Abstract
Background
Cross-sectional and retrospective studies have associated major depressive disorder with glial activation and injury as well as blood–brain barrier disruption, but these associations have not been assessed prospectively. Here, we aimed to determine the relationship between changes in depressive symptom severity and in blood levels of S-100 calcium-binding protein B (S-100B), high-sensitivity C-reactive protein, and interleukin-6 following an inflammatory challenge.
Methods
Fifty unselected participants were recruited from a randomized, controlled trial comparing coronary artery bypass grafting procedures performed with versus without cardiopulmonary bypass for the risk of neurocognitive decline. Depressive symptom severity was measured at baseline, discharge, and six-month follow-up using the Beck Depression Inventory II (BDI-II). The primary outcome of the present biomarker study was acute change in depressive symptom severity, defined as the intra-subject difference between baseline and discharge BDI-II scores. Blood biomarker levels were determined at baseline and 2 days postoperative.
Results
Changes in S-100B levels correlated positively with acute changes in depressive symptom severity (Spearman ρ, 0.62; P = 0.0004) and accounted for about one-fourth of their observed variance (R2, 0.23; P = 0.0105). This association remained statistically significant after adjusting for baseline S-100B levels, age, weight, body-mass index, or β-blocker use, but not baseline BDI-II scores (P = 0.064). There was no statistically significant association between the primary outcome and baseline S-100B levels, baseline high-sensitivity C-reactive protein or interleukin-6 levels, or changes in high-sensitivity C-reactive protein or interleukin-6 levels. Among most participants, levels of all three biomarkers were normal at baseline and markedly elevated at 2 days postoperative.
Conclusions
Acute changes in depressive symptom severity were specifically associated with incremental changes in S-100B blood levels, largely independent of covariates associated with either. These findings support the hypothesis that glial activation and injury and blood–brain barrier disruption can be mechanistically linked to acute exacerbation of depressive symptoms in some individuals.
Introduction
A large body of evidence substantiates the association between inflammation and major depressive disorder [1]–[13]. Cross-sectional and retrospective studies of depression have documented microglial activation and proliferation [3], [4], [14] as well as astroglial loss in biopsied and post-mortem brain tissue [15]–[18], and increased Th1 proinflammatory cytokines in cerebrospinal fluid (CSF) and blood samples [6]–[12] (recently reviewed in [1]). In addition, population-level studies identify autoimmune diseases and severe infections as risk factors for depression [19], [20]. Prospective etiologic [5], [21]–[31] and treatment studies [3], [32]–[35] have provided some of the most direct evidence supporting the hypothesis that inflammation can be causally related to depressive symptoms. The prototypical etiologic studies in this regard are the so-called inflammation-challenge paradigms, such as interferon-α administration [36]. Among those treated with interferon-α, typically for hepatitis C or cancer, about half experience acute-onset depression in association with systemic inflammatory and neuroimaging abnormalities [5], [21]–[31]. These inflammation-challenge paradigms have also been extended to include orthopedic surgery [37], and more recently–cardiac surgery [38], [39], in which the occurrence of a robust systemic inflammatory response is well-established [40].
A related and emerging area of research in psychoimmunology has focused on elucidating the connections among inflammatory abnormalities as well as glial activation and injury and blood–brain barrier disruption in depression [2], [3]. Like inflammation, evidence of glial activation and injury in depression has been extensively documented [1]. Despite this, however, and notwithstanding the necessary functions of astroglial–endothelial interactions in maintaining blood–brain barrier integrity [35], only recently has evidence linking blood–brain barrier disruption to depression begun to accumulate [2], [3]. Evidence to this effect includes histological and ultrastructural findings in biopsied and post-mortem brain tissue [3], [41]; elevated CSF-to-serum albumin ratios [42]–[44]; reduced flow-mediated dilation (a surrogate of endothelial dysfunction) [45]; increased vascular disease comorbidity [46], [47]; and elevated blood levels of endothelial dysfunction biomarkers (e.g., intercellular adhesion molecule 1, vascular adhesion molecule 1, E-selectin, and P-selectin) [48]–[61].
S-100 calcium-binding protein B (S-100B) is a 10 kDa protein, consisting of 92 amino acids and two EF-hand domains, that is implicated in the pathophysiology of major depressive disorder [55], [59], [62]–[71] and various neurological disorders [72]–[74]. S-100B is produced and secreted by both intracerebral (i.e., mainly astrocytes, and to a lesser degree, oligodendrocytes) and extracerebral sources (e.g., adipocytes and myocytes) [72], and is involved in numerous regulatory and immune pathways [75], [76]. Elevated blood S-100B levels are considered a marker of glial activation and injury [72], [74], [77]–[87] as well as blood–brain barrier disruption [72], [81], [88]–[93], and have been associated with suicidality [56], lower Glasgow Coma Scale scores [73], greater post-concussive symptom severity [74], ictal events [72], and greater risk of postoperative cognitive decline [94]–[97] (in addition to numerous other clinically significant outcomes). To date, however, no studies have simultaneously assessed the relationships between depression and glial activation and injury, blood–brain barrier disruption, and inflammation using a prospective design. Here, we aimed to address this gap in the literature by evaluating perioperative changes in blood levels of S-100B, high-sensitivity C-reactive protein (hs-CRP), and interleukin-6 for changes in depressive symptom severity in the inflammation-challenge paradigm of cardiac surgery.
Materials and Methods
Design, setting, and participants
This is a prospective cohort study nested within a randomized, controlled trial. An unselected group of 50 participants was recruited from among 201 patients undergoing coronary artery bypass grafting in the context of a randomized, controlled trial comparing conventional vs off-pump procedures for the risk of neurocognitive decline (Scarecrow trial, 2001–2004) [98]. Inclusion criteria were ages 40 to 80 years, clinical indication for urgent or elective coronary artery bypass grafting, referral to the Cardiothoracic Surgery service at Eastern Maine Medical Center (Bangor, Maine, United States), and capacity to provide written informed consent. Exclusion criteria were concomitant surgical procedures, severe calcification of the ascending aorta or deep intra-myocardial left anterior descending coronary artery, recent administration of an inotropic agent in excess of 3 µg/kg/min, requiring a cardiac-assist device for hemodynamic instability, and not providing written informed consent. Details of the surgical and anesthesia protocols used in the trial have been described previously [98]. All participants provided written informed consent. The Institutional Review Board of Eastern Maine Medical Center approved this study.
Biochemical measurements
Blood samples were collected by standard venipuncture at baseline and approximately 2 days postoperative. Upon collection, samples were stored at −80°C and then transferred on dry ice to the Laboratory for Clinical and Biochemical Research (Colchester, Vermont, United States) where S-100B, hs-CRP, and interleukin-6 blood levels were determined in either plasma or serum. Enzyme-linked immunosorbent assays were used to determine S-100B (#364701D, DiaSorin, Bromma, Sweden) and interleukin-6 blood levels (#HS600B, R&D Systems, Minnesota, United States). An automated BNII nephelometer was used to perform an immunonephelometric assay to determine hs-CRP blood levels (Siemens Healthcare Diagnostics, Illinois, United States). All laboratory personnel were blinded to participant characteristics and clinical information.
Lower limits of detection were 0.02 µg/l for S-100B, 0.16 mg/l for hs-CRP, and 0.16 pg/ml for interleukin-6. Inter-assay coefficients of variance at baseline and 2 days postoperative were 6.01% and 3.31% for S-100B, 2.93% and 1.82% for hs-CRP, and 7.95% and 8.71% for interleukin-6. In determining S-100B and interleukin-6 blood levels, each sample was run twice, the arithmetic mean of which was taken as the final value. In determining hs-CRP blood levels, each sample was run once, as the assays were automated. The approximate upper limits of normal blood levels for each biomarker are 0.15 µg/l for S-100B, 10.0 mg/l for hs-CRP, and 5.0 pg/ml for interleukin-6. hs-CRP levels less than or equal to 1.0 mg/l are indicative of low risk for ischemic heart disease, 1.0 to 3.0 mg/l moderate risk, and 3.0 to 10.0 mg/l high risk. hs-CRP levels greater than or equal to 10.0 mg/l are indicative of acute systemic inflammation of a non-cardiac origin (e.g., as occurs with infection or cardiac surgery). The change (i.e., intra-subject difference) between blood levels of each biomarker at baseline and 2 days postoperative was computed for each participant. Instances where 2-day postoperative blood levels were greater than baseline levels (for a given biomarker and participant), were considered a positive change (i.e., perioperative increases). Likewise, when 2-day postoperative blood levels were less than baseline levels, this was considered a negative change (i.e., perioperative decreases).
Main outcomes
Depressive symptom severity was assessed at baseline, discharge, and six-month month follow-up using the Beck Depression Inventory II (BDI-II). Scores ranging from 0 to 13 indicate subclinical or minimal depressive symptom severity, 14 to 19 mild, 20 to 28 moderate, and 29 to 63 severe [99]. Participants, family members, treating clinicians, BDI-II test examiners, and all other trial staff in contact with participants were blinded to the results of blood biomarker levels. The primary outcome of the present biomarker study, acute change in depressive symptom severity, was defined as the intra-subject difference between baseline and discharge BDI-II scores. The secondary outcome, delayed change in depressive symptom severity, was defined as the intra-subject difference between baseline and six-month follow-up BDI-II scores.
