Abstract
Component exchange in reversible polymers allows the generation of dynamic constitutional diversity. The polycondensation of dihydrazides with dialdehydes generates polyacylhydrazones, to which the acylhydrazone functionality formed confers both hydrogen-bonding and reversibility features through the amide and imine groups, respectively. Polyacylhydrazones are thus dynamic polyamides. They are able to reversibly exchange either one or both of their repeating monomer units in the presence of different monomers, thus presenting constitutional dynamic diversity. The polymers subjected to monomer exchange/interchange may be brought to exhibit physical properties vastly different from those of the original polymer. The principle may be extended to other important classes of polymers, giving access, for instance, to dynamic polyureas or polycarbamates. These reversible polymers are therefore able to incorporate, decorporate, or reshuffle their constituting monomers, namely in response to environmental physical or chemical factors, an adaptability feature central to constitutional dynamic chemistry.
Keywords: constitutional dynamic chemistry, reversible polymers, dynamic materials
Blending supramolecular chemistry with materials science defines a field of supramolecular materials that rests on the explicit implementation of intermolecular interactions in the design and synthesis of novel materials presenting novel properties.
Because supramolecular chemistry is by nature a dynamic chemistry in view of the lability of the noncovalent interactions connecting the molecular components of a supramolecular entity, supramolecular materials are by nature dynamic materials (1–3).
Importing such dynamic features, characteristic of supramolecular chemistry, into molecular chemistry implies looking at molecules as labile entities, in contrast to the usual longing for stability, thus opening novel perspectives to covalent chemistry. It requires searching for reversible reactions that allow the making and breaking of covalent bonds, preferentially under mild conditions. These developments are embodied in the recent emergence of dynamic combinatorial chemistry as a powerful means for generating dynamic, effector-responsive molecular diversity (2, 4).
Thus, on both the supramolecular and molecular levels, dynamic materials may be defined as materials whose constituents are linked through reversible connections (noncovalent or covalent) and are able to undergo continuous reorganization through assembly/disassembly processes, incorporation, extrusion, or reshuffling of components in a given set of conditions, usually under thermodynamic control (1, 2).
The resulting constitutional plasticity defines a constitutional dynamic chemistry (3) encompassing both covalent molecular (2, 5) and noncovalent supramolecular entities.
Focusing on a specific class of materials, polymers, the pathway leads from supramolecular polymers (6–8) to dynamic covalent polymers (see ref. 5, pp. 902–915). One may designate under the term, Dynamers (7), both the polymers that are dynamic by nature (supramolecular) and by design (molecular) due to the presence of either a noncovalent or a covalent reversibility cassette, respectively (Scheme 1). In view of their ability to build up by self-assembly (3, 9, 10) and to select in principle their constituents in response to external stimuli or to environmental factors, they behave as adaptive materials (1, 7).
Scheme 1.
Schematic representation of main-chain dynamers in which monomers are linked by either supramolecular or molecular reversibility cassettes X, Y, and Z, conferring dynamic character through reversible connections, noncovalent interactions or reversible covalent bonds, respectively. X, Y, and Z may be the same or different. In conventional polymers, they represent covalent bonds that are not reversible in the conditions considered.
Reversibility, Exchange, and Diversity
Molecular dynamers open a range of perspectives to polymer chemistry. Even though exchange reactions have long been known in macromolecular chemistry (5) and have been put to use even in large-scale industrial processes (involving, e.g., transesterification), the exploitation of reversible polymerization has been hampered by the difficulties in controlling depolymerization (11). The primary focus of polymer methodology has been on trying to avoid exchange reactions and to produce chemically well defined, unique polymers, in particular, for industry. Taking the opposite standpoint consists in deliberately pursuing constitutional diversity rather than emphasizing unicity. It leads to exploration of the generation of diversity through reversibility, which allows component exchange leading to highly diverse populations, combinatorial libraries, of polymeric constituents in dynamic equilibrium. Reversible reactions are presently receiving increasing attention and provide a means for developing new materials exhibiting unusual features. Recent covalent polymers investigated for their reversible properties have included entities based on various reactions, such as transesterification (12–15), transetherification (16), Diels–Alder reaction (17, 18), [2 + 2] photodimerization (19), radical reaction (20), and boronate ester formation (21).
Among the known reversible covalent reactions (2, 4, 5), amino/carbonyl condensations to give C N products, such as imines, hydrazones, and oximes, are particularly attractive in view of the very wide range of structural variations available, the easy synthetic accessibility, the control through conditions of yields, rates, and reversibility, and their role and potential for application in both biological/medicinal (2, 4, 22) and materials sciences.
N products, such as imines, hydrazones, and oximes, are particularly attractive in view of the very wide range of structural variations available, the easy synthetic accessibility, the control through conditions of yields, rates, and reversibility, and their role and potential for application in both biological/medicinal (2, 4, 22) and materials sciences.
