Abstract
Lysine is a limiting amino acid in diets based on wheat as the staple. In experimental animals, prolonged dietary lysine inadequacy increases stress-induced anxiety. If observed in humans, such a result would have a strong implication for the relationship between nutrition and communal quality of life and mental health. As part of a 3-month randomized double-blind study, we tested whether lysine fortification of wheat reduces anxiety and stress response in family members in poor Syrian communities consuming wheat as a staple food. In the lysine-fortified group, the plasma cortisol response to the blood drawing as a cause of stress was reduced in females, as was sympathetic arousal in males as measured by skin conductance. Lysine fortification also significantly reduced chronic anxiety as measured by the trait anxiety inventory in males. These results suggest that some stress responses in economically weak populations consuming cereal-based diets can be improved with lysine fortification.
Dietary inadequacy of an essential amino acid leads to nonspecific signs of protein deficiency, such as lowered resistance to disease, decreased blood proteins, and stunting in children. The risk of lysine inadequacy is high where low socioeconomic groups depend on wheat for their protein supply (1), but experimental evidence of the nutritional benefits of increasing the lysine content of wheat flour is limited (2–8). This study, which was a part of a larger wheat fortification trial, was based on a hypothesis that fortification of a lysine inadequate diet in poor communities may reduce anxiety and improve stress response, thereby potentially improving the quality of life. The hypothesis originated from studies that found worsening of stress-induced anxiety and colonic health in rats fed a lysine-deficient diet (9) and an improvement of stress responses in rats and pigs receiving lysine loads (10, 11). The anxiogenic response to lysine inadequacy in rats was mediated via serotonin alterations in the central amygdala (9), the brain region functionally comparable in rodents and humans (12). Therefore, we extrapolated the animal studies to humans consuming lysine-deficient diets and measured stress-associated responses in rural communities in Northwestern Syria. This study is a clinical investigation of neuroendocrine and psychological responses to lysine fortification.
Materials and Methods
Study Design and Sample Size. A 3-month-long fortification trial was conducted between March and July of 2003 in a randomized double-blind manner. Households were recruited in the five villages in the Khannaser valley, Northwestern Syria, ≈80 km southeast of the city of Aleppo. The area is classified as semiarid and is located at the fringe of the Syrian steppe. Principal crops grown in this area are barley and wheat; however, the primary source of income for the families is agricultural-wage labor. Socioeconomic and dietary assessments were conducted 1 year before the trial by using a structured questionnaire that included a survey on yearly income for 2001 and household characteristics. A separate anthropometric assessment indicated relatively high levels of stunting (23.0%) and underweight (14.3%) among the children (13).
The dietary data were collected by using a 7-day inventory method, in which household intake and waste were recorded on two occasions 1 week apart. Household sizes and exact number of individuals consuming each meal were averaged over the week and expressed as adult portion sizes and per capita adult male availability of energy and nutrients. This procedure was necessary because of the use of a common plate that did not allow reporting food intake on an individual basis. The average total daily intake of protein in males was 70.1 ± 2.7 g, of which >65% was of cereal origin. Only dietary intake was used as a measure of lysine adequacy because plasma lysine is a poor indicator of dietary changes (14). The diet of the studied communities was characterized by only marginal total protein deficiency; thus, we were able to separate the probable effects of lysine inadequacy from those of general protein malnutrition.
Criteria for including households were baking bread at the household level, diet with an availability of dietary lysine <42 mg/g protein, a presence of at least one 6- to 14-year-old child, the willingness to participate in the trial, and the ability to understand informed consent. Community meetings were held with household heads present to explain the study in detail. The household head was then given an informed consent form, which was signed by potential participants. All families were visited on a fortnightly basis to record any complaints and monitor morbidity and wheat flour use. Households in each village were randomly assigned to control or lysine-fortified groups. The duration of the fortification was 3 months. Thirteen households dropped out in the first month of fortification. At the end of the study, there remained 48 control and 45 lysine-fortified households. Only those subjects who were measured before and after fortification were included in the final data analysis.