Statistical analysis
Analyses were done using Stata version 12.1 for Mac (StataCorp, LP. College Station, Texas, United States). Participant characteristics were summarized as the arithmetic mean ± standard deviation for continuous data and frequency and percentage for categorical data. These descriptive statistics were then compared between the biomarker and non-biomarker cohorts that comprised the Scarecrow trial with the use of two-tailed Student t tests for continuous data and Cochran–Mantel–Haenszel χ2 tests for categorical data. To identify covariates of blood biomarker measures at baseline and their differences between baseline and postoperative assessments and/or the main outcomes, Spearman rank correlation coefficients (Spearman ρ) were calculated for all possible two-term combinations, applying the pairwise deletion method for comparisons involving missing data. Participant characteristics that yielded statistically significant correlations were entered into the relevant multiple linear regression models (see below).
Spearman ρ values were calculated and scatterplots generated for all possible two-term combinations between and among blood biomarker measures at baseline and postoperative assessments, and the difference between them and BDI-II scores at baseline, discharge, six-month follow-up, the difference between baseline and discharge BDI-II scores (primary outcome), and the difference between baseline and six-month follow-up BDI-II scores (secondary outcome). For statistically significant correlations between blood biomarker measures and the main outcomes, we did sensitivity analysis by recalculating Spearman ρ values using Bonferroni-adjusted significance levels and again using Sidak-adjusted significance levels. Last, the main outcomes were regressed on blood biomarker measures at baseline and the difference between blood biomarker levels at baseline and postoperative assessments using ordinary-least squares simple and multiple linear regressions. Before entering terms into the models, variable transformations were first applied to all variables that did not have a normal distribution. The transformation used for a given variable was based on the lowest χ2 value yielded by a ladder of powers analysis [100].
Results
Biomarker cohort
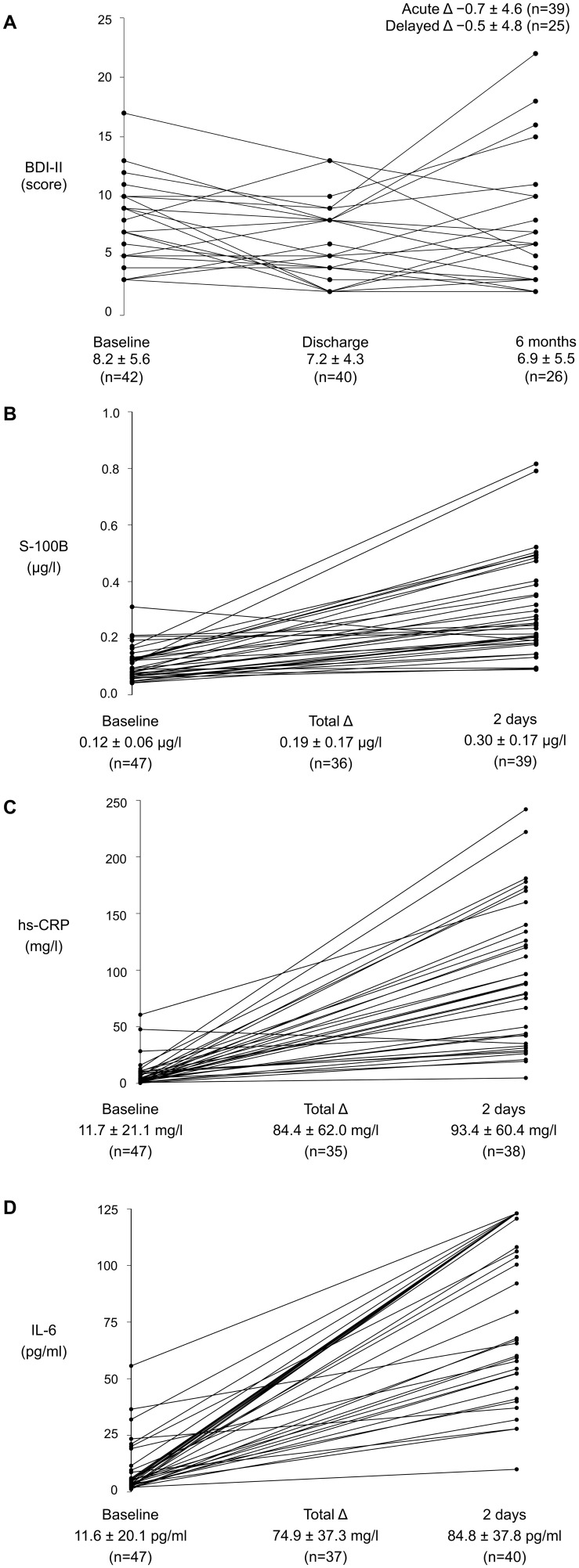
The pairwise distributions of BDI-II scores and blood biomarker levels are displayed in Figure 1. Participant characteristics and BDI-II scores were generally similar among those comprising the biomarker cohort relative to the remaining participants in the Scarecrow trial (Table 1). One exception was discharge BDI-II scores, which were lower on average among those in the biomarker cohort (Table 1).
Figure 1. Paired distributions of Beck Depression Inventory II scores and blood biomarker measures.
(A) Beck Depression Inventory II scores at baseline, discharge, six-month follow-up; (B) S-100 calcium-binding protein B levels at baseline, 2 days postoperative, and the total change between them; (C) high-sensitivity C-reactive protein levels at baseline 2 days postoperative, and the total change between them; (D) inteleukin-6 levels at baseline 2 days postoperative, and the total change between them. Values given below the x-axis are arithmetic mean ± standard deviation. BDI-II = Beck Depression Inventory II. hs-CRP = high-sensitivity C-reactive protein. IL-6 = interleukin-6. S-100B = S-100 calcium-binding protein B.
Table 1. Prospective biomarker cohort, nested within the Scarecrow trial (2001–2004).
| Biomarker cohort | Non-biomarker cohort | P Value* | |
| (n = 50) | (n = 151) | ||
| Age (years) | 63.0±8.3 | 64.2±9.5 | 0.43 |
| Female | 6 (12.0) | 34 (22.5) | 0.11 |
| Weight (kg) | 87.7±12.9 | 88.3±14.9 | 0.83 |
| Height (cm) | 172.5±9.3 | 172.8±8.8 | 0.87 |
| Body-mass index (kg/m2) | 29.5±3.8 | 29.5±4.2 | 0.95 |
| History of vascular disease | 8 (16.0) | 34 (22.5) | 0.33 |
| History of diabetes mellitus | 17 (34.0) | 49 (32.5) | 0.84 |
| History of COPD | 3 (6.0) | 13 (8.6) | 0.56 |
| History of smoking | 10 (20.0) | 28 (18.5) | 0.82 |
| Charlson comorbidity index | 0.62±0.70 | 0.77±0.92 | 0.28 |
| Myocardial infarction† | 8 (16.0) | 17 (11.3) | 0.38 |
| Unstable angina at admission | 35 (70.0) | 88 (58.3) | 0.14 |
| Two-vessel disease | 18 (36.0) | 43 (28.5) | 0.32 |
| Three-vessel disease | 27 (54.0) | 85 (56.3) | 0.78 |
| Left-ventricular ejection fraction (%)‡ | 61.4±10.8 | 58.9±13.3 | 0.25 |
| Troponin T at 24 hours (ng/dl) | 0.62±0.61 | 0.58±1.01 | 0.77 |
| NYHA class (I-IV)§ | 1.70±0.83 | 1.75±0.85 | 0.75 |
| Serum creatinine (mg/dl) | 1.05±0.24 | 1.15±0.73 | 0.33 |
| β-blocker | 39 (78.0) | 112 (74.2) | 0.59 |
| ACE-inhibitor | 11 (22.0) | 49 (32.5) | 0.16 |
| Ca2+ channel blocker | 11 (22.0) | 24 (15.9) | 0.32 |
| Acetylsalicylic acid† | 39 (78.0) | 106 (70.2) | 0.29 |
| Randomized to off-pump procedure | 23 (46.0) | 76 (50.3) | 0.60 |
| Underwent off-pump procedure | 21 (42.0) | 70 (46.4) | 0.59 |
| Urgent priority procedure | 30 (60.0) | 95 (62.9) | 0.71 |
| Time from baseline to procedure (days) | 3.1±3.6 | 2.6±3.3 | 0.34 |
| Time from procedure to discharge (days) | 6.0±2.6 | 7.3±4.9 | 0.08 |
| BDI-II (score), baseline (n = 42; n = 145) | 8.2±5.6 | 9.6±6.9 | 0.23 |
| BDI-II (score), discharge (n = 40; n = 121) | 7.2±4.3 | 9.8±6.7 | 0.026 |
| BDI-II (score), 6 months (n = 26; n = 105) | 6.9±5.5 | 7.8±7.4 | 0.59 |
| BDI-II (score), acute Δ (n = 39; n = 120)|| | −0.7±4.6 | 0.0±5.4 | 0.43 |
| BDI-II (score), delayed Δ (n = 25; n = 104)¶ | −0.5±4.8 | −1.8±5.7 | 0.29 |
Values are arithmetic mean ± standard deviation for continuous data, and frequency (percentage) for categorical data. The Scarecrow trial compared CABG performed with vs. without cardiopulmonary bypass for the risk of postoperative neurocognitive decline (defined as a ≥20% reduction on ≥20% of 19 psychometrics) among 201 patients at Eastern Maine Medical Center. Abbreviations: ACE = angiotensin-converting enzyme. CABG = coronary artery bypass grafting. COPD = chronic obstructive pulmonary disease. hs-CRP = high-sensitivity C-reactive protein. NYHA = New York Heart Association. S-100B = S-100 calcium-binding protein B.
