Abstract
Rab3 is a subfamily of the small GTP-binding protein Rab family and plays an important role in exocytosis. Several potential effectors of Rab3, including rabphilin3 and Rims (Rim1 and Rim2), have been isolated and characterized. Noc2 was identified originally in endocrine pancreas as a molecule homologous to rabphilin3, but its role in exocytosis is unclear. To clarify the physiological function of Noc2 directly, we have generated Noc2 knockout (Noc2-/-) mice. Glucose intolerance with impaired insulin secretion was induced in vivo by acute stress in Noc2-/- mice, but not in wild-type (Noc2+/+) mice. Ca2+-triggered insulin secretion from pancreatic isles of Noc2-/- mice was markedly impaired, but was completely restored by treatment with pertussis toxin, which inhibits inhibitory G protein Gi/o signaling. In addition, the inhibitory effect of clonidine, an α2-adrenoreceptor agonist, on insulin secretion was significantly greater in Noc2-/- islets than in Noc2+/+ islets. Impaired Ca2+-triggered insulin secretion was rescued by adenovirus gene transfer of wild-type Noc2 but not by that of mutant Noc2, which does not bind to Rab3. Accordingly, Noc2 positively regulates insulin secretion from endocrine pancreas by inhibiting Gi/o signaling, and the interaction of Noc2 and Rab3 is required for the effect. Interestingly, we also found a marked accumulation of secretory granules in various exocrine cells of Noc2-/- mice, especially in exocrine pancreas with no amylase response to stimuli. Thus, Noc2, a critical effector of Rab3, is essential in normal regulation of exocytosis in both endocrine and exocrine cells.
Regulated exocytosis is a key biological process in secretory cells, and has been extensively studied in neurons, in which neurotransmitters are released from synaptic vesicles (1, 2). Many nonneuronal cell types such as endocrine and exocrine cells contain secretory vesicles identified as dense-core granules, the contents of which exert a variety of biological effects (3). Secretory vesicle exocytosis occurs in the secretion of hormones in amine/peptide-containing endocrine cells (4, 5) and digestive enzymes in exocrine cells (6). Rab3 is a subfamily of the small GTP-binding protein Rab family (7), and plays an important role in targeting, docking, priming, and fusion processes in exocytosis (8). There are four isoforms (A–D) in the Rab3 family, all of which have been associated with regulated exocytosis (9–12). Several potential effectors of Rab3 also have been identified, including rabphilin3 (13), Rims (Rim1 and Rim2) (14, 15), granuphilin (16), and Noc2 (17). Rim1 and rabphilin3 are expressed predominantly in brain (13, 14), suggesting involvement in synaptic vesicle exocytosis. While studies of Rim1-deficient (Rim1-/-) mice and Caenorhabditis elegans suggest that Rim1 is involved in priming of synaptic vesicles (18–20), the role of rabphilin3 in synaptic vesicle exocytosis is not clear (21, 22). In neuroendocrine and endocrine cells, Rim2 (15), granuphilin (16), and Noc2 (17) are expressed predominantly, suggesting involvement in secretory granule exocytosis (3). We have shown previously that Rim2, interacting with cAMP-GEFII (Epac2) and Piccolo, is responsible for cAMP-dependent, protein kinase A-independent exocytosis of insulin granules (15, 23, 24). The physiological function of Noc2 in exocytosis, however, remains unclear. By overexpressing Noc2 in PC12 cells, we and another group found that Noc2 has positive (17) and negative (25) effects on Ca2+-triggered exocytosis, respectively. We have generated Noc2 knockout (Noc2-/-) mice to determine the physiological role of Noc2 directly.
Methods
Generation of Noc2-/- Mice. The targeting vector was constructed from a genomic fragment of Noc2 isolated from a 129Sv mouse genomic library (Fig. 1a) and was introduced into embryonic stem cell line R1. Three independent mouse lines were established from the independent embryonic stem cell clones under standardized methods. The mice were backcrossed to mouse strain C57BL6 at least over five generations. All animal procedures were approved by the Chiba University Animal Care Committee.