Based on the dietary surveys and a previous clinical trial (7), the fortification level was set at 4.2 g of lysine HCl per kg of wheat flour. This fortification ensured that the dietary lysine requirements were satisfied (1–3). To fortify wheat, a mixer and a grain pump were purchased locally and installed at the local mill. Wheat was collected from the households 1 day before the baseline blood collection and returned fortified with an appropriate premix, either control or lysine, on the day of prefortification blood drawing. The flour and the exact amount of premix to be added were weighed and mixed in a 500-liter chrome blender. The content of lysine in both the fortified and control wheat was reexamined at the end of the trial.
The protocol was reviewed and approved by an institutional review board for use of humans as experimental subjects at the International Center for Agricultural Research in the Dry Areas (Aleppo, Syria).
Blood Collection and Skin Conductance Measurement. Blood samples were collected from fasted subjects between 5:00 a.m. and 8:00 a.m., with venipuncture as a cause of stress. For the serum cortisol determinations, blood was collected in plain tubes without coagulant and transported by car to the American University of Beirut (Beirut, Lebanon) and centrifuged (3,000 rpm for 10 min). Total time from start of collection to drop-off at American University of Beirut was 7–8 h. Samples were stored at -70°C. Cortisol was analyzed with an Immulite 1000 automated immunoassay analyzer and an Immulite cortisol kit (Diagnostic Products, Los Angeles). Skin conductance responses were measured noninvasively before, during, and after blood sampling (total measurement time, 5 min). Two Ag/AgCl electrodes were placed in a contact area of 6 mm diameter on the middle phalanxes of the fore and index fingers of the left hand by using an adhesive collar. Hypoallergenic gel provided good skin contact, and a computerized module (both from BioResearch Center, Tokyo) amplified the electrical signal by a circuit of constant voltage (0.6 V). An artifact-free change in skin conductance ≥0.1 μS was considered a response.
Chronic Anxiety. Chronic anxiety was evaluated by an Arabic version of trait-STAI (t-STAI), an inventory composed of 20 items (15, 16). Because of high levels of illiteracy, the adult subjects replied orally to the t-STAI items read to them. The t-STAI was administered separately from the blood drawing during the first week of fortification and immediately after the trial termination. A time difference of at least 3 days was allowed between the blood collection and obtaining t-STAI responses.
Results and Discussion
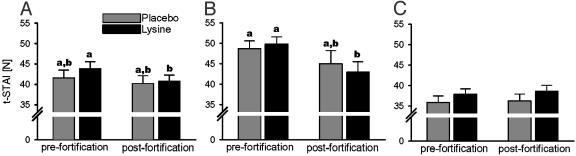
Lysine fortification significantly reduced t-STAI in males (Fig. 1A) without any effects in females (data not shown). The anxiolytic effects were observed only in a subgroup with high baseline anxiety (Fig. 1 B and C), indicating that the fortification did not reduce normal levels of alertness and social apprehension. The fortification-induced improvement in t-STAI score was seemingly small (Δ 6.9 ± 1.8), but it corresponded to the changes observed in clinical tests with anxiety-reducing pharmacological agents such as fluoxetine, which reduced the t-STAI score by Δ 8.1 ± 5.0 points (17), and diazepam, which has been reported to reduce to t-STAI by Δ 6.8 ± 2.0 points (18). Besides t-STAI, no other anxiety questionnaires were used because of technical, linguistic, and cultural barriers. Chronic anxiety disorders encompass a wide variety of diagnoses, and their prevalence in Western countries is high (19). Rigorous data on the situation in the developing world are missing, but it is hypothesized that general anxiety prevalence is also high in poor developing regions. Although anxiety is efficiently treated with benzodiazepines and 5-hydroxytryptamine (5-HT) drugs, prevention is currently ineffective, partly because a framework that incorporates concepts of relevant disciplines, including social science and nutrition, is lacking (20).