*Two-tailed Student t test for continuous data; χ2 test for categorical data.
Within 7 days before the index procedure.
Missing data for: n = 3; n = 19.
Missing data for: n = 3; n = 3.
Acute Δ refers to the primary outcome, acute change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and discharge.
Delayed Δ refers to the secondary outcome, delayed change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and six-month follow-up.
Covariate assessment
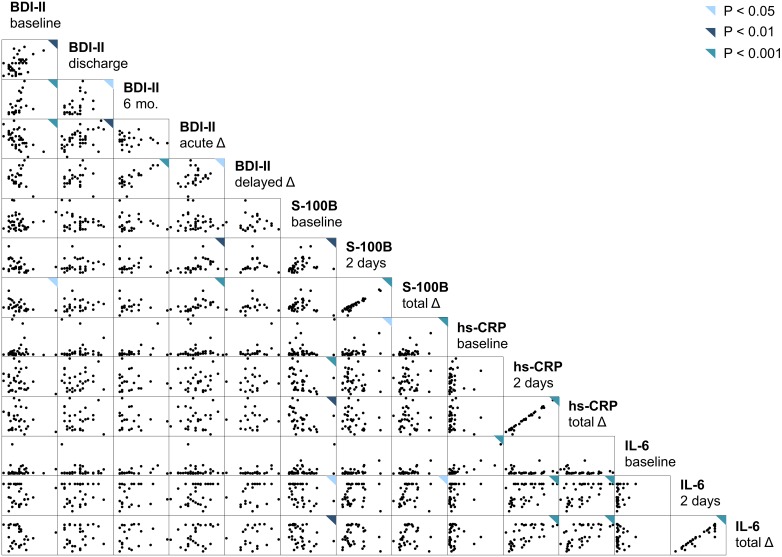
Spearman ρ values for comparisons between participant characteristics and the main outcomes and blood biomarker measures are displayed in Table 2. None of the participant characteristics showed a statistically significant correlation with either outcome (Table 2). Intra-subject differences between baseline and postoperative S-100B levels correlated positively with age and negatively with weight and body-mass index, and were significantly lower on average among those taking β-blockers (Table 2). Perioperative increases in hs-CRP levels correlated negatively with age. Perioperative increases in hs-CRP and interleukin-6 levels were significantly greater on average among participants randomized to (intention-to-treat) and having undergone (as-treated) off-pump procedures, as compared with those randomized to and having undergone conventional procedures (Table 2). Spearman ρ values and scatterplots for all possible two-term combinations between and among BDI-II scores and blood biomarker measures are displayed in Table 3 and Figure 2.
Table 2. Spearman rank correlation coefficients for comparisons between participant characteristics and main outcomes as well as blood biomarker levels.
| BDI-II (score) | S-100B (µg/l) | hs-CRP (mg/l) | IL-6 (pg/ml) | |||||
| Acute Δ | Delayed Δ | Baseline | Total Δ | Baseline | Total Δ | Baseline | Total Δ | |
| (n = 39)† | (n = 25)‡ | (n = 47) | (n = 36)§ | (n = 47) | (n = 35)§ | (n = 47) | (n = 37)§ | |
| Age (years) | 0.30 | 0.04 | 0.23 | 0.39* | 0.15 | –0.35* | 0.23 | –0.22 |
| Female | 0.07 | 0.05 | 0.14 | 0.16 | 0.08 | 0.13 | 0.08 | 0.12 |
| Weight (kg) | –0.14 | –0.05 | –0.21 | –0.38* | –0.04 | 0.07 | 0.00 | 0.16 |
| Height (cm) | –0.01 | 0.15 | –0.12 | –0.06 | –0.07 | 0.10 | –0.01 | 0.10 |
| Body-mass index (kg/m2) | –0.22 | –0.21 | –0.15 | –0.34* | 0.05 | –0.01 | 0.06 | 0.06 |
| History of vascular disease | –0.04 | –0.11 | 0.07 | 0.10 | –0.08 | 0.12 | 0.00 | 0.08 |
| History of diabetes mellitus | 0.03 | –0.19 | –0.16 | –0.07 | –0.33* | –0.05 | –0.05 | –0.10 |
| History of COPD | 0.04 | 0.16 | 0.11 | 0.20 | –0.21 | 0.04 | –0.08 | –0.12 |
| History of smoking | –0.06 | 0.00 | –0.11 | 0.03 | 0.08 | –0.09 | 0.10 | 0.12 |
| Charlson comorbidity index | –0.03 | –0.14 | –0.06 | 0.16 | –0.37* | 0.05 | –0.05 | 0.01 |
| Myocardial infarction|| | –0.15 | 0.39 | –0.04 | –0.10 | 0.34* | 0.14 | 0.42** | 0.24 |
| Unstable angina at admission | 0.11 | –0.09 | –0.08 | 0.15 | 0.36* | 0.14 | 0.40** | 0.23 |
| Two-vessel disease | 0.07 | –0.20 | –0.01 | –0.26 | 0.07 | 0.02 | 0.15 | 0.13 |
| Three-vessel disease | –0.18 | 0.22 | –0.10 | 0.26 | 0.02 | 0.06 | –0.14 | 0.03 |
| Left-ventricular ejection fraction (%)†† | –0.08 | –0.06 | 0.17 | –0.23 | –0.09 | –0.19 | –0.24 | –0.28 |
| Troponin T at 24 hours (ng/dl) | –0.10 | 0.02 | 0.30* | 0.21 | 0.30* | –0.21 | 0.16 | –0.14 |
| NYHA class (I-IV)‡‡ | 0.04 | –0.19 | 0.21 | 0.29 | –0.14 | –0.25 | 0.04 | –0.24 |
| Serum creatinine (mg/dl)§§ | 0.05 | –0.05 | 0.09 | –0.02 | 0.06 | –0.08 | 0.15 | –0.09 |
| β-blocker | –0.05 | –0.13 | 0.07 | –0.39* | –0.30* | –0.17 | –0.20 | –0.15 |
| ACE-inhibitor | –0.21 | 0.04 | –0.21 | –0.01 | –0.22 | 0.19 | –0.06 | 0.19 |
| Ca2+ channel blocker | 0.10 | 0.23 | –0.13 | 0.15 | 0.20 | 0.24 | –0.21 | 0.45** |
| Acetylsalicylic acid† | 0.29 | 0.01 | 0.12 | 0.12 | –0.05 | –0.30 | –0.26 | –0.21 |
| Randomized to off-pump procedure | 0.21 | –0.16 | –0.39** | –0.01 | –0.01 | 0.50** | –0.05 | 0.54*** |
| Underwent off-pump procedure | 0.26 | –0.06 | –0.41** | 0.02 | –0.07 | 0.50** | –0.09 | 0.53*** |
| Urgent priority procedure | 0.01 | 0.24 | 0.19 | 0.29 | 0.23 | 0.00 | 0.59*** | 0.10 |
| Time from baseline to procedure (days) | 0.02 | 0.24 | 0.18 | 0.34* | 0.19 | 0.00 | 0.41** | 0.16 |
| Time from procedure to discharge (days) | 0.20 | –0.15 | –0.06 | 0.09 | –0.02 | 0.14 | 0.31* | 0.05 |
Pairwise case deletion was used for missing observations. Abbreviations: ACE = angiotensin-converting enzyme. BDI-II = Beck Depression Inventory II. CABG = coronary artery bypass grafting. COPD = chronic obstructive pulmonary disease. hs-CRP = high-sensitivity C-reactive protein. IL-6 = interleukin-6. NYHA = New York Heart Association. S-100B = S-100 calcium-binding protein B.
*P<0.05; **P<0.01; ***P<0.001.
Acute Δ refers to the primary outcome, acute change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and discharge.
Delayed Δ refers to the secondary outcome, delayed change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and six-month follow-up.
Total Δ refers to the intra-subject difference in blood biomarker levels between baseline and 2 days postoperative.
Within 7 days before the index procedure.
Missing data (from left to right): n = 3; n = 1; n = 3; n = 2; n = 2; n = 3; n = 2; n = 3; n = 2.
Missing data (from left to right): n = 0; n = 1; n = 3; n = 2; n = 2; n = 3; n = 2; n = 3; n = 3.
Missing data (from left to right): n = 0; n = 1; n = 0; n = 0; n = 0; n = 0; n = 0; n = 0; n = 0.