Polyacylhydrazones
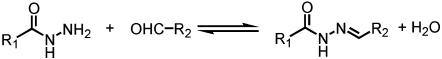
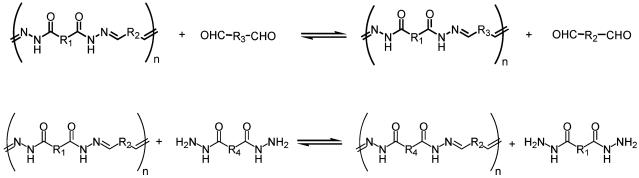
The formation of hydrazones and acylhydrazones by condensation of hydrazines and hydrazides, respectively, with carbonyl groups has been implemented in the generation of biologically active substances (22, 23) and of polymeric materials [polyhydrazones (24–29), polyacylhydrazones (30–40), and for an oligoimine case see ref. 41]. More specifically, acylhydrazone formation (Scheme 2) displays reversibility under mild conditions (23, 42–44) with acid catalysis, regenerating the starting reagents. This reversibility can be exploited in the presence of additional aldehydes or hydrazides to produce new acylhydrazones as a result of aldehyde or hydrazide exchange promoted by acid catalysis and/or heat.
Scheme 2.
Reversible acylhydrazone formation involving condensation of a hydrazide and an aldehyde.
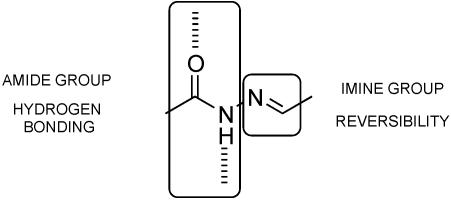
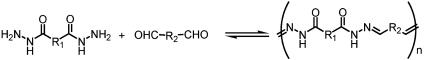
The acylhydrazone functionality provides both dynamic character through the reversibility of the imino bond and hydrogen-bonding sites through the amide group (Scheme 3). When dihydrazides are reacted with dicarbonyl compounds, polycondensation occurs and polyacylhydrazone polymers are formed (Scheme 4) that present particularly attractive features for polymer chemistry: (i) they are strict alternating copolymers; (ii) they form in high yield under mild conditions; (iii) they are dynamic covalent polymers (molecular dynamers) due to the reversibility of the acylhydrazone bond, and (iv) they are therefore capable of exchanging their components; and (v) they contain an amide group providing hydrogen bonding interactions, as in polyamides.
Scheme 3.
Representation of the specific features of the acylhydrazone functionality displaying both a hydrogen-bonding amide group and a reversible imine bond.
Scheme 4.
Polyacylhydrazone formation by condensation of a dihydrazide with a dicarbonyl compound; here, a dialdehyde.
Thus, by virtue of the special properties of the acylhydrazone unit, polyacylhydrazones may be considered as reversible polyamides, endowing this most important class of polymers with dynamic character and behavior of living polymer type. Through monomer exchange, they allow the generation of novel, constitutionally modified alternating copolymers, exhibiting physical properties different from those of the original unexchanged polymer. They are able to incorporate, decorporate, or reshuffle their constituting monomers, in particular, in response to external stimuli and environmental physical or chemical factors (heat, light, chemical entities, etc.). They also offer the possibility of obtaining new crossover entities by mixing different dynamers. The polycondensation and exchange processes may be performed under mild and adjustable conditions. Thus, the features of polyacylhydrazone dynamers provide a powerful and easily implemented methodology for the generation of new materials presenting adaptive behavior.
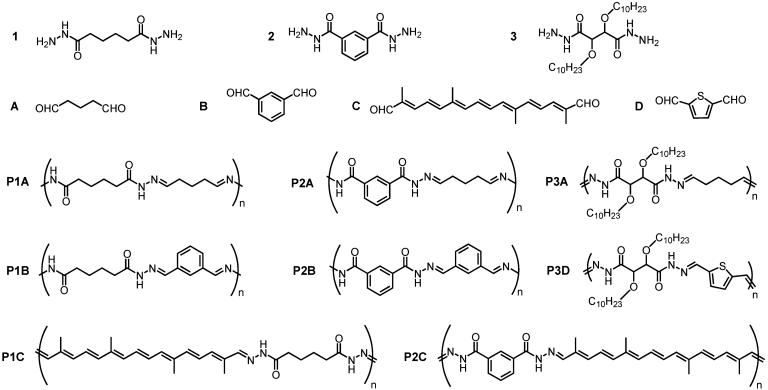
Within a broad investigation of dynamic polymers (ref. 45 and N. Giuseppone, T. Nobori, T. Ono, and J.-M.L., unpublished work), we present results of a study of the polyacylhydrazones derived from the dihydrazides 1–3 and the dialdehydes A, B, and C (Fig. 1). A few reports in the literature (30–36) and in patents (37–40) describe the preparation of such polymers, but we wish to stress here the potential offered by the special dynamic features conferred to them by their reversible nature. We shall only consider main-chain dynamic polymers; of course, reversible side-chain attachment is also possible.