Fig. 1.
t-STAI scores obtained in adult men immediately before and after a 3-month wheat fortification with lysine. A decrease in t-STAI score indicates reduction of long-term anxiety. (A) Summarized scores obtained from all male subjects participating in both the pre- and postfortification evaluation. Means + SEM of 20 and 22 subjects are shown. The bars with different letters differ significantly (two-way ANOVA followed by paired or unpaired t test as appropriate). Thereafter, the subjects were divided into two equally sized subgroups according to the prefortification t-STAI score (B and C). The results for the subgroup with high prefortification t-STAI are shown in B (means + SEM of 9 and 11 subjects), and the results obtained from the subgroup with low-to-medium t-STAI score are shown in C (means + SEM of 10 and 11 subjects).
Our research raises questions concerning the physiological mechanism. To the extent that it is possible to extrapolate from animal results (9, 21) to humans (Fig. 1), we can associate the current results with changes within the central 5-HT4 system. The 5-HT4 receptors are located mainly within the gastrointestinal tract and the limbic area of the brain, and they play a specific prostress role by enhancing behavioral and gastrointestinal stress responses, such as diarrhea and anxiety (22). Although lysine does not interfere with the metabolism of 5-HT, it acts like a 5-HT4 receptor antagonist and suppresses 5-HT4 receptor-mediated anxiety (21) without affecting plasma 5-HT, comparably to synthetic antagonists (23). Older reports showed that chronically administered lysine has also tranquilizing properties mediated via the benzodiazepine receptors (24). The above nutritional-pharmacological studies (21, 24) indicate that abolishing lysine inadequacy might suppress the 5-HT4 system, potentiate the benzodiazepine system, and consequently reduce chronic anxiety. Caution must be used in extrapolating to aggressiveness or chronic anxiety in communities living within different geographic, social, and cultural environments, although the t-STAI has been successfully used in multiethnic neighborhoods (15).
To confirm that not only long-term anxiety but also acute stress response is lessened by lysine fortification, we measured the hypothalamus-pituitary-adrenal (HPA) responses and sympathetic arousal during blood drawing. From experience in developing country populations (8), we considered that the process of blood drawing was a sufficient stressor in the studied population. Besides the plasma cortisol, blood drawing-related sympathetic arousal was evaluated by skin conductance. This is a response increased during stress caused by hydration of the exocrine sweat glands of the fingertips and is exclusively innervated by the sympathetic nerves. The response of sympathetic and HPA axes in humans decreases (habituates) after exposure to a 9-s stressor of the same type (25, 26), and impaired stress response habituation predicts proneness to various stress-triggered diseases (26).
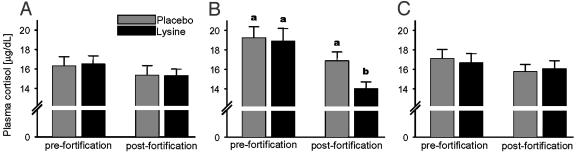
Baseline (prestress) concentration of plasma cortisol in humans is within the range of 5–10 μg/dl (25–27). We did not determine baseline levels because indwelling catheters could not be used in the field. The stress values indicated that the blood collection and its anticipation was stressful because the concentration of plasma cortisol ranged from 16.4 ± 3.0 μg/dl in males to 19.0 ± 0.9 μg/dl in females. Substantial habituation in the HPA axis response was found only in the lysine-fortified female and not in male subjects (Fig. 2). Additionally, the females were characterized by significantly higher prefortification cortisol, as compared to males (P < 0.05). The literature on gender differences in plasma cortisol varies (26, 27) and offers few applicable clues. Both the effect of lysine on the HPA habituation in females and the high prefortification values of cortisol may be related to endocrine interactions of stress and sex hormones, or to gender-dependent cognitive processing of stress.
Fig. 2.
Plasma cortisol obtained in men (A), women (B), and children (<14 years old) of both genders (C). Lysine, lysine-fortified group. Pre- and postfortification measurements were conducted by the local staff. Means + SEM of 28 and 34 males, 48 and 44 females, and 36 and 30 children are shown. The bars with different letters differ significantly (two-way ANOVA followed by paired or unpaired t test as appropriate).