Table 3. Spearman rank correlation coefficients for comparisons between and among Beck Depression Inventory II scores and blood biomarker measures.
| BDI-II (score) | S-100B (µg/l) | hs-CRP (mg/l) | IL-6 (pg/ml) | ||||||||||||
| Baseline | Discharge | 6 months | Acute Δ | Delayed Δ | Baseline | 2 days | Total Δ | Baseline | 2 days | Total Δ | Baseline | 2 days | Total Δ | ||
| BDI-II (score) | Baseline | . | 0.46** | 0.66*** | −0.52*** | −0.03 | 0.02 | −0.31 | −0.43* | −0.01 | −0.01 | −0.21 | 0.24 | −0.26 | −0.22 |
| . | 39 | 25 | 39 | 25 | 39 | 33 | 30 | 39 | 39 | 29 | 39 | 34 | 31 | ||
| Discharge | 0.46** | . | 0.48* | 0.46** | 0.34 | −0.01 | 0.11 | 0.18 | 0.10 | 0.04 | −0.10 | 0.13 | 0.06 | 0.07 | |
| 39 | . | 26 | 39 | 25 | 37 | 31 | 28 | 37 | 30 | 27 | 37 | 32 | 29 | ||
| 6 months | 0.66*** | 0.48* | . | −0.14 | 0.67*** | −0.13 | −0.06 | 0.03 | 0.28 | −0.01 | −0.12 | 0.26 | 0.14 | 0.13 | |
| 25 | 26 | . | 25 | 25 | 25 | 22 | 21 | 25 | 21 | 20 | 25 | 23 | 22 | ||
| Acute Δ | −0.52*** | 0.46** | −0.14 | . | 0.40* | 0.02 | 0.55** | 0.62*** | 0.21 | 0.05 | −0.00 | 0.06 | 0.34 | 0.29 | |
| 39 | 39 | 25 | . | 25 | 36 | 31 | 28 | 36 | 30 | 27 | 36 | 32 | 29 | ||
| Delayed Δ | −0.03 | 0.34 | 0.67*** | 0.40* | . | 0.10 | 0.47* | 0.42 | 0.32 | −0.03 | −0.07 | 0.10 | 0.29 | 0.26 | |
| 25 | 25 | 25 | 25 | . | 24 | 22 | 21 | 24 | 21 | 20 | 24 | 23 | 22 | ||
| S-100B (µg/l) | Baseline | 0.02 | −0.01 | −0.13 | 0.02 | 0.10 | . | 0.51** | 0.10 | −0.09 | −0.56*** | −0.53** | 0.02 | 0.39* | −0.42** |
| 39 | 37 | 25 | 36 | 24 | . | 36 | 36 | 47 | 35 | 35 | 47 | 37 | 37 | ||
| 2 days | −0.31 | 0.11 | −0.06 | 0.55** | 0.47* | 0.51** | . | 0.89*** | 0.41* | −0.22 | −0.25 | 0.25 | 0.21 | 0.07 | |
| 33 | 31 | 22 | 31 | 22 | 36 | . | 36 | 36 | 38 | 35 | 36 | 39 | 36 | ||
| Total Δ | −0.43* | 0.18 | 0.03 | 0.62*** | 0.42 | 0.10 | 0.89*** | . | 0.54*** | 0.02 | −0.05 | 0.28 | 0.37* | 0.24 | |
| 30 | 28 | 21 | 28 | 21 | 36 | 36 | . | 36 | 35 | 35 | 36 | 36 | 36 | ||
| hs-CRP (mg/l) | Baseline | −0.01 | 0.10 | 0.28 | 0.21 | 0.32 | −0.09 | 0.41* | 0.54*** | . | 0.23 | 0.05 | 0.59*** | 0.29 | 0.10 |
| 39 | 37 | 25 | 36 | 24 | 47 | 36 | 36 | . | 35 | 35 | 47 | 37 | 37 | ||
| 2 days | −0.11 | 0.04 | −0.01 | 0.05 | −0.03 | −0.56*** | −0.22 | 0.02 | 0.23 | . | 0.97*** | −0.02 | 0.52*** | 0.63*** | |
| 32 | 30 | 21 | 30 | 21 | 35 | 38 | 35 | 35 | . | 35 | 35 | 38 | 35 | ||
| Total Δ | −0.21 | −0.10 | −0.12 | −0.00 | −0.07 | −0.53** | −0.25 | −0.05 | 0.05 | 0.97*** | . | −0.15 | 0.54*** | 0.62*** | |
| 29 | 27 | 20 | 27 | 20 | 35 | 35 | 35 | 35 | 35 | . | 35 | 35 | 35 | ||
| IL-6 (pg/ml) | Baseline | 0.24 | 0.13 | 0.26 | 0.06 | 0.10 | 0.02 | 0.25 | 0.28 | 0.59*** | −0.02 | −0.15 | . | 0.21 | −0.08 |
| 39 | 37 | 25 | 36 | 24 | 47 | 36 | 36 | 47 | 35 | 35 | . | 37 | 37 | ||
| 2 days | −0.26 | 0.06 | 0.14 | 0.34 | 0.29 | 0.39* | 0.21 | 0.37* | 0.29 | 0.52*** | 0.54*** | 0.21 | . | 0.93*** | |
| 34 | 32 | 23 | 32 | 23 | 37 | 39 | 36 | 37 | 38 | 35 | 37 | . | 37 | ||
| Total Δ | −0.22 | 0.07 | 0.13 | 0.29 | 0.26 | −0.42** | 0.07 | 0.24 | 0.10 | 0.63*** | 0.62*** | −0.08 | 0.93*** | . | |
| 31 | 29 | 22 | 29 | 22 | 37 | 36 | 36 | 37 | 35 | 35 | 37 | 37 | . | ||
Values in the cells for each comparison are (from top to bottom): the Spearman rank correlation coefficient (Spearman ρ) and number of observations. Pairwise case deletion was used for missing observations. Abbreviations: ACE = angiotensin-converting enzyme. BDI-II = Beck Depression Inventory II. CABG = coronary artery bypass grafting. COPD = chronic obstructive pulmonary disease. hs-CRP = high-sensitivity C-reactive protein. NYHA = New York Heart Association. S-100B = S-100 calcium-binding protein B.
*P<0.05; **P<0.01; ***P<0.001.
Acute Δ refers to the primary outcome, acute change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and discharge.
Delayed Δ refers to the secondary outcome, delayed change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and six-month follow-up.
Total Δ refers to the intra-subject difference in blood biomarker levels between baseline and 2 days postoperative.
Figure 2. Scatterplot matrix of all possible combinations between and among Beck Depression Inventory II scores and blood biomarker measures.
BDI-II scores were assessed at baseline, discharge, and 6 months. Differences between baseline and discharge BDI-II scores (primary outcome) and baseline and 6-month BDI-II scores (secondary outcome) were computed for each participant. Biomarker levels were determined at baseline and 2 days postoperatively, and change scores computed. BDI-II = Beck Depression Inventory II. hs-CRP = high-sensitivity C-reactive protein. IL-6 = interleukin-6. S-100B = S-100 calcium-binding protein B.
Acute change in depressive symptom severity
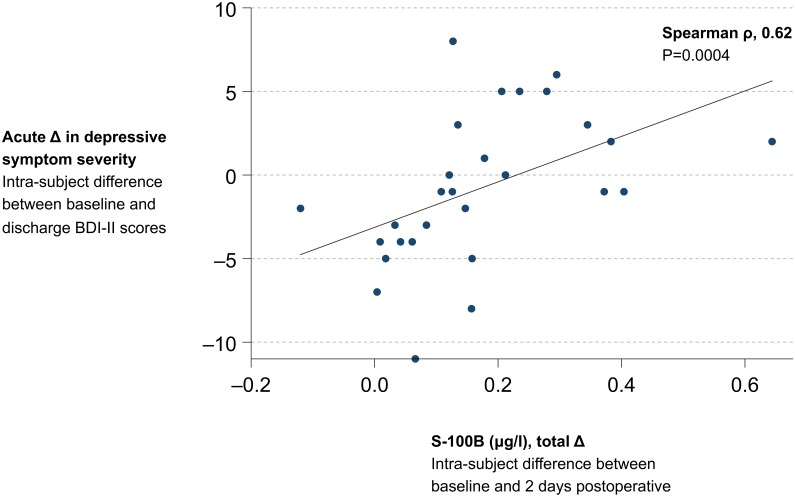
Intra-subject differences between baseline and postoperative S-100B levels exhibited a strong positive correlation with intra-subject differences between baseline and discharge BDI-II scores (Spearman ρ, 0.62; P = 0.0004; Table 3; Figure 3). Neither the strength nor the statistical significance of this correlation was affected by Bonferroni- or Sidak-adjusted significance levels (for both adjustments: Spearman ρ, 0.62; P = 0.0004). There were no other statistically significant correlations between blood biomarker measures and the primary outcome (Table 3). Significant negative correlations were observed between baseline BDI-II scores and changes in S-100B levels and between baseline BDI-II scores and acute changes in depressive symptom severity (Table 3).
Figure 3. Association between perioperative increases in blood levels of S-100 calcium-binding protein B and acute changes in depressive symptom severity.
The x-axis refers to the intra-subject difference between S-100 calcium binding protein B (S-100B) levels measured at baseline and 2 days postoperative. The y-axis refers to the primary outcome, acute change in depressive symptom severity, defined as the intra-subject difference between baseline and discharge Beck Depression inventory II (BDI-II) scores. BDI-II = Beck Depression Inventory II. S-100B = S-100 calcium-binding protein B. Spearman rho = Spearman rank correlation coefficient.