Fig. 1.
Structures of the monomers and of the polyacylhydrazone dynamers investigated.
Materials and Methods
Dihydrazides 1–3 were obtained in high yield by treatment of the corresponding methylesters with hydrazine hydrate in ethanol. Because of their very low solubility in ethanol, they precipitate on formation and can be isolated easily by filtration, followed by washing with absolute ethanol and water. Their analytical and spectroscopic properties were in agreement with the assigned structures.
General Method of Polyacylhydrazone Synthesis. Typically between 100 and 200 mg of dihydrazide monomer were added to between 250 and 350 ml of absolute ethanol. The heterogeneous solution is rendered homogeneous by heating the reaction mixture for 15 min followed by placing it in an ultrasonic bath for ≈15 min. Complete dissolution is achieved by repeating this process once or twice. While the homogeneous solution was still warm, a stoichiometric amount of dialdehyde was added, after which the medium immediately became cloudy. The addition of a catalyst in the form of acid is not required but drastically decreases the reaction time. Acid catalysts may be hydrochloric acid, acetic acid, or trifluoroacetic acid (TFA) and between 2 and 10 drops are sufficient to promote polymerization and the subsequently ensuing precipitation. The polymeric slurry is stirred at room temperature from 3 to 12 h, with the desired polymer being obtained by filtration. The polymers were isolated in the form of cakes and were washed with an abundant amount of water and then dried under vacuum. Characterization and experimental conditions for the polyacylhydrazone synthesis are included in Supporting Text, which is published on the PNAS web site.
In the more soluble P3A the following procedure was used: Dihydrazide 3 (14 mg, 0.026 mmol) was dissolved in 0.5 ml of dimethylformamide (DMF), then diluted with 6 ml of dichloromethane. Glutaraldehyde (50%, 6.3 mg, 0.026 mmol) was diluted in 5 ml of water and added to the dihydrazide solution. The biphasic mixture was strongly stirred for 48 h at room temperature after the addition of a catalytic amount of benzyltriethyl ammonium chloride. The organic layer was removed and evaporated to yield a slightly yellow oil, which was immediately diluted in 4 ml of spectroscopic grade DMF and used for monomer exchange and subsequently for gel permeation chromatography (GPC) analyses relative to polystyrene standards, giving Mw = 72,962 g/mol, degree of polymerization (DP) = 129.
General Procedure of Monomer Exchange in Polyacylhydrazones. Typically, a polymeric stock solution was prepared by dissolving 24 mg of polyacylhydrazone in 2.5 ml of deuterated DMSO through heating followed by ultrasonication. Stock solutions (7 mM) of dialdehyde and dihydrazide monomers were prepared separately in deuterated DMSO. Exactly 0.3 ml of the polymer solution was charged into a NMR tube followed by 0.05 ml of either the dialdehyde or dihydrazide stock solution, representing one equivalent relative to the constitutional monomer within the polymer. The resulting mixture was heated between 90° and 110°C for between 0.5 and 2 min to induce the monomer exchange within the polymer and then observed by proton NMR. A catalytic amount of <1 μl of TFA along with mild heating was required to exchange the monomer units contained in the polyhydrazones. Specific exchange conditions and procedures used are found in the Supporting Text.
In the P3A the following procedure was used: In 1 ml of a solution of polymer P3A in DMF was added one equivalent of 2,5-thiophene dicarboxaldehyde and a catalytic amount of TFA. The mixture was heated at 120°C for varying durations, and the molecular weight was determined with a Waters GPC system equipped with a refractive index detector and a flow rate of 1 ml/min DMF with a Polymer Laboratoires (Amherst Fields Research Park, Amherst, MA) organic 5-μm mixed C GPC column.
In a UV-visible spectrophotometric cuvette were introduced 1.5 ml (0.5 mmol) of a DMSO solution of the polymer P2A (3.5 mg in 5 ml, ≈0.33 mM), 1.5 ml (0.65 mmol) of stock solution of crocetin dialdehyde C (4.3 mM), and 2 μl of acetic acid. The cuvette was heated to 75°C and the new polymer formed resulting from dialdehyde monomer exchange was observed at 345 and 355 nm for determination of the pseudofirst order rate constant of exchange.
Results and Discussion
Synthesis of the Acylhydrazone Polymers. The synthesis of the polyacylhydrazones P1A–P3D (Fig. 1) is performed by direct condensation of dialdehydes and dihydrazides (Scheme 4). It proceeds smoothly at room temperature under catalytic acid conditions. The major difficulty results from their insolubility in commonly used organic solvents, an indication of extensive hydrogen bonding between chains. The low solubility of the starting dihydrazides also poses a problem in conventional solvents. They were first added to a large amount of absolute ethanol and rendered soluble through repeated cycles of heating and sonication. Once completely dissolved, a trace amount of acid was added, immediately followed by the corresponding dialdehyde. Within minutes, the resulting polymers normally precipitated from the reaction medium giving the polyacylhydrazones as white or off-white powders that could be easily purified by washing with common organic solvents or water.
