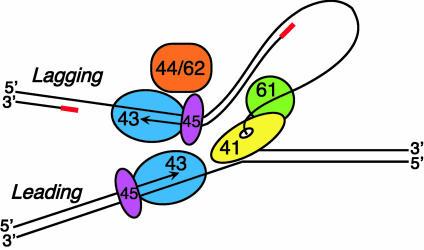
The exchange of polymerase subunits in the T4 replisome is mediated by the gp45 sliding clamp.
DNA polymerases are full of surprises. It is widely accepted that replicative polymerases are highly processive, thanks, in large part, to accessory subunits that act as sliding clamps and tether the polymerase to its DNA template. The bacteriophage T4 replication system is typical in that processivity measurements indicate that a substantial fraction of replication complexes should remain associated with DNA for the ≈15 min required to copy an entire genome length (1). However, an elegant study by Yang et al. (2) in this issue of PNAS challenges our notion of processivity by showing that, within the highly processive T4 replisome, individual DNA polymerase molecules are exchanging rapidly, maybe 90 times per genome length under the conditions prevailing in vivo. Thus, if the replisome is visualized as a molecular factory, the workers are making frequent shift changes.
Processivity Measurements
The bacteriophage T4 replisome, on which these studies were carried out, serves as a simple model incorporating the essential features of multisubunit replication systems. The components of the T4 system are illustrated in cartoon form in Fig. 1. The high overall processivity of the replisome does not necessarily mean that all subunits are equally processive, and two approaches have been informative in measuring the life-times of individual components within an actively synthesizing replisome. The simplest is to determine the sensitivity of DNA synthesis to dilution of individual components. This approach has demonstrated that the helicase (gp41) is very stably associated with the replication fork (1). By contrast, lagging strand synthesis was sensitive to dilution of the clamp (gp45), clamp loader (gp44/62 complex), and primase (gp61) (5, 6), reflecting the remodeling of the lagging strand machinery that must take place as each new Okazaki fragment is started. Significantly, dilution of the polymerase (gp43) did not affect lagging strand synthesis, implying that it remains associated with the replisome throughout the synthesis of multiple Okazaki fragments.
Fig. 1.
Simplified representation of the proteins at the T4 replication fork. The replisome contains two DNA polymerase subunits (gp43), one for the leading and one for the lagging strand, each with its own sliding clamp (gp45), and with a clamp loader (gp44/62 complex) associated with the lagging strand polymerase. Helicase (gp41) drives the replication fork and is associated with primase (gp61). Not shown are gp59, which functions to load helicase onto the fork, and gp32, which binds to single-stranded DNA generated during replication. The RNA primers that initiate DNA synthesis on the lagging strand are shown in red (not to scale). The components and assembly pathway of the T4 replisome have been established from work in many laboratories, exemplified by refs. 3 and 4.
A second approach to investigate the processivity of components of the replisome is the use of protein traps, mutant forms of the subunit under investigation, which are catalytically inactive but otherwise unchanged in their ability to bind to DNA or other replisome components. Such mutant proteins can bind to their normal target and then inhibit the replisome. This approach confirmed the results from dilution experiments by showing a rapid shutdown of lagging strand replication when inactive forms of the primase or clamp loader were introduced (6).
In this issue of PNAS, Yang et al. (2) describe the surprising results obtained when investigating the lifetime of the DNA polymerase (gp43) in the T4 replisome. In a dilution experiment, where the final concentration of the polymerase was too low to permit rebinding after dissociation, there was only a very gradual decrease in both leading and lagging strand synthesis, indicating that the two polymerase subunits, like helicase, apparently remain associated with the replisome for synthesis of all or most of the phage genome. A reasonable prediction from this result is that replication should be inhibited very little by an inactive polymerase mutant acting as a protein trap, because dissociation of the replicating polymerase, and exchange with the inactive trap, should be very infrequent. Surprisingly, the opposite result was obtained. Addition of a gp43 active site mutant to an ongoing replication reaction caused rapid inhibition of both leading and lagging strand synthesis within approximately one minute, implying that, despite their apparent high processivity, both polymerases were available to exchange with the inactive mutant. Control experiments ruled out indirect effects caused by the mutant polymerase interfering with other components of the replisome.
Polymerase Exchange Mechanisms
The paradoxical aspect of the trapping experiment is that the onset of inhibition by the inactive polymerase was faster than dissociation of polymerase from the replisome (as determined by the dilution experiment). The kinetics of trapping can be explained by an “active exchange” mechanism in which the trap polymerase in some way assists the displacement of the replicating polymerase. This interpretation makes biological sense because it ensures that a replicating polymerase will not dissociate from the replisome unless a replacement polymerase is ready to take over, avoiding spontaneous loss of the replicating polymerase and the need to recruit a replacement from bulk solution.
A possible mechanism for the binding of additional polymerase molecules to the replisome is suggested by a key observation of Yang et al. (2) that a T4 DNA polymerase mutant with a small C-terminal deletion did not act as a trap. The C-terminal deletion, which prevents binding to the sliding clamp but does not otherwise affect polymerase-DNA binding (7), removes a conserved sequence, present on several other polymerases, that has been shown to interact with the clamp (8-10). Yang et al. therefore infer that the exchange of polymerase subunits in the T4 replisome is mediated by means of the trimeric gp45 sliding clamp, and they present two structural models to explain how the sliding clamp may bind both the replicating polymerase and a “spare” polymerase that is available to replace it. Regardless of the precise details of the binding geometry, sliding clamps, being multimeric, clearly have the potential to provide additional binding sites for associated proteins. The active exchange inferred from the kinetics of inhibition in the trapping experiment could result from an allosteric destabilization of the replicating polymerase caused by binding of a polymerase to a second site on the sliding clamp. Alternatively, the replicating polymerase may transiently let go of the DNA in the normal course of synthesis, and the spare polymerase, by virtue of its proximity, would be well placed to take advantage of this. Transient release of the primer terminus by the polymerase is to be expected based on the rather low processivity of T4 gp43 (and other replicative polymerases) in the absence of accessory factors (11). The enhanced processivity conferred by the sliding clamp derives not from preventing the initial release of the primer terminus but from tethering the polymerase and thus increasing the probability of rebinding to the primer terminus.