The results of simple and multiple linear regressions of the primary outcome on blood biomarker measures are displayed in Table 4. A simple linear regression model showed that each 0.10-µg/l increase in intra-subject differences between baseline and postoperative S-100B levels was associated with a 1.36-point increase in intra-subject differences between baseline and discharge BDI-II scores (regression coefficient [R] = 13.60, coefficient of determination [R2] = 0.23, t = 2.76, P = 0.010, standardized regression coefficient [β] = 0.48, F (1,26) = 7.61, P = 0.0105; Table 4; Figure 3). This association remained statistically significant after individual adjustments for baseline S-100B levels, age, weight, body-mass index, and β-blocker use, but not baseline BDI-II scores (R = 9.18, R2 = 0.40, t = 1.94, P = 0.064) adjusting for which improved the explanatory power of the model overall (β = 0.32, R2 adjusted = 0.35, F (2,25) = 8.29, P model = 0.0017; Table 4). There were no statistically significant associations between the primary outcome and the remaining blood biomarker measures (Table 3; Table 4).
Table 4. Linear regression of the primary outcome on blood biomarker measures and covariates.
| Independent variables | Acute Δ in depressive symptom severity* | ||||||
| Biomarker measures | Covariates | β | n | R2 | R2 adjusted | RMSE | P Value† |
| S-100B (µg/l), baseline | Unadjusted | −0.02 | 36 | 0.00 | −0.03 | 4.72 | 0.92 |
| BDI-II (scores), baseline | −0.16 | 36 | 0.30 | 0.25 | 4.02 | 0.91 | |
| Underwent off-pump procedure | 0.22 | 36 | 0.15 | 0.09 | 4.43 | 0.07 | |
| Urgent priority procedure | −0.03 | 36 | 0.01 | −0.05 | 4.76 | 0.82 | |
| Troponin T at 24 hours (ng/dl) | 0.07 | 36 | 0.05 | −0.01 | 4.68 | 0.47 | |
| S-100B (µg/l), total Δ | Unadjusted | 0.48 | 28 | 0.23 | 0.20 | 4.03 | 0.010 |
| S-100B (µg/l), baseline | 0.47 | 28 | 0.24 | 0.18 | 4.09 | 0.012 | |
| BDI-II (scores), baseline | 0.32 | 28 | 0.40 | 0.35 | 3.63 | 0.06 | |
| Age (years) | 0.42 | 28 | 0.26 | 0.20 | 4.02 | 0.030 | |
| Weight (kg) | 0.40 | 28 | 0.25 | 0.20 | 4.04 | 0.046 | |
| Body-mass index (kg/m2) | 0.44 | 28 | 0.23 | 0.17 | 4.09 | 0.025 | |
| β-blocker | 0.53 | 28 | 0.24 | 0.18 | 4.08 | 0.011 | |
| hs-CRP (mg/l), baseline | Unadjusted | 0.07 | 36 | 0.01 | −0.02 | 4.71 | 0.67 |
| BDI-II (scores), baseline | 0.08 | 36 | 0.30 | 0.26 | 4.00 | 0.58 | |
| Charlson comorbidity index | 0.13 | 36 | 0.02 | −0.04 | 4.73 | 0.48 | |
| Myocardial infarction† | 0.07 | 36 | 0.01 | −0.05 | 4.77 | 0.69 | |
| Troponin T at 24 hours (ng/dl) | 0.12 | 36 | 0.05 | −0.00 | 4.66 | 0.50 | |
| hs-CRP (mg/l), total Δ | Unadjusted | 0.03 | 27 | 0.00 | −0.04 | 4.67 | 0.87 |
| hs-CRP (mg/l), baseline | 0.05 | 27 | 0.09 | 0.02 | 4.55 | 0.79 | |
| BDI-II (scores), baseline | −0.03 | 27 | 0.31 | 0.25 | 3.96 | 0.86 | |
| Age (years) | 0.15 | 27 | 0.13 | 0.05 | 4.46 | 0.47 | |
| Underwent off-pump procedure | −0.17 | 27 | 0.06 | −0.02 | 4.63 | 0.52 | |
| Urgent priority procedure | −0.02 | 27 | 0.13 | 0.06 | 4.45 | 0.92 | |
| IL-6 (pg/ml), baseline | Unadjusted | −0.05 | 36 | 0.00 | −0.03 | 4.71 | 0.76 |
| BDI-II (scores), baseline | −0.10 | 36 | 0.31 | 0.26 | 3.99 | 0.50 | |
| Myocardial infarction‡ | −0.05 | 36 | 0.01 | −0.05 | 4.77 | 0.77 | |
| Unstable angina at admission | 0.04 | 36 | 0.03 | −0.02 | 4.71 | 0.82 | |
| IL-6 (pg/ml), total Δ | Unadjusted | 0.23 | 29 | 0.06 | 0.02 | 4.37 | 0.22 |
| IL-6 (pg/ml), baseline | 0.23 | 29 | 0.08 | 0.01 | 4.41 | 0.23 | |
| BDI-II (scores), baseline | 0.13 | 29 | 0.32 | 0.27 | 3.77 | 0.44 | |
| Acetylsalicylic acid‡ | 0.27 | 29 | 0.08 | 0.01 | 4.39 | 0.18 | |
| Underwent off-pump procedure | 0.18 | 29 | 0.06 | −0.01 | 4.44 | 0.47 | |
| Urgent priority procedure | 0.19 | 29 | 0.16 | 0.09 | 4.21 | 0.31 | |
BDI-II = Beck Depression Inventory II. β = standardized regression coefficient. hs-CRP = high-sensitivity C-reactive protein. IL-6 = interleukin-6. R2 = coefficient of determination. R2 adjusted = adjusted coefficient of determination. RMSE = root-mean-square error. S-100B = S-100 calcium-binding protein B.
*Acute Δ refers to the primary outcome, acute change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and discharge
Corresponds to the blood biomarker term in the model.
Within 7 days before the index procedure.
Delayed change in depressive symptom severity
Spearman ρ values and scatterplots for all possible two-term combinations between the secondary outcome and blood biomarker measures are displayed in Table 3 and Figure 2. There were no statistically significant correlations between blood biomarker levels and the secondary outcome (Table 3). The results of simple and multiple linear regressions of the secondary outcome on blood biomarker levels are displayed in Table 5. None of these models, unadjusted or adjusted, yielded a statistically significant relationship between blood biomarker levels and delayed change in depressive symptom severity (Table 5).
Table 5. Linear regression of the secondary outcome on blood biomarker measures and covariates.
| Independent variables | Delayed Δ in depressive symptom severity* | ||||||
| Biomarker measures | Covariates | β | n | R2 | R2 adjusted | RMSE | P Value† |
| S-100B (µg/l), baseline | Unadjusted | −0.04 | 24 | 0.00 | −0.04 | 5.01 | 0.86 |
| BDI-II (scores), baseline | −0.39 | 24 | 0.02 | −0.08 | 5.09 | 0.85 | |
| BDI-II (scores), acute Δ | −0.10 | 24 | 0.12 | 0.03 | 4.83 | 0.63 | |
| Underwent off-pump procedure | −0.12 | 24 | 0.02 | −0.07 | 5.08 | 0.64 | |
| Urgent priority procedure | −0.07 | 24 | 0.05 | −0.04 | 5.01 | 0.74 | |
| Troponin T at 24 hours (ng/dl) | −0.04 | 24 | 0.00 | −0.09 | 5.13 | 0.88 | |
| S-100B (µg/l), total Δ | Unadjusted | 0.22 | 21 | 0.05 | −0.00 | 4.63 | 0.35 |
| S-100B (µg/l), baseline | 0.21 | 21 | 0.05 | −0.05 | 4.74 | 0.37 | |
| BDI-II (scores), baseline | 0.12 | 21 | 0.07 | −0.03 | 4.69 | 0.64 | |
| BDI-II (scores), acute Δ | 0.03 | 21 | 0.15 | −0.05 | 4.49 | 0.90 | |
| Age (years) | 0.21 | 21 | 0.05 | −0.06 | 4.75 | 0.38 | |
| Weight (kg) | 0.16 | 21 | 0.08 | −0.02 | 4.66 | 0.50 | |
| Body-mass index (kg/m2) | 0.18 | 21 | 0.06 | −0.04 | 4.71 | 0.46 | |
| β-blocker | 0.25 | 21 | 0.05 | −0.06 | 4.74 | 0.25 | |
| hs-CRP (mg/l), baseline | Unadjusted | 0.28 | 24 | 0.08 | 0.04 | 4.81 | 0.18 |
| BDI-II (scores), baseline | 0.29 | 24 | 0.10 | 0.01 | 4.88 | 0.18 | |
| BDI-II (scores), acute Δ | 0.21 | 24 | 0.15 | 0.07 | 4.74 | 0.31 | |
| Charlson comorbidity index | 0.29 | 24 | 0.08 | −0.01 | 4.93 | 0.20 | |
| Myocardial infarction† | 0.31 | 24 | 0.15 | 0.07 | 4.74 | 0.14 | |
| Troponin T at 24 hours (ng/dl) | 0.30 | 24 | 0.09 | −0.00 | 4.91 | 0.18 | |
| hs-CRP (mg/l), total Δ | Unadjusted | −0.07 | 20 | 0.00 | −0.05 | 4.77 | 0.78 |
| hs-CRP (mg/l), baseline | −0.07 | 20 | 0.00 | −0.11 | 4.91 | 0.79 | |
| BDI-II (scores), baseline | −0.08 | 20 | 0.06 | −0.05 | 4.77 | 0.73 | |
| BDI-II (scores), acute Δ | 0.00 | 20 | 0.16 | 0.06 | 4.51 | 0.99 | |
| Age (years) | −0.05 | 20 | 0.01 | −0.11 | 4.90 | 0.85 | |
| Underwent off-pump procedure | −0.10 | 20 | 0.01 | −0.11 | 4.91 | 0.77 | |
| Urgent priority procedure | −0.07 | 20 | 0.01 | −0.11 | 4.90 | 0.78 | |
| IL-6 (pg/ml), baseline | Unadjusted | −0.10 | 24 | 0.01 | −0.04 | 4.99 | 0.64 |
| BDI-II (scores), baseline | −0.15 | 24 | 0.04 | −0.06 | 5.04 | 0.50 | |
| BDI-II (scores), acute Δ | −0.13 | 24 | 0.12 | 0.04 | 4.81 | 0.54 | |
| Myocardial infarction‡ | −0.13 | 24 | 0.07 | −0.02 | 4.96 | 0.54 | |
| Unstable angina at admission | −0.25 | 24 | 0.08 | −0.01 | 4.94 | 0.32 | |
| IL-6 (pg/ml), total Δ | Unadjusted | 0.16 | 22 | 0.03 | −0.02 | 4.89 | 0.47 |
| IL-6 (pg/ml), baseline | 0.17 | 22 | 0.03 | −0.07 | 5.01 | 0.47 | |
| BDI-II (scores), baseline | 0.14 | 22 | 0.06 | −0.04 | 4.94 | 0.54 | |
| BDI-II (scores), acute Δ | 0.11 | 22 | 0.15 | 0.06 | 4.69 | 0.63 | |
| Acetylsalicylic acid‡ | 0.17 | 22 | 0.03 | −0.07 | 5.01 | 0.46 | |
| Underwent off-pump procedure | 0.31 | 22 | 0.06 | −0.04 | 4.92 | 0.28 | |
| Urgent priority procedure | 0.15 | 22 | 0.04 | −0.06 | 4.97 | 0.52 | |
BDI-II = Beck Depression Inventory II. β = standardized regression coefficient. hs-CRP = high-sensitivity C-reactive protein. IL-6 = interleukin-6. R2 = coefficient of determination. R2 adjusted = adjusted coefficient of determination. RMSE = root-mean-square error. S-100B = S-100 calcium-binding protein B.