Owing to their very poor solubility, these polymers (except P3A) cannot be readily characterized by standard techniques such as GPC or matrix-assisted laser desorption ionization–time- of-flight mass spectrometry for molecular weight determination. High-boiling-point solvents capable of disrupting the polyacylhydrazone hydrogen bond network, such as DMSO, DMF, or N-methylpyrrolidone, are required for solubilization. With mild heating and sonication, the polyacylhydrazones dissolved in DMSO, allowing their characterization by proton NMR. This method is equally suitable for determining the average DP and the polymer molecular weight Mn by integration of the terminal aldehyde proton signal with respect to the aromatic proton region or to the hydrazone ( N—NH—) proton (Eq. 1). The signals of the reagents and products occur at different chemical shifts providing an unobscured window (Figs. 2 and 3) in which accurate integration is possible. Such calculations lead to Mn and to a lower limit of the polyacylhydrazone molecular weight, based on the average DP. DP values in the 10–25 range were obtained for the polymers derived from the dihydrazides 1 and 2 (see Materials and Methods).
N—NH—) proton (Eq. 1). The signals of the reagents and products occur at different chemical shifts providing an unobscured window (Figs. 2 and 3) in which accurate integration is possible. Such calculations lead to Mn and to a lower limit of the polyacylhydrazone molecular weight, based on the average DP. DP values in the 10–25 range were obtained for the polymers derived from the dihydrazides 1 and 2 (see Materials and Methods).
 |
[1] |
The solubility of the polyacylhydrazones can be greatly enhanced by incorporating groups such as long alkyl chains into the monomer, as in dihydrazide 3 that can be dissolved in most common organic solvents under moderate warming. Dihydrazide 3 readily polymerizes with glutaraldehyde to yield the corresponding polymer P3A. The resulting polymer is soluble in most organic solvents and exhibits a high molecular weight of 72,962 g/mol corresponding to a DP of 219.
Fig. 2.
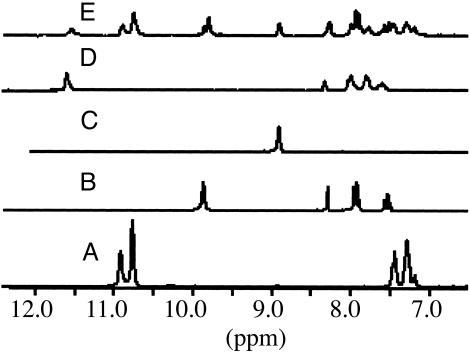
The 200-MHz proton NMR spectra of polyacylhydrazones in deuterated DMSO: sample A, starting material P1A; sample B, compound 2; sample C, compound 1; sample D, compound P2A; and sample E, sample A mixed with sample B and heating for 30–45 sec at between 90 and 110°C, 47% conversion.
Fig. 3.
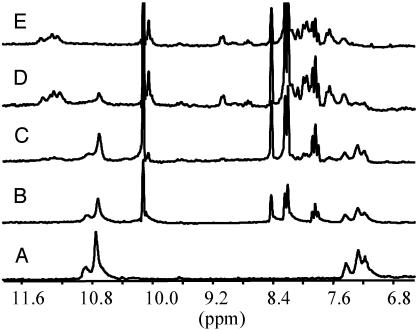
The 200-MHz proton NMR spectra of polyacylhydrazones in deuterated DMSO: sample A, P1A; sample B, P1A containing B; sample C, sample B after heating for 20 sec at 90 and 110°C, 15% exchange; sample D, sample B after heating for 40 sec at 90 and 110°C, 79% exchange; and sample E, sample B after heating for 30 sec at 90 and 110°C, 100% exchange.
Constitutional Dynamics: Monomer Exchange in Polyacylhydrazones. Polyacylhydrazone alternating copolymers, with two repeating units resulting from the condensation of a dialdehyde and a dihydrazide, were examined for their capacity to reversibly incorporate other monomer units (Scheme 5). The reversibility of the covalent hydrazone functionality, in principle, makes the exchange of either an aldehyde or an hydrazide component possible. Incorporation of a different monomer unit by displacing an unit already contained in the polymer backbone causes a modification of constitution and is expected to yield polymers containing various amounts of the different components and presenting properties different from those of the original one.
Scheme 5.
Dynamer constitutional modification by dialdehyde exchange (Upper) and dihydrazide exchange (Lower) in a polyacylhydrazone.