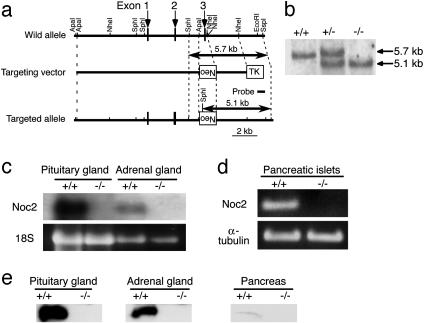
Fig. 1.
Generation of Noc2 knockout mice. (a) Schematic representation of the mouse Noc2 gene, targeting vector, and targeted allele. Exons are indicated by arrows. Neo and TK indicate a neomycin-resistant gene and a herpes simplex virus thymidine kinase gene, respectively. Restriction sites are indicated. The probe used for Southern blot analysis is shown. (b) Southern blot analysis of F2 offspring. Genomic DNA was digested with SphI and SspI and was hybridized with the probe. Lanes: +/+, wild-type; +/-, heterozygote; -/-, homozygote. (c) Northern blot analysis. Total RNA (15 μg) from the pituitary and adrenal glands of Noc2+/+ mice and Noc2-/- mice was used. (d) RT-PCR analysis of pancreatic islets of Noc2+/+ mice and Noc2-/- mice. Lanes: +/+, Noc2+/+; -/-, Noc2-/-. Noc2 transcript was not detected in Noc2-/- mice. (e) Western blot analysis. Homogenates of mouse pituitary gland, adrenal gland, and pancreas (20 μg) were subjected to SDS/PAGE and were immunoblotted with anti-Noc2 antibody [raised by immunizing rabbits with 17-mer peptide (QGGTPAQPEPRVPGKRH) corresponding to amino acid residues 279–295 of mouse Noc2]. Lanes: +/+, Noc2+/+; -/-, Noc2-/-.
RT-PCR Analysis. First-strand cDNAs were synthesized from total RNAs (10 μg) of isolated pancreatic islets. After reverse transcription, the cDNAs for Noc2, Rim2, granuphilin, rabphilin3, and α-tubulin were amplified by PCR using the following set of primers (from 5′ to 3′): Noc2 (GenBank accession no. AB158403), sense GCAGTGGAAATGATCAGTGG, antisense CATCACGTTCCTCTGCATTG; Rim2 (AB021131), sense AGTTCAGACCAGTCCGAGT, antisense TTAATCTGAGGCTCAGACCA; granuphilin (NM_013757), sense ACAGACTTTGGTCATCCATG, antisense GAGGACTCAGTACTGATCTT; rabphilin3 (AB158403), sense ACCCTGTGTGGAATGAGACA, antisense TCCTCCTCATAGAGAGCCAT; and α-tubulin (NM_009448), sense TGCCAATAACTATGCCCGTG, antisense TTGTCTACCATGAAGGCACA. The PCR products were separated in agarose gel (2%) by electrophoresis.
Animal Studies in Vivo. An oral glucose tolerance test was performed on male mice fasted for 16 h at 12–20 weeks of age. Water immersion stress experiment was performed as described (26), by using mice that were immobilized individually in a restraint holder and were vertically immersed in water at 20°C after glucose load. Blood glucose levels were measured in whole blood with Antisense Glucose II (Sankyo). Serum insulin levels were determined by and ultra-high sensitivity rat insulin ELISA kit (Morinaga, Yokohama, Japan).
Generation of Chimeric Mice. Chimeric mice were generated by aggregating the four cell-stage-fertilized eggs from Noc2-/- parents and the wild-type (Noc2+/+) eggs expressing GFP. The Noc2+/+ eggs expressing GFP were prepared by using male transgenic (homozygous for the transgene) mice expressing GFP (GFP-Tg) under the control of CAG promoter (27). Chimerism was determined by PCR or genomic Southern blot analysis.
Measurement of Insulin Secretion from Isolated Pancreatic Islets. Pancreatic islets were isolated by the collagenase digestion method as described (28). The isolated pancreatic islets were subsequently cultured for 48 h in RPMI medium 1640 in the presence or absence of pertussis toxin (PTX; 30 ng/ml). Batch incubation was performed as described (28). Insulin released into medium was measured by an RIA (Eiken Chemical, Tokyo). To test the effect of clonidine on insulin secretion, 0.1, 1, or 10 ng/ml clonidine (Sigma) was added to the preincubation and incubation buffer. Recombinant adenoviruses carrying LacZ, wild-type Noc2 (Noc2wt), or mutant Noc2 (Noc2AAA) cDNA were generated according to the manufacturer's instructions (Stratagene). The pancreatic islets of Noc2-/- mice were infected with these adenoviruses immediately after isolation for 48 h. Insulin secretion experiments were also performed by using freshly prepared pancreatic islets without culture.