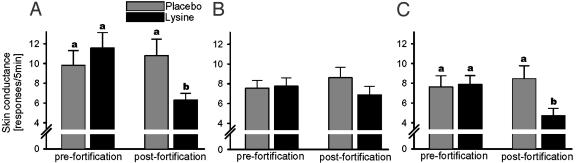
Prolonged lysine supplementation reduced plasma cortisol in animals by inhibiting long-term anxiety (11) but without directly affecting the adrenal gland (20). Correspondingly, we believe that the current modification of the human stress reaction (Fig. 2) was not a direct endocrine effect; rather, it was elicited by anxiolytic mechanisms outlined above. Sympathetic arousal during the second, postfortification blood drawing decreased significantly in the lysine-fortified males and children, but not controls (Fig. 3). Similarly to the perception of chronic anxiety, acute stress responses are gender-specific (25), and it is recognized that the sympathetic system is more stress-reactive in males than in females (27).
Fig. 3.
The number of skin conductance responses measured 3 min before, during, and 2 min after blood drawing in men (A), women (B), and children (<14 years old) of both genders (C). Lysine, lysine-fortified group. Pre- and postfortification measurements were conducted by the local staff. Means + SEM of 20 and 23 males, 33 and 25 females, and 24 and 19 children are shown. The bars with different letters differ significantly (two-way ANOVA followed by paired or unpaired t test as appropriate).
Although the populations studied apparently consumed enough low-quality protein to meet minimal requirements, improvement in the quality of their protein intake by lysine fortification of their cereal staple is presumed to have been responsible for the favorable effects observed on protein and immune status and child growth in two similar previous studies (7, 8). It might, therefore, be that an equivalent improvement in chronic anxiety and stress responsiveness would have been achievable by supplying lysine-rich proteins. We argue against this. Reduced plasma levels of specific amino acids, notably glutamine, characterize catabolic stress, such as that resulting from accidental injury. It is relevant that the amino acids needed to defend the body during catabolic stress, and those provided by normal protein breakdowns are not identical (28).
Extending the model of catabolic stress to our experimental conditions, we believe that the amino acid needed for improving the measured characteristics in populations consuming a lysine-inadequate diet may be more readily available from lysine fortification than the digestion of dietary protein. In any case, the currently observed effects seemed to be caused by abolition of lysine inadequacy (9, 10) via 5-HT and benzodiazepine mechanisms (21, 24). Whether these results are translatable to a lower rate of stress-related ills, ranging from higher anxiety and aggressiveness to infectious diseases, should be explored in other populations.
Acknowledgments
Financial support was provided by Ajinomoto Co., Inc., Tokyo.
Abbreviations: 5-HT, 5-hydroxytryptamine; HPA, hypothalamus-pituitary-adrenal; t-STAI, trait-STAI.
References
- 1.Young, V. R. & Pellett, P. L. (1990) Food Nutr. Bull. 12, 289-300. [Google Scholar]
- 2.Kurpad, A. V., Raj, T., El-Khoury, A., Beaumier, L., Kuriyan, R., Srivatsa, A., Borgonha, S., Selvaraj, A., Regan, M. M. & Young, V. R. (2001) Am. J. Clin. Nutr. 73, 900-907. [DOI] [PubMed] [Google Scholar]