*Delayed Δ refers to the secondary outcome, delayed change in depressive symptom severity, defined as the intra-subject difference between BDI-II scores at baseline and six-month follow-up
Corresponds to the blood biomarker term in the model.
Within 7 days before the index procedure.
Discussion
This is the first study, to our knowledge, to have prospectively assessed the relationships between blood biomarkers of glial activation and injury, blood–brain barrier disruption, and depressive symptom severity in the context of the systemic inflammatory challenge paradigm of coronary artery bypass grafting. We found that perioperative increases in the glial activation and injury and blood–brain barrier-disruption biomarker, S-100B, exhibited a strong positive correlation with acute changes in depressive symptom severity, largely independent of covariates associated with either. Given the magnitude of the test statistic for this correlation (P = 0.0004), the statistical significance of this finding cannot readily be attributed to the fact that we did multiple pre-specified comparisons that included assessing the relationship between depressive symptoms and two other biomarkers besides S-100B (α/comparisons = [0.05]/[3] = 0.001>0.0004). We also found that baseline BDI-II scores correlated negatively with both perioperative increases in S-100B levels as well as acute changes in depressive symptom severity, and that adjusting for baseline BDI-II scores diminished the strength and statistical significance of their association (the term for perioperative increases in S-100B levels still approached statistical significance at P = 0.064). Adjusting for baseline BDI-II scores also increased the proportion of variance in the primary outcome explained by the model (from 23% to 35%). Exploratory analyses indicated that the association between perioperative increases in S-100B levels and acute changes in depressive severity remained statistically significant after adjusting for baseline BDI-II scores among participants with BDI-II scores greater than or equal to 5 at baseline (Spearman ρ, 0.66; P = 0.0007). This was not the case, however, among those with BDI-II scores less than 5 at baseline (Spearman ρ, 0.15; P = 0.15).
Two earlier studies assessed relationships between depression and inflammation in the context of coronary artery bypass grafting [38], [39]. In the first of these, Yang and colleagues found that mean levels of hs-CRP at baseline were significantly higher among those with clinical depression at six-month follow-up (defined as a Patient Health Questionnaire (PHQ-9) score ≥9) compared with those who were not [38]. By comparison, the correlation between baseline blood levels of hs-CRP blood levels and six-month BDI-II scores in the present study was not statistically significant. A possible explanation for this apparent discrepancy is that six-month follow-up depressive symptom severity was defined as a dichotomous outcome in Yang and colleagues’ study, whereas it was defined as a continuous outcome in the present study. Supporting this interpretation, results of an exploratory analysis we performed showed that baseline hs-CRP blood levels were significantly higher among participants who were clinically depressed at six-month follow-up (defined as a BDI-II score ≥14), as compared with participants who were not (26.9±41.1 mg/l (n = 4) vs 5.6±6.3 mg/l (n = 21); mean difference, 21.3 mg/l; 95% confidence interval, 3.3 to 39.3 mg/l; P = 0.022). The same between-group comparison for interleukin-6 blood levels at baseline approached statistical significance (16.0±15.4 pg/ml (n = 4) vs 7.0±7.9 pg/ml (n = 21); mean difference, 8.5 pg/ml; 95% confidence interval, 1.4 to 19.4 pg/ml; P = 0.09). In the second study, Poole and colleagues found that elevated hs-CRP levels at postoperative day four mediated the association between depression and an increased length of stay [39]. Differences in the aims and designs of this study compared with the present one limit the ability to directly compare and contrast their findings.
The present study has limitations. First, some studies have shown that cardiopulmonary bypass can produce a transient elevation in blood S-100B levels, potentially confounding the observed correlation between perioperative increases in S-100B levels and acute changes in depressive symptom severity [101]. However, subsequent studies, which performed serial assays of blood S-100B levels among patients undergoing coronary artery bypass grafting found that increases in blood S-100B levels due to cardiopulmonary bypass are no longer apparent after 24 hours [102], [103]. Accordingly, because we collected postoperative blood samples at about 48 hours, it is unlikely that perioperative increases in S-100B levels in our study were related to cardiopulmonary bypass. Supporting this interpretation, perioperative increases in S-100B levels among participants in our study did not differ significantly between patients who had cardiopulmonary bypass and those who did not. Second, whether the observed perioperative increases in blood S-100B levels are reliably attributable to glial activation and injury and blood-brain barrier disruption rather than non-cardiac extracerebral sources remains subject to interpretation. This concern arises primarily in the context of two animal model studies having shown a disconnect between S-100B levels in blood and those in CSF or brain parenchyma [68], and one recent cross-sectional study that found a negative correlation between CSF levels of S-100B and depressive symptom severity among patients with non-inflammatory neurological disorders [62]. Arguing in the opposite direction, however, other studies have shown that extracerebral sources do not influence blood S-100B levels [104]. In addition, a recent study of patients undergoing coronary artery bypass grafting found that S-100B levels in both blood and CSF increased together with those of the quintessential astroglial marker glial fibrillary acidic protein as well as CSF-to-serum albumin ratios [81]. Last, since data on antidepressant and statin use were not collected for the original Scarecrow trial, the effect of these drugs on the observed correlation between changes in S-100B levels and acute changes in depressive symptom severity cannot be excluded.
In conclusion, perioperative increases in S-100B levels exhibited a strongly positive correlation with acute changes in depressive symptom severity in the systemic inflammatory challenge paradigm of cardiac surgery. There were no significant associations between either acute or delayed changes in depressive symptom severity and baseline S-100B levels, baseline hs-CRP or interleukin-6 levels, or perioperative changes in hs-CRP or interleukin-6 levels. Among most participants, levels of all three biomarkers were normal at baseline and markedly elevated at 2 days postoperative. Taken together, these findings are consistent with the hypothesis that depression can be mechanistically linked to glial activation and injury as well as blood-brain barrier disruption in the context of systemic inflammation challenge paradigms, to include cardiac surgery. Large prospective cohort studies that can inform the reproducibility of these findings are warranted.
Acknowledgments
We thank all participants and staff involved in the Scarecrow trial.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. For approved reasons, some access restrictions apply to the data underlying the findings. Data are available upon request for researchers who enter into a data use agreement with Eastern Maine Medical Center (Bangor, Maine, United States) as well as Dartmouth College (Hanover, New Hampshire, United States) and obtain Institutional Review Board approval for access to the data. Requests for the data should be sent to Jeremiah R. Brown, PhD (jbrown@dartmouth.edu).