Owing to the poor solubility of the polyacylhydrazones in standard solvents, DMSO-d6 was selected as solvent for examining monomer component exchange within the polymer backbone by proton NMR spectroscopy. Among the various studies performed (45), those involving the monomers and the polyacylhydrazones represented in Fig. 1 were selected for dynamer exchange investigations. They exhibit a clear window without spectral overlap of other monomers in their 1H NMR spectra, allowing unequivocal assignment of a given substance (Figs. 2 and 3). Moreover, they also provide data on the influence of alkyl and aryl substitution on the dynamer exchange process. Typically, hydrazides exhibit NH chemical shifts in the region of ≈9–10 ppm, whereas in the acylhydrazones these signals are shifted to ≈11–12 ppm. These fingerprint regions allow easy determination of the dihydrazide or dialdehyde from the polymer backbone concomitant with the inclusion of a different one leading to a new polyacylhydrazone.
The polyacylhydrazone P1A was examined for its capability of undergoing monomer exchange and for determining the reaction conditions required for such a process. It contains only aliphatic spacers; therefore, incorporation of a new monomer consisting of either an aryl dihydrazide or an aryl dialdehyde would provide insight into the exchange reaction. P1A was dissolved in DMSO, to which was added one equivalent of the aryl dihydrazide 2. Fig. 2 shows that the starting materials (spectra A and B) and the expected exchanged products (spectra C and D) possess unique chemical shifts, allowing for the unequivocal identification of the dihydrazide exchange which proceeds on heating and in the absence of a catalyst. The substitution of an alkyl dihydrazide for an aryl one within P1A is seen by the appearance of new proton signals in the 11.6-ppm (arylhydrazone) and 8.3-ppm (alkyl hydrazide) regions. This change is concomitant with the disappearance of the aryl hydrazide signal at 9.8 ppm, giving rise to 47% exchange under limited heating times of 45 sec at ≈90°C.
Like a dihydrazide moiety can be incorporated into the polymer, the other alternating unit, the dialdehyde, can also be exchanged. The dialdehyde units and the resulting exchanged products also exhibit characteristic chemical shifts within the aromatic region of the 1H NMR spectrum, allowing for unequivocal characterization of starting and exchanged products (Fig. 3). Polyacylhydrazone P1A dissolved in DSMO-d6 along with one equivalent of aryl dialdehyde B exhibits a distinctive proton NMR signal at 10.1 ppm. Incorporation of dialdehyde B occurs on heating the sample in the absence of an acid catalyst. The resulting proton NMR spectrum displays the new hydrazone proton signals in the chemical shift region of 11.3 ppm and the disappearance of the signals at 10.8 ppm as a result of dialdehyde exchange. Incorporation of B into P2A required catalytic TFA, without heating, however.
The exchange process involves the random insertion of the monomer into the polymer backbone, leading to polymer fragmentation. As it progresses, the molecular weight of the original polymer decreases, causing an increase in solubility. Indeed, the excess of either of the components acts as end-capping group, thus leading to a shortening of the polymeric chains after the analysis performed for supramolecular polymers (46). Removal of a (volatile) monomer from the reaction medium is of course expected to shift the equilibrium in favor of the new exchanged polymer, leading to high-molecular-weight polymers. The DP describes the extent of reaction; and, of course, the concentration influences the DP of the polymer because these systems are pseudoliving.
Since dynamers are condensation polymers, they are expected to obey the kinetics of polymer condensation and to be subject to conventional condensation limitations (11, 46), with high DPs only attainable with high conversions and strict control of the stoichiometry, as can be achieved when the monomer expelled from the polymer backbone is removed from the reaction medium.
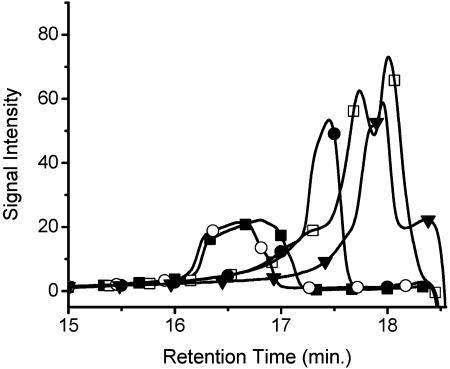
This exchange mechanism is illustrated by the incorporation of 2,5-thiophene dicarboxaldehyde into P3A to afford cross-dynamers of P3A and P3D evolving toward P3D. Table 1 and Fig. 4 show the GPC analysis for the exchange process. Monomer insertion into the polyacylhydrazone leads to polymer dissociation and decrease in molecular weight (Table 1). Owing to the high molecular weight of the starting polymer, a longer incorporation time was required to exchange the glutaraldehyde for the thiophene unit. Moreover, since the glutaraldehyde is still present in the reaction medium, the correct stoichiometry required for repolymerization is not adhered. Consequently, only low-molecular-weight oligomers are formed.