Measurement of Amylase Secretion from Isolated Pancreatic Acinar Cells. Pancreatic acini were prepared by the collagenase digestion method. Amylase secretion experiments were performed according to Ohnishi et al. (29) with slight modifications. Briefly, isolated acini were suspended in incubation buffer as described (29), and were preincubated at 37°C for 30 min. After preincubation, the acini were centrifuged, resuspended in fresh incubation buffer, and incubated at 37°C in the presence or absence of 30 pM cholecystokinin (CCK) or 1 μM carbachol. Amylase released into the supernatant during incubation was quantified by using an amylase B test (Wako Pure Chemical, Osaka).
In Vitro Binding Assay. Full-length Noc2wt and Noc2AAA were expressed as GST-fusion proteins and were purified according to the manufacturer's instructions (Amersham Pharmacia). Full-length Rab3A, B, C, and D and Rab5 cDNAs were subcloned into pFLAG-CMV-2 (Sigma). For cosedimentation assays, COS-1 cells were transfected with each plasmid by using Lipofectamine (Invitrogen). After transfection, cells were sonicated in buffer [20 mM Hepes, pH 7.4/200 mM NaCl/1 mM DTT/5 mM MgCl2/1 mM ATP/0.26% (vol/vol) 3-[(3-cholamidopropy-l)dimethylammonio]-1-propanesulfonate]. In vitro binding assay was performed as described (15).
Histological Analysis. The pancreas and various portions of the gastrointestinal tract were removed from Noc2+/+ and Noc2-/- mice, and were immersion-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Fixed tissues were dehydrated and embedded in paraffin by conventional procedure. Five-micromole-thick paraffin sections were stained with hematoxylin/eosin, Azan, or periodic acid Schiff for secretory granules, and immunostained for pancreatic hormones. Small tissue pieces from the pancreas, stomach, and salivary glands were postfixed with 2.5% glutaraldehyde and 1% OsO4 and embedded in an epoxy resin. Semithin and ultrathin sections were stained with toluidine blue and uranyl acetate/lead citrate for observation under both a light microscope and electron microscope, respectively.
Results and Discussion
Genomic Southern blot of F2 offspring confirmed homologous recombination of the targeted allele (Fig. 1b). Absence of Noc2 expression in Noc2-/- mice was confirmed by Northern blot analysis, RT-PCR, or Western blot analysis (Fig. 1 c–e). Noc2-/- mice develop normally and are fertile, with no apparent abnormalities in general appearance or behavior. Because Noc2 is expressed at high levels in pancreatic islets (17), we first examined endocrine pancreatic function. Blood glucose and serum insulin levels after oral glucose load in Noc2+/+ and Noc2-/- mice are similar when small blood samples were obtained under normal conditions (Fig. 2a). However, when a larger amount of blood was withdrawn, we found incidentally that whereas Noc2+/+ mice exhibit both normal glucose levels and insulin response after glucose load, Noc2-/- mice exhibit significantly higher blood glucose levels with reduced insulin response compared with Noc2+/+ mice (data not shown). A large amount of blood loss is known to elicit various stress responses (30). To investigate the response of endocrine pancreas to stress in Noc2-/- mice, we measured blood glucose and insulin responses to water immersion stress (26). After oral glucose load, water immersion stress elicited a sustained rise in blood glucose levels and markedly reduced insulin secretion in Noc2-/- mice but not in Noc2+/+ mice (Fig. 2b), indicating that Noc2+/+ mice can maintain normal blood glucose levels in response to water immersion stress by enhancing insulin secretion, whereas Noc2-/- mice cannot. Because water immersion stress is known to trigger adrenergic response (26), and activation of inhibitory G protein Gi/o signaling through α2-adrenergic receptors in pancreatic β cells inhibits insulin secretion (31–33), the defective insulin response in vivo in Noc2-/- mice might be associated with activation of Gi/o signaling in pancreatic β cells. To investigate the mechanism underlying defective insulin secretion in Noc2-/- mice, we examined insulin secretion in isolated pancreatic islets. As shown in Fig. 2c, glucose-stimulated and Ca2+-triggered