Funding Statement
DMP received funding from the Audrey and Theodor Geisel School of Medicine (Hanover, New Hampshire, United States). JRB was supported in part by a grant from the Agency for Healthcare Research and Quality (K01HS018443). For the original randomized, controlled trial (Scarecrow trial, 2001–2004), FH received donated funds, donated supplies, biomarker assays, and loaned equipment from Medtronic Inc. (Minneapolis, Minnesota, United States), Genzyme Surgical (Fall River, Massachusetts, United States), and Somanetics Corporation (Troy, Michigan, United States). FH also received biomarker assays from the Northern New England Cardiovascular Disease Study Group (Hanover, New Hampshire, United States). Loaned equipment included the Genzyme Off-Pump Coronary Artery Bypass (OPCAB) Elite apparatus and the Medtronic Octopus system, both of which used to stabilize target vessels during off-pump coronary artery bypass grafting procedures performed in the trial. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O (2013) Neuroinflammation and psychiatric illness. J Neuroinflammation 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D (2013) Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation 10: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Najjar S, Pearlman DM, Hirsch S, Friedman K, Strange J, et al. (2014) Brain biopsy findings link major depressive disorder to neuroinflammation, oxidative stress, and neurovascular dysfunction: a case report. Biol Psychiatry 75: e23–26. [DOI] [PubMed] [Google Scholar]
- 4. Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, et al. (2011) Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186. [DOI] [PubMed] [Google Scholar]
- 7. Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, et al. (2005) Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol 4: 371–380. [DOI] [PubMed] [Google Scholar]
- 8. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 9. Hiles SA, Baker AL, de Malmanche T, Attia J (2012) Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol Med 42: 2015–2026. [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Ho RC, Mak A (2012) Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 139: 230–239. [DOI] [PubMed] [Google Scholar]
- 11. Ford DE, Erlinger TP (2004) Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 164: 1010–1014. [DOI] [PubMed] [Google Scholar]
- 12. Hickman RJ, Khambaty T, Stewart JC (2014) C-reactive protein is elevated in atypical but not nonatypical depression: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Behav Med 37: 621–629. [DOI] [PubMed] [Google Scholar]
- 13. Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, et al. (2008) Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42: 151–157. [DOI] [PubMed] [Google Scholar]
- 15. Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, et al. (2000) Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry 48: 861–873. [DOI] [PubMed] [Google Scholar]
- 16. Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, et al. (2010) Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord 12: 541–549. [DOI] [PubMed] [Google Scholar]
- 17. Webster MJ, O'Grady J, Kleinman JE, Weickert CS (2005) Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neurosci 133: 453–461. [DOI] [PubMed] [Google Scholar]
- 18. Johnston-Wilson NL, Sims CD, Hofmann JP, Anderson L, Shore AD, et al. (2000) Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry 5: 142–149. [DOI] [PubMed] [Google Scholar]
- 19. Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, et al. (2013) Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70: 812–820. [DOI] [PubMed] [Google Scholar]
- 20. Kayser M (2014) Severe infection and autoimmune disease are associated with increased risk of mood disorders. Evid Based Ment Health 17: 20. [DOI] [PubMed] [Google Scholar]
- 21. Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, et al. (2003) Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry 160: 1342–1345. [DOI] [PubMed] [Google Scholar]
- 22. Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, et al. (2010) Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry 15: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, et al. (2005) Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry 66: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, et al. (2005) Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 19: 23–27. [DOI] [PubMed] [Google Scholar]
- 25. Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, et al. (2009) Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry 65: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ (2002) Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 147: 1142–1146. [DOI] [PubMed] [Google Scholar]
- 27. Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, et al. (2012) Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 73: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 28. Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, et al. (2001) Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 344: 961–966. [DOI] [PubMed] [Google Scholar]
- 29. Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, et al. (2001) Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 158: 1252–1257. [DOI] [PubMed] [Google Scholar]
- 30. Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, et al. (2007) Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 25: 1163–1174. [DOI] [PubMed] [Google Scholar]
- 31. Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, et al. (2010) Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry 68: 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martins JG (2009) EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 28: 525–542. [DOI] [PubMed] [Google Scholar]
- 33. Bloch MH, Hannestad J (2012) Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 17: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Na KS, Lee KJ, Lee JS, Cho YS, Jung HY (2014) Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 48: 79–85. [DOI] [PubMed] [Google Scholar]
- 35. Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, et al. (2006) The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 11: 680–684. [DOI] [PubMed] [Google Scholar]
- 36. Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, et al. (2010) Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cremeans-Smith JK, Soehlen S, Greene K, Alexander T, Delahanty DL (2009) In-hospital levels of C-reactive protein and IL-6 predict post-operative depressive symptoms among patients undergoing total knee replacement surgery. Brain Behav Immun 23: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 38. Yang L, Wang J, Zhang L, Hou J, Yuan X, et al. (2012) Preoperative high-sensitivity C-reactive protein predicts depression in patients undergoing coronary artery bypass surgery: a single-center prospective observational study. J Thorac Cardiovasc Surg 144: 500–505. [DOI] [PubMed] [Google Scholar]
- 39. Poole L, Kidd T, Leigh E, Ronaldson A, Jahangiri M, et al. (2014) Depression, C-reactive protein and length of post-operative hospital stay in coronary artery bypass graft surgery patients. Brain Behav Immun 37: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newman MF, Mathew JP, Grocott HP, Mackensen GB, Monk T, et al. (2006) Central nervous system injury associated with cardiac surgery. Lancet 368: 694–703. [DOI] [PubMed] [Google Scholar]
- 41. Miguel-Hidalgo JJ, Overholser JC, Jurjus GJ, Meltzer HY, Dieter L, et al. (2011) Vascular and extravascular immunoreactivity for intercellular adhesion molecule 1 in the orbitofrontal cortex of subjects with major depression: age-dependent changes. J Affect Disord 132: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gudmundsson P, Skoog I, Waern M, Blennow K, Palsson S, et al. (2007) The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry 15: 832–838. [DOI] [PubMed] [Google Scholar]
- 43. Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, et al. (2010) Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res 44: 321–330. [DOI] [PubMed] [Google Scholar]
- 44. Niklasson F, Agren H (1984) Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol Psychiatry 19: 1183–1206. [PubMed] [Google Scholar]
- 45. Lavoie KL, Pelletier R, Arsenault A, Dupuis J, Bacon SL (2010) Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med 72: 20–26. [DOI] [PubMed] [Google Scholar]
- 46. Valkanova V, Ebmeier KP (2013) Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry 73: 406–413. [DOI] [PubMed] [Google Scholar]
- 47. Serlin Y, Levy J, Shalev H (2011) Vascular pathology and blood-brain barrier disruption in cognitive and psychiatric complications of type 2 diabetes mellitus. Cardiovasc Psychiatry Neurol 2011: 609202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Permoda-Osip A, Dorszewska J, Skibinska M, Chlopocka-Wozniak M, Rybakowski JK (2013) Hyperhomocysteinemia in bipolar depression: clinical and biochemical correlates. Neuropsychobiology 68: 193–196. [DOI] [PubMed] [Google Scholar]
- 49. Zill P, Baghai TC, Schule C, Born C, Frustuck C, et al. (2012) DNA methylation analysis of the angiotensin converting enzyme (ACE) gene in major depression. PLoS ONE 7: e40479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Do DP, Dowd JB, Ranjit N, House JS, Kaplan GA (2010) Hopelessness, depression, and early markers of endothelial dysfunction in U.S. adults. Psychosom Med 72: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dimopoulos N, Piperi C, Salonicioti A, Mitsonis C, Liappas I, et al. (2006) Elevation of plasma concentration of adhesion molecules in late-life depression. Int J Geriatr Psychiatry 21: 965–971. [DOI] [PubMed] [Google Scholar]
- 52. Schaefer M, Horn M, Schmidt F, Schmid-Wendtner MH, Volkenandt M, et al. (2004) Correlation between sICAM-1 and depressive symptoms during adjuvant treatment of melanoma with interferon-alpha. Brain Behav Immun 18: 555–562. [DOI] [PubMed] [Google Scholar]
- 53. Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, et al. (2003) Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry 18: 7–13. [DOI] [PubMed] [Google Scholar]
- 54. Thomas AJ, Ferrier IN, Kalaria RN, Woodward SA, Ballard C, et al. (2000) Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am J Psychiatry 157: 1682–1684. [DOI] [PubMed] [Google Scholar]
- 55. Gos T, Schroeter ML, Lessel W, Bernstein HG, Dobrowolny H, et al. (2013) S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res 47: 1694–1699. [DOI] [PubMed] [Google Scholar]