Table 1. GPC results for the exchange reaction of polyacylhydrazone P3A with 2,5-thiophene dicarboxaldehyde at 120°C in DMF (see Materials and Methods).
| Heating time, sec | Mw,* g/mol | Mn,* g/mol | PDI,* | DP |
|---|---|---|---|---|
| 0 | 72,962 | 67,218 | 1.09 | 129 |
| 30 | 67,482 | 60,508 | 1.10 | 119 |
| 60 | 43,283 | 38,195 | 1.13 | 76 |
| 120 | 32,865 | 26,620 | 1.22 | 58 |
| 240 | 26,851 | 22,904 | 1.17 | 47 |
| Overnight† | 6,568 | 6,490 | 1.01 | 12 |
Relative to polystyrene standards. Mw, average-weight molecular weight; Mn, average-number molecular weight; PDI, polydispersity index
90°C
Fig. 4.
GPC traces of polyacylhydrazone P3A with 2,5-thiophene dicarboxaldehyde (○), heating at 120°C with a catalytic amount of TFA for 30 sec (▪), 60 sec (•), 120 sec (□), and 240 sec (▾), leading to the new exchanged polymer P3B.
The polyacylhydrazone P1A consisting of only alkyl units readily exchanges either its dihydrazide or dialdehyde component on heating. Acid catalysis is needed when the entity to be incorporated into the polymer comprises an aryl unit. This reflects the lesser reactivity of the hydrazone group involving an aryl unit, requiring harsher exchange conditions.
The catalysts used to promote monomer exchange were typically, but not exclusively, TFA or acetic acid. Heating is required for exchange to occur when using catalytic amounts of acid. The heating temperature varies depending on the solvent selected and on the thermal stability of the dynamers, ranging from 50 to 150°C with an optimum value for practical purposes in the region of 70–95°C. The reaction time for exchange to occur varied according to the stability of the original and exchanged polymers, the amounts of initial polymer, difunctional monomer, and acid catalyst added, the solvent, and the reaction temperature.
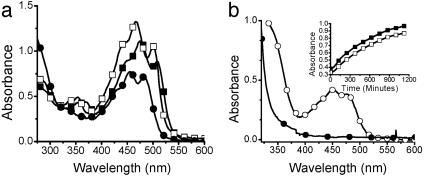
Kinetics of Component Exchange in Polyacylhydrazones. Since NMR spectroscopy is less suitable for long-term kinetic studies at elevated temperatures, UV-visible spectroscopy was used for kinetic studies. Because an additional chromophore was required to follow the reaction kinetics, the incorporation of a highly conjugated monomer into the polymer was examined. Crocetin dialdehyde C was a compound of choice because it exhibits a strong absorption in the visible spectral region and only a weak absorption in the UV-spectral region. Moreover, on condensation with a dihydrazide, a band appears at ≈355 nm resulting from the hydrazone formation, regardless of the choice of hydrazide (Fig. 5a). Observing the changes of this band, the exchange kinetic process was monitored at 345 and 355 nm. In the reaction conditions used, a pseudo first-order rate constant of 2.1 × 10-5 M-1·sec-1 was measured at 75°C. As expected, the rates of exchange were accelerated on increasing the concentration of either the dihydrazide or dialdehyde monomer. The integration of the crocetin dialdehyde C into the polyacylhydrazone P2A is seen from the spectra shown in Fig. 5b.
Fig. 5.
(a) UV-visible absorption spectra of P1C (▪), P2C (□), and C (•) in DMSO. (b) UV-visible spectra of P2A before (•) and after (○) the addition of C and of the dialdehyde exchange reaction at 75°C in DMSO for 12 h giving P2C.(Inset) Rate of dialdehyde exchange measured at 345 (▪) and 355 (□)nm at 75°C.
Extension Outlook. The dynamic features resulting from the exchange reaction readily allow for the introduction of one or several different new difunctional aldehyde or hydrazide monomers into the polymer backbone at the expense of displacing monomer units initially present. The end result is the generation of a highly diverse dynamic combinatorial library (2, 4) of alternating copolymers, containing variable amounts of the new and the displaced monomers, whose properties differ from those of the original one. Adequate selection of the monomeric components, in principle, makes possible the modulation of the properties of the polymer for specific purposes by incorporation/decorporation processes. Physical properties that may change on exchanging monomers include solubility, hydrophobicity, hydrophilicity, molecular weight, absorbance, fluorescence, conductivity, castability, thermal behavior, viscosity, and elasticity. Designed modification of such features is both of basic interest and of significant potential in applied materials science, as indicated by the mechanical properties of several recently obtained polyacylhydrazone polymers (T. Nobori, T. Ono, J.-M.L., unpublished work). Thus, a polycondensation product, similar to P1A, of H2NNHCO—(CH2)6—CONHNH2 with OHC—(CH2)4—CHO represents a dynamic analog of nylon, which one might term dynalon.