Biological Implications
A dynamic relationship between the polymerase subunit and the replisome may be advantageous in a variety of biological situations. The most obvious is in replication past a blocking lesion on the template. The exchange mechanism proposed by Yang et al. (2) provides a means of transferring the primer terminus from a stalled replicative polymerase to a specialized lesion bypass polymerase. One can envisage a straightforward targeting process. Stalling of the replicative polymerase at a site of DNA damage could enhance release of the primer terminus for capture by a specialized lesion bypass polymerase. If the bypass polymerase has a lower intrinsic affinity for the primer terminus than does the replicative polymerase, this scenario would facilitate exchange subsequent to lesion bypass, thus avoiding the synthesis of long tracts of DNA by the less accurate bypass polymerase. It also has been suggested that transient release of DNA by a replicating polymerase could relieve torsional stress at the replication fork and could facilitate transfer of a mispaired primer terminus to the editing site (9, 12).
Recent work suggests that the Escherichia coli sliding clamp can bind a large number of replication proteins, including all five bacterial polymerases, the clamp loader, and DNA ligase (8). All of these interactions use the same site on the clamp, and many, although not all, involve the conserved C-terminal peptide mentioned earlier. These findings reinforce the idea of the sliding clamp as a “tool belt” carrying additional replication proteins whose activities may be required under specific circumstances (13). Given the limited number of potential binding sites on the sliding clamp, frequent exchange of the tools (or multiple tool belts) may be necessary to ensure the availability of all of the appropriate enzymatic activities when needed.
Dynamic Processivity
An important message from the article by Yang et al. (2) is the need to adopt a more nuanced view of the concepts of dissociation and processivity and to recognize that different experimental approaches may yield apparently contradictory answers because they interrogate different steps in the process of dissociation. The loss of a subunit from a large assembly is likely to be a multistep process, with early steps involving the release from contacts with immediate neighbors and leading eventually to complete escape of the subunit into the surrounding medium. Dilution experiments address dissociation in a macroscopic sense because they measure capture of the target subunit from the bulk solution. On the other hand, proteintrapping experiments may probe an earlier stage provided that, as in the present case, the protein trap has access to the macromolecular complex. Thus, the T4 polymerase trapping experiment reveals not only the dynamics of the DNA polymerase subunits within the T4 replisome but also the availability of binding sites for additional polymerase molecules.
How dynamic then is the T4 replisome? It is well established that lagging strand components, other than the polymerase, are recruited continuously from solution. The current study by Yang et al. (2) shows that the polymerase is exchangeable within the replisome complex but only rarely leaves the complex if a replacement polymerase is not available. The helicase appears to be the most solidly fixed component and has been suggested to be the “cornerstone” of the replisome (1). Although the high processivity of the helicase has not been challenged by a protein-trapping approach, it is difficult to imagine a biological rationale for helicase exchange or a mechanism by which it could take place. Finally, the many analogies between the T4 replication system and the more complex bacterial and eukaryotic systems suggest that the dynamic notion of polymerase processivity put forward by Yang et al. should be considered in these systems too.
See companion article on page 8289.
References
- 1.Schrock, R. D. & Alberts, B. (1996) J. Biol. Chem. 271, 16678-16682. [DOI] [PubMed] [Google Scholar]
- 2.Yang, J., Zhuang, Z., Roccasecca, R. M., Trakselis, M. A. & Benkovic, S. J. (2004) Proc. Natl. Acad. Sci. USA 101, 8289-8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benkovic, S. J., Valentine, A. M. & Salinas, F. (2001) Annu. Rev. Biochem. 70, 181-208. [DOI] [PubMed] [Google Scholar]
- 4.Jones, C. E., Mueser, T. C., Dudas, K. C., Kreuzer, K. N. & Nossal, N. G. (2001) Proc. Natl. Acad. Sci. USA 98, 8312-8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadyrov, F. A. & Drake, J. W. (2001) J. Biol. Chem. 276, 29559-29566. [DOI] [PubMed] [Google Scholar]
- 6.Trakselis, M. A., Roccasecca, R. M., Yang, J., Valentine, A. M. & Benkovic, S. J. (2003) J. Biol. Chem. 278, 49839-49849. [DOI] [PubMed] [Google Scholar]
- 7.Berdis, A. J., Soumillion, P. & Benkovic, S. J. (1996) Proc. Natl. Acad. Sci. USA 93, 12822-12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez de Saro, F. J., Georgescu, R. E., Goodman, M. F. & O'Donnell, M. (2003) EMBO J. 22, 6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shamoo, Y. & Steitz, T. A. (1999) Cell 99, 155-166. [DOI] [PubMed] [Google Scholar]
- 10.Bunting, K. A., Roe, S. M. & Pearl, L. H. (2003) EMBO J. 22, 5883-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delagoutte, E. & von Hippel, P. H. (2003) J. Biol. Chem. 278, 25435-25447. [DOI] [PubMed] [Google Scholar]
- 12.Hingorani, M. M. & O'Donnell, M. (2000) Curr. Biol. 10, R25-R29. [DOI] [PubMed] [Google Scholar]
- 13.Pages, V. & Fuchs, R. P. P. (2002) Oncogene 21, 8957-8966. [DOI] [PubMed] [Google Scholar]