- 56. Falcone T, Fazio V, Lee C, Simon B, Franco K, et al. (2010) Serum S100B: a potential biomarker for suicidality in adolescents? PLoS ONE 5: e11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schroeter ML, Abdul-Khaliq H, Sacher J, Steiner J, Blasig IE, et al. (2010) Mood disorders are glial disorders: evidence from in vivo studies. Cardiovasc Psychiatry Neurol 2010: 780645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE (2008) Serum markers support disease-specific glial pathology in major depression. J Affect Disord 111: 271–280. [DOI] [PubMed] [Google Scholar]
- 59. Arolt V, Peters M, Erfurth A, Wiesmann M, Missler U, et al. (2003) S100B and response to treatment in major depression: a pilot study. Eur Neuropsychopharmacol 13: 235–239. [DOI] [PubMed] [Google Scholar]
- 60. Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D (2013) Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry 73: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uher T, Bob P (2012) Cerebrospinal fluid S100B levels reflect symptoms of depression in patients with non-inflammatory neurological disorders. Neurosci Lett 529: 139–143. [DOI] [PubMed] [Google Scholar]
- 63. Zhang Y, Rothermundt M, Peters M, Wiesmann M, Hoy L, et al. (2009) S100B serum levels and word memory processing in remitted major depression as reflected by brain potentials. Neuropsychobiology 59: 172–177. [DOI] [PubMed] [Google Scholar]
- 64. Yang K, Xie GR, Hu YQ, Mao FQ, Su LY (2008) The effects of gender and numbers of depressive episodes on serum S100B levels in patients with major depression. J Neural Transm 115: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 65. Jang BS, Kim H, Lim SW, Jang KW, Kim DK (2008) Serum S100B Levels and Major Depressive Disorder: Its Characteristics and Role in Antidepressant Response. Psychiatry Investig 5: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dietrich DE, Hauser U, Peters M, Zhang Y, Wiesmann M, et al. (2004) Target evaluation processing and serum levels of nerve tissue protein S100B in patients with remitted major depression. Neurosci Lett 354: 69–73. [DOI] [PubMed] [Google Scholar]
- 67. Goncalves FM, Freitas AE, Peres TV, Rieger DK, Ben J, et al. (2013) Vatairea macrocarpa lectin (VML) induces depressive-like behavior and expression of neuroinflammatory markers in mice. Neurochem Res 38: 2375–2384. [DOI] [PubMed] [Google Scholar]
- 68. Luo KR, Hong CJ, Liou YJ, Hou SJ, Huang YH, et al. (2010) Differential regulation of neurotrophin S100B and BDNF in two rat models of depression. Prog Neuropsychopharmacol Biol Psychiatry 34: 1433–1439. [DOI] [PubMed] [Google Scholar]
- 69. Ye Y, Wang G, Wang H, Wang X (2011) Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett 503: 15–19. [DOI] [PubMed] [Google Scholar]
- 70. Veena J, Srikumar BN, Raju TR, Shankaranarayana Rao BS (2009) Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett 455: 178–182. [DOI] [PubMed] [Google Scholar]
- 71. Rothermundt M, Arolt V, Wiesmann M, Missler U, Peters M, et al. (2001) S-100B is increased in melancholic but not in non-melancholic major depression. J Affect Disord 66: 89–93. [DOI] [PubMed] [Google Scholar]
- 72. Bargerstock E, Puvenna V, Iffland P, Falcone T, Hossain M, et al. (2014) Is peripheral immunity regulated by blood-brain barrier permeability changes? PLoS ONE 9: e101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mondello S, Müller U, Jeromin A, Streeter J, Hayes RL, et al. (2011) Blood-based diagnostics of traumatic brain injuries. Expert Rev Mol Diagn 11: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shahim P, Tegner Y, Wilson DH, Randall J, Skillback T, et al. (2014) Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 71: 684–692. [DOI] [PubMed] [Google Scholar]
- 75. Ponath G, Schettler C, Kaestner F, Voigt B, Wentker D, et al. (2007) Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol 184: 214–222. [DOI] [PubMed] [Google Scholar]
- 76. Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB (2010) Systemic markers of inflammation are independently associated with S100B concentration: results of an observational study in subjects with acute ischaemic stroke. J Neuroinflammation 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Szelenyi A, Heukamp C, Seifert V, Marquardt G (2014) S100B, intraoperative neuromonitoring findings and their relation to clinical outcome in surgically treated intradural spinal lesions. Acta Neurochir (Wien) 156: 733–739. [DOI] [PubMed] [Google Scholar]
- 78. Blennow K, Jonsson M, Andreasen N, Rosengren L, Wallin A, et al. (2011) No neurochemical evidence of brain injury after blast overpressure by repeated explosions or firing heavy weapons. Acta Neurol Scand 123: 245–251. [DOI] [PubMed] [Google Scholar]
- 79. Derkach DN, Okamoto H, Takahashi S (2000) Neuronal and astroglial injuries in patients undergoing coronary artery bypass grafting and aortic arch replacement during hypothermic cardiopulmonary bypass. Anesth Analg 91: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 80. Kinoshita H, Iranami H, Fujii K, Yamazaki A, Shimogai M, et al. (2003) The use of bone cement induces an increase in serum astroglial S-100B protein in patients undergoing total knee arthroplasty. Anesth Analg 97: 1657–1660. [DOI] [PubMed] [Google Scholar]
- 81. Reinsfelt B, Ricksten SE, Zetterberg H, Blennow K, Freden-Lindqvist J, et al. (2012) Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann Thorac Surg 94: 549–555. [DOI] [PubMed] [Google Scholar]
- 82. Ashraf S, Bhattacharya K, Tian Y, Watterson K (1999) Cytokine and S100B levels in paediatric patients undergoing corrective cardiac surgery with or without total circulatory arrest. Eur J Cardiothorac Surg 16: 32–37. [DOI] [PubMed] [Google Scholar]
- 83. Sellner J, Davies NW, Howard RS, Petzold A (2014) Neurofilament heavy chain as a marker of neuroaxonal pathology and prognosis in acute encephalitis. European Journal of Neurology 21: 845–850. [DOI] [PubMed] [Google Scholar]
- 84. Skold MK, Risling M, Holmin S (2006) Inhibition of vascular endothelial growth factor receptor 2 activity in experimental brain contusions aggravates injury outcome and leads to early increased neuronal and glial degeneration. Eur J Neurosci 23: 21–34. [DOI] [PubMed] [Google Scholar]
- 85. Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, et al. (2004) Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma 21: 1113–1122. [DOI] [PubMed] [Google Scholar]
- 86. Laskowitz DT, Grocott H, Hsia A, Copeland KR (1998) Serum markers of cerebral ischemia. J Stroke Cerebrovasc Dis 7: 234–241. [DOI] [PubMed] [Google Scholar]
- 87. Kessler FH, Woody G, Portela LV, Tort AB, De Boni R, et al. (2007) Brain injury markers (S100B and NSE) in chronic cocaine dependents. Revista Brasileira de Psiquiatria 29: 134–139. [DOI] [PubMed] [Google Scholar]
- 88. Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, et al. (2007) Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 48: 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Reiber H (1998) Cerebrospinal fluid–physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler 4: 99–107. [DOI] [PubMed] [Google Scholar]
- 90. Reiber H (2001) Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta 310: 173–186. [DOI] [PubMed] [Google Scholar]
- 91. Marchi N, Fazio V, Cucullo L, Kight K, Masaryk T, et al. (2003) Serum transthyretin monomer as a possible marker of blood-to-CSF barrier disruption. J Neurosci 23: 1949–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, et al. (2003) Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci 21: 109–121. [PMC free article] [PubMed] [Google Scholar]
- 93. Zongo D, Ribereau-Gayon R, Masson F, Laborey M, Contrand B, et al. (2012) S100-B protein as a screening tool for the early assessment of minor head injury. Ann Emerg Med 59: 209–218. [DOI] [PubMed] [Google Scholar]
- 94. Johnsson P, Lundqvist C, Lindgren A, Ferencz I, Alling C, et al. (1995) Cerebral complications after cardiac surgery assessed by S-100 and NSE levels in blood. J Cardiothorac Vasc Anesth 9: 694–699. [DOI] [PubMed] [Google Scholar]
- 95. Grocott HP, Arrowsmith JE (2001) Serum S100 protein as a marker of cerebral damage during cardiac surgery. Br J Anaesth 86: 289–290. [PubMed] [Google Scholar]
- 96. Bayram H, Hidiroglu M, Cetin L, Kucuker A, Iriz E, et al. (2013) Comparing S-100 beta protein levels and neurocognitive functions between patients undergoing on-pump and off-pump coronary artery bypass grafting. J Surg Res 182: 198–202. [DOI] [PubMed] [Google Scholar]
- 97. Whitaker DC, Green AJ, Stygall J, Harrison MJ, Newman SP (2007) Evaluation of an alternative S100b assay for use in cardiac surgery: relationship with microemboli and neuropsychological outcome. Perfusion 22: 267–272. [DOI] [PubMed] [Google Scholar]
- 98. Hernandez F Jr, Brown JR, Likosky DS, Clough RA, Hess AL, et al. (2007) Neurocognitive outcomes of off-pump versus on-pump coronary artery bypass: a prospective randomized controlled trial. Ann Thorac Surg 84: 1897–1903. [DOI] [PubMed] [Google Scholar]
- 99. Beck AT, Steer RA, Garbin MG (1988) Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 8: 77–100. [Google Scholar]
- 100.Tukey JW (1977) Exploratory data analysis. Reading, MA: Addison–Wesley.
- 101. Fazio V, Bhudia SK, Marchi N, Aumayr B, Janigro D (2004) Peripheral detection of S100beta during cardiothoracic surgery: what are we really measuring? Ann Thorac Surg 78: 46–52 discussion 52–43. [DOI] [PubMed] [Google Scholar]
- 102. Jonsson H, Johnsson P, Alling C, Westaby S, Blomquist S (1998) Significance of serum S100 release after coronary artery bypass grafting. Ann Thorac Surg 65: 1639–1644. [DOI] [PubMed] [Google Scholar]
- 103. Baufreton C, Allain P, Chevailler A, Etcharry-Bouyx F, Corbeau JJ, et al. (2005) Brain injury and neuropsychological outcome after coronary artery surgery are affected by complement activation. Ann Thorac Surg 79: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 104. Pham N, Fazio V, Cucullo L, Teng Q, Biberthaler P, et al. (2010) Extracranial sources of S100B do not affect serum levels. PLoS ONE 5: e12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. For approved reasons, some access restrictions apply to the data underlying the findings. Data are available upon request for researchers who enter into a data use agreement with Eastern Maine Medical Center (Bangor, Maine, United States) as well as Dartmouth College (Hanover, New Hampshire, United States) and obtain Institutional Review Board approval for access to the data. Requests for the data should be sent to Jeremiah R. Brown, PhD (jbrown@dartmouth.edu).