It is clear that the dynamic polyamide features presented by polyacylhydrazones may be extended to other classes of polymers, in particular, dynamic polyureas and dynamic polyurethanes may be obtained by reaction of difunctional components containing the corresponding groups, —NH—CO—NHNH2 (or H2NHN—CO—NHNH2) and —O—CO—NHNH2, respectively, with a dicarbonyl component.
One may also stress that the hydrazide/hydrazone functionalities implement amide and amine/imine groups, which are both playing a major role in biological molecules and processes. Extension toward the generation of dynamic biopolymers may therefore be envisaged (D. Hickman, S. Lohman, Y. Ruff, N. Sreenivasachary, and J.-M.L., unpublished work). Furthermore, the reversibility of dynamers makes these materials of interest also for “dynamic formulation,” allowing slow release of active ingredients in pharmaceutical chemistry and medicine (controlled drug delivery), in agrochemistry, and in home and personal care.
Finally, dynamers present environmental and biological degradability, a feature of much significance in waste management.
Conclusion
Alternating polyacylhydrazones copolymers, generated by polycondensation of dialdehydes (or, in principle, any dicarbonyl compound) with dihydrazides, are dynamic covalent polymers able to reversibly exchange their components against different ones, thus allowing the incorporation or decorporation of either a dihydrazide or a dialdehyde into the polymer backbone and leading to a set of copolymers presenting diverse compositions and properties. Such processes give access to easy doping and tuning of dynamer constitution by selective incorporation, decorporation, or reshuffling of different monomeric components yielding ultimately polymers with “dial-in” properties. Furthermore, this dynamic constitutional diversity allows for component selection, thus conferring to the polymeric material the ability to respond to external stimuli and to environmental conditions [i.e., adaptability, a major feature of constitutional dynamic chemistry (3)].
Acknowledgments
We thank Dr. D. Hickman for helpful suggestions. W.G.S. received a postdoctoral fellowship from Natural Sciences and Engineering Research Council (Canada) and additional financial support from the Université Louis Pasteur.
Abbreviations: DP, degree of polymerization; DMF, dimethylformamide; TFA, trifluoroacetic acid; GPC, gel permeation chromatography.
References
- 1.Lehn, J.-M. (1999) in Supramolecular Science: Where It Is and Where It Is Going, eds. Ungaro, R. & Dalcanale, E. (Kluwer, Dordrecht, The Netherlands), pp. 287-304.
- 2.Lehn, J.-M. (1999) Chem. Eur. J. 5, 2455-2463. [Google Scholar]
- 3.Lehn, J.-M. (2002) Proc. Natl. Acad. Sci. USA 99, 4763-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousins, G. R. L., Poulsen, S. A. & Sanders, J. K. M. (2000) Curr. Opin. Chem. Biol. 4, 270-279. [DOI] [PubMed] [Google Scholar]
- 5.Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. (2002) Angew. Chem. Int. Ed. Engl. 41, 898-952. [DOI] [PubMed] [Google Scholar]
- 6.Ciferri, A., ed. (2000) Supramolecular Polymers (Dekker, New York).
- 7.Lehn, J.-M. (2002) Polym. Int. 51, 825-839. [Google Scholar]
- 8.Brunsveld, L., Folmer, B. J. B., Meijer, E. W. & Sijbesma, R. P. (2001) Chem. Rev. 101, 4071-4097. [DOI] [PubMed] [Google Scholar]
- 9.Lehn, J.-M. (1995) Supramolecular Chemistry: Concepts and Perspectives (VCH, Weinheim, Germany), pp. 139-197.
- 10.Philp, D. & Stoddart, J. F. (1996) Angew. Chem. Int. Ed. Engl. 35, 1154-1196. [Google Scholar]
- 11.Allcock, H. R. & Lampe, F. W. (1990) Contemporary Polymer Chemistry (Prentice–Hall, London), 2nd Ed.
- 12.Kricheldorf, H. R. (2003) Macromolecules 36, 2302-2308. [Google Scholar]
- 13.Xie, J. & Hsieh, Y.-L. (2001) J. Polym. Sci. Polym. Chem. Ed. 39, 1931-1939. [Google Scholar]
- 14.Berkane, C., Mezoul, G., Lalot, T., Brigodiot, M. & Maréchal, E. (1997) Macromolecules 30, 7729-7734. [Google Scholar]
- 15.Lavalette, A., Lalot, T., Brigodiot, M. & Maréchal, E. (2002) Biomacromolecules 3, 225-228. [DOI] [PubMed] [Google Scholar]
- 16.Colquhoun, H. M., Lewis, D. F., Ben-Haida, A. & Hodge, P. (2003) Macromolecules 36, 3775-3778. [Google Scholar]
- 17.Chen, X., Dam, M. A., Ono, K., Mal, A., Shen, H., Nutt, S. R., Sheran, K. & Wudl, F. (2002) Science 295, 1698-1702. [DOI] [PubMed] [Google Scholar]
- 18.Chen, X. & Ruckenstein, E. (2000) J. Polym. Sci. Polym. Chem. Ed. 38, 1662-1672. [Google Scholar]
- 19.Chen, Y. & Chen, K.-H. (1997) J. Polym. Sci. Polym. Chem. Ed. 35, 613-624. [Google Scholar]
- 20.Otsuka, H., Aotani, K., Higaki, Y. & Takahara, A. (2003) J. Am. Chem. Soc. 125, 4064-4065. [DOI] [PubMed] [Google Scholar]
- 21.Nakazawa, I., Suda, S., Masuda, M., Asai, M. & Shimizu, T. (2000) Chem. Commun. 881-882.