- 3.Kurpad, A. V., Regan, M. M., Raj, T., El-Khoury, A., Kuriyan, R., Vaz, M., Chandakudlu, D., Venkataswamy, V. G., Borgonha, S. & Young, V. R. (2003) Am. J. Clin. Nutr. 77, 101-108. [DOI] [PubMed] [Google Scholar]
- 4.Scrimshaw, N. S., Taylor, Y. & Young, V. R. (1973) Am. J. Clin. Nutr. 26, 965-972. [DOI] [PubMed] [Google Scholar]
- 5.Ghai, P. & Chaudhuri, S. N. (1971) Indian J. Med. Res. 59, 756-759. [PubMed] [Google Scholar]
- 6.Reddy, V. (1971) Am. J. Clin. Nutr. 24, 1246-1249. [DOI] [PubMed] [Google Scholar]
- 7.Zhao, W., Zhai, F., Zhang, D., An, Y., Liu, Y., He, Y., Ge, K. & Scrimshaw, N. S. (2004) Food Nutr. Bull. 25, in press. [DOI] [PubMed]
- 8.Hussain, T., Abbas, S., Khan, M. A. & Scrimshaw, N. S. (2004) Food Nutr. Bull. 25, in press. [DOI] [PubMed]
- 9.Smriga, M., Kameishi, M., Uneyama, H. & Torii, K. (2002) J. Nutr. 132, 3744-3746. [DOI] [PubMed] [Google Scholar]
- 10.Smriga, M. & Torii, K. (2003) Nutr. Neurosci. 6, 125-127. [DOI] [PubMed] [Google Scholar]
- 11.Srinongkote, S., Smriga, M., Nakagawa, K. & Toride, Y. (2003) Nutr. Neurosci. 6, 283-289. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh, S., Aw-Hassan, A. & Pellett, P. (2004) Ecol. Food Nutr. 43, 107-148. [Google Scholar]
- 13.Barton, R. A., Aggleton, J. P. & Grenyer, R. (2003) Proc. R. Soc. London Ser. B 270, 539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungqvist, B. G., Svanberg, U. S. & Young, V. R. (1978) Res. Exp. Med. (Berlin) 174, 13-28. [DOI] [PubMed] [Google Scholar]
- 15.Hishinuma, E. S., Miyamoto, R. H., Nishimura, S. T., Goebert, D. A., Yuen, N. Y., Makini, G. K., Jr., Andrade, N. N., Johnson, R. C. & Carlton, B. S. (2001) J. Anxiety Disord. 15, 511-533. [DOI] [PubMed] [Google Scholar]
- 16.Spielberger, D., Gorsuch, R. L. & Lushene, R. E. (1970) Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, Palo Alto, CA).
- 17.Simeon, D., Stein, D. J., Gross, S., Islam, N., Schmeidler, J. & Hollander, E. A. (1997) J. Clin. Psychiatry 58, 341-347. [DOI] [PubMed] [Google Scholar]
- 18.Suetsugi, M., Mizuki, Y., Ushijima, I., Kobayashi, T. & Watanabe, Y. (2001) Neuropsychobiology 43, 49-53. [DOI] [PubMed] [Google Scholar]
- 19.Kessler, R. C., Crum, R. M., Warner, L. A., Nelson, C. B., Schulenberg, J. & Anthony, J. C. (1994) Arch. Gen. Psychiatry 51, 8-19. [DOI] [PubMed] [Google Scholar]
- 20.Nesse, R. M. (1999) Neurosci. Biobehav. Rev. 23, 895-903. [DOI] [PubMed] [Google Scholar]
- 21.Smriga, M. & Torii, K. (2003) Proc. Natl. Acad. Sci. USA 100, 15370-15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eglen, R. M., Wong, E. H. F., Dumuis, A. & Bockaert, J. (1995) Trends Pharmacol. Sci. 16, 391-398. [DOI] [PubMed] [Google Scholar]
- 23.Kennett, G. A., Bright, F., Trail, B., Blackburn, T. P. & Sanger, G. J. (1997) Neuropharmacology 36, 707-712. [DOI] [PubMed] [Google Scholar]
- 24.Chang, Y. F. & Gao, X. M. (1995) Neurochem. Res. 20, 931-937. [DOI] [PubMed] [Google Scholar]
- 25.Thiagarajan, A. B., Gleiter, C. H., Mefford, I. N., Eskay, R. L. & Nutt, D. J. (1989) Psychopharmacology 97, 548-552. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum, C., Wurst, S. & Hellhammer, D. (1992) Psychosom. Med. 54, 648-657. [DOI] [PubMed] [Google Scholar]
- 27.Klein, L. C. & Corwin, E. J. (2002) Curr. Psychiatr. Rep. 4, 441-448. [DOI] [PubMed] [Google Scholar]
- 28.Obled, C., Papet, I. & Breuille, D. (2002) Curr. Opin. Clin. Nutr. Metab. Care 5, 189-197. [DOI] [PubMed] [Google Scholar]