insulin secretion (assessed by high K+ stimulation) in pancreatic islets cultured for 48 h after isolation from Noc2-/- mice is decreased markedly. To determine whether the reduced insulin secretion in Noc2-/- mice is due to activation of Gi/o signaling, we examined the effect of PTX, which blocks Gi/o signaling, on glucose-stimulated and Ca2+-triggered insulin secretion (34). PTX treatment of pancreatic islets completely restored the reduced Ca2+-triggered insulin secretion in Noc2-/- mice, suggesting the involvement of Noc2 in Gi/o signaling in insulin secretion. The PTX treatment enhanced glucose-stimulated insulin secretion in both Noc2+/+ and Noc2-/- mice. Unlike high K+-stimulated insulin secretion, glucose-stimulated insulin secretion in Noc2-/- mice was not restored to the level of Noc2+/+ mice by PTX treatment. Different effects of PTX might occur under high K+ stimulation and glucose stimulation because high K+ stimulation elicits principally a Ca2+ signal, whereas glucose stimulation generates various intracellular signals (35), some of which could mediate Noc2-associated but PTX-insensitive insulin secretion. To ascertain that Noc2 participates in Gi/o signaling in Ca2+-triggered insulin secretion, we examined the inhibitory effect of clonidine, an agonist specific for an α2-adrenoreceptor that mediates Gi/o signaling, on high K+-stimulated insulin secretion. The inhibitory effect of clonidine on insulin secretion is significantly greater in Noc2-/- than in Noc2+/+ islets at all concentrations examined (Fig. 2d), indicating that Noc2 has a suppressive effect on Gi/o signaling in insulin secretion. In contrast to the results in cultured pancreatic islets, Ca2+-triggered insulin secretion in pancreatic islets freshly prepared from Noc2+/+ mice and Noc2-/- mice was similar (data not shown). The discrepancy between cultured islets and freshly prepared pancreatic islets suggests that Gi/o signaling is activated in Noc2-/- islets during culture, although the mechanism is unknown. These findings demonstrate that Noc2 functions in the maintenance of normal insulin secretion by inhibiting Gi/o signaling in pancreatic β cells. Disruption of Noc2 unmasks the Gi/o signal, thereby suppressing insulin secretion. Noc2, which is highly homologous to the N-terminal region of rabphilin3 (16), has been shown to bind Rab3A (25). There are four isoforms of Rab3 (Rab3A–D), all of which have been associated with regulated exocytosis (9–12). We find that Noc2 binds to all of the isoforms of Rab3 in a GTP-dependent manner, but does not bind to Rab5A (Fig. 3a), suggesting that Noc2 binds to members of the Rab3 family. In addition to Noc2, rabphilin3, Rim (Rim1 and Rim2), and granuphilin also are Rab3 effectors. We then examined to find whether these other Rab3 effectors might compensate for the lack of Noc2 expression in pancreatic islets. Neither Rim2 or granuphilin mRNA expression levels, as assessed by RT-PCR, were different in Noc2+/+ and Noc2-/- islets, and rabphilin3 was not detected in Noc2-/- islets (Fig. 3b), as was reported (17). This finding suggests that the other Rab3 effectors do not compensate for the lack of Noc2 expression in pancreatic islets, at least at the transcriptional level. To determine whether the effect of Noc2 in exocytosis requires Rab3, we generated adenovirus vectors carrying Noc2wt and Noc2AAA (25) that does not bind to any isoform of Rab3 (Fig. 3c), and infected pancreatic islets with these vectors. The defective Ca2+-triggered insulin secretion in cultured pancreatic islets of Noc2-/- mice is completely restored by Noc2wt gene transfer, whereas the mutant Noc2 has no effect (Fig. 3d), indicating that the effect of Noc2 on Ca2+-triggered insulin secretion requires interaction with Rab3. Rab3A-/- mice also have been shown to have a defect in insulin secretion (36), complementing the present findings and further suggesting the necessity of interaction between Noc2 and Rab3 in the maintenance of normal insulin secretion. The trimeric G protein signal that couples to Rab3-mediated exocytosis has not been identified, but our present results show that Gi/o signaling is closely associated with Noc2/Rab3 interaction in pancreatic β cells.