- 22.Ramström, O. & Lehn, J.-M. (2002) Nat. Rev. Drug. Discov. 1, 26-36. [DOI] [PubMed] [Google Scholar]
- 23.Bunyapaiboonsri, T., Ramström, O., Lohmann, S., Lehn, J.-M., Peng, L. & Goeldner, M. (2001) ChemBioChem 2, 438-444. [DOI] [PubMed] [Google Scholar]
- 24.Korshak, V. V. & Pozhil'tsova, E. A. (1950) Izv. Akad. Nauk. SSSR Otd. Khim. Nauk, 412-417.
- 25.Imai, Y., Ueda, M., Aizawa, T. & Ohnuma, M. (1976) Kobunshi Ronbunshu 33, 633-641. [Google Scholar]
- 26.Gol'din, G. S. & Tsiomo, S. N. (1975) Vysokomol. Soedin. Ser. B 17, 463-465. [Google Scholar]
- 27.Simon, H., Moldenhauer, W. & Kraus, A. (1969) Chem. Ber. 102, 2777-2786. [Google Scholar]
- 28.Hayden, L. M., Kim, W. K., Chafin, A. P. & Lindsay, G. A. (2001) Macromolecules 34, 1493-1495. [Google Scholar]
- 29.Schmitt, J.-L. & Lehn, J.-M. (2003) Helv. Chim. Acta 86, 3417-3426. [Google Scholar]
- 30.Euler, H. V. & Hagglund, B. (1945) Ark. Kemi Mineral. Geol. A19, 1-10. [Google Scholar]
- 31.Oikawa, E., Tamura, S., Arai, Y. & Aoki, T. (1995) J. Appl. Polym. Sci. 58, 1205-1219. [Google Scholar]
- 32.Kim, K. S., Lee, Y. W. & Lee, H. D. (1985) J. Korean Chem. Soc. 29, 543-551. [Google Scholar]
- 33.Hirose, S., Hatakeyama, H. & Hatakeyama, T. (1983) Sen'i Gakkaishi 39, T496-T500. [Google Scholar]
- 34.Roberts, G. A. & Thomas, I. M. (1981) Makromol. Chem. 182, 2611-2617. [Google Scholar]
- 35.Korshak, V. V., Krongauz, E. S., Berlin, A. M., Gribkova, P. N. & Sheina, V. E. (1964) Izv. Akad. Nauk SSSR Ser. Khim. 7, 1281-1288. [Google Scholar]
- 36.Michel, R. H. & Murphey, W. A. (1963) J. Appl. Polym. Sci. 7, 617-624. [Google Scholar]
- 37.Hirose, S. & Hatakeyama, H. (1982) Jpn. Patent 57088156A; Chem. Abstr. 97, 163707. [Google Scholar]
- 38.Emmons, W. D. (1980) U.S. Patent 4,210,565; (1980) Chem. Abstr . 60, 75763. [Google Scholar]
- 39.De Witt, H. D. (1964) U.S. Patent 3,124,559.
- 40.Michel, R. H. (1967) U.S. Patent 3,354,122.
- 41.Oh, K., Jeong, K.-S. & Moore, J. S. (2003) J. Org. Chem. 68, 8397-8403. [DOI] [PubMed] [Google Scholar]
- 42.Furlan, R. L. E., Cousins, G. R. L. & Sanders, J. K. M. (2000) Chem. Commun., 1761-1762.
- 43.Nguyen, R. & Huc, I. (2003) Chem. Commun., 942-943. [DOI] [PubMed]
- 44.Lohmann, S. (2003) Ph. D. thesis (Université Louis Pasteur, Strasbourg, France).
- 45.Lehn, J.-M. & Skene, W. G. (2002) U.S. Patent Appl. 60,392,308; (2004) Patent Cooperation Treaty Int. Appl. WO2004003044.
- 46.Berl, V., Schmutz, M., Krische, M. J., Khoury, R. G. & Lehn, J.-M. (2002) Chem. Eur. J. 8, 1227-1244. [DOI] [PubMed] [Google Scholar]