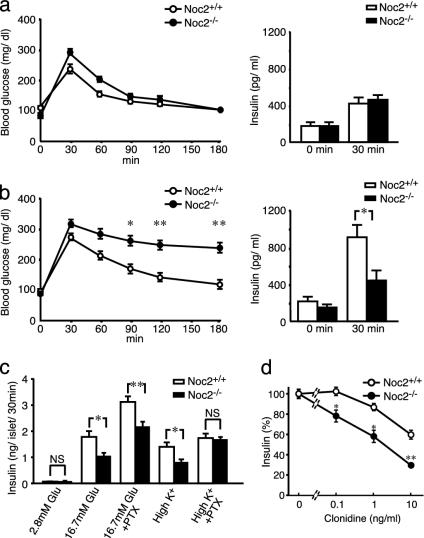
Fig. 2.
Blood glucose and insulin responses in vivo and insulin secretion in vitro. (a) Blood glucose levels (Left) and serum insulin levels (Right) after glucose load under normal conditions. There are no statistical differences in blood glucose and serum insulin levels between Noc2+/+ mice and Noc2-/- mice. (b) Blood glucose levels (Left) and serum insulin levels (Right) after glucose load under water immersion stress. Blood glucose levels at 90, 120, and 180 min after glucose load are significantly higher in Noc2-/- mice than in Noc2+/+ mice (n = 13 at each time point; *, P < 0.01 at 90 min; **, P < 0.001 at 120 and 180 min). Serum insulin levels at 30 min after glucose load are significantly lower in Noc2-/- mice than in Noc2+/+ mice (Noc2+/+ mice: 919.5 ± 123.8 pg/ml, n = 22; Noc2-/- mice: 451.7 ± 101.9 pg/ml, n = 22, *, P < 0.001). (c) Insulin secretion from isolated pancreatic islets. There is no difference in insulin secretion at 2.8 mM glucose (basal state). Glucose (16.7 mM)-stimulated insulin secretion in Noc2-/- mice is significantly lower than in Noc2+/+ mice (Noc2+/+ mice: 1.82 ± 0.21 ng per islet per 30 min n = 16; Noc2-/- mice: 1.06 ± 0.11 ng per islet for 30 min, n = 15). High K+ (60 mM)-stimulated insulin secretion of Noc2-/- mice is also significantly lower than in Noc2+/+ mice (Noc2+/+ mice: 1.43 ± 0.16 ng per islet for 30 min; Noc2-/- mice: 0.83 ± 0.10 ng per islet per 30 min, n = 12). However, there is no significant difference in high K+-stimulated insulin secretion between Noc2+/+ mice and Noc2-/- mice when pancreatic islets are treated with PTX (30 ng/ml, 48 h) after isolation. Open circles and columns, Noc2+/+ mice; filled circles and columns, Noc2-/- mice. Values are means ± SEM. *, P < 0.005; **, P < 0.001. (d) Inhibition of insulin secretion by clonidine. Results are expressed as means ± SEM of percent insulin secretion relative to that in the absence of clonidine. The inhibitory effect of clonidine on insulin secretion is significantly greater in Noc2-/- mice than in Noc2+/+ mice at all concentrations examined. *, P < 0.005; **, P < 0.0005.
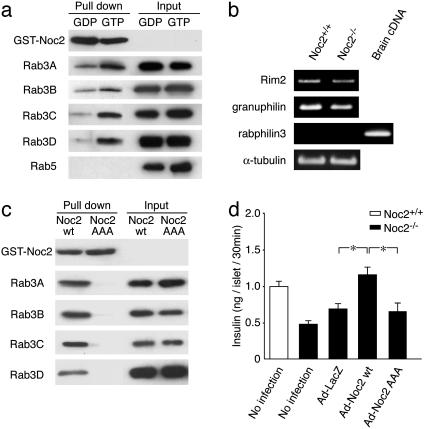
Fig. 3.
Interaction of Noc2 and Rab3, Rab3 effector expressions, and rescue of impaired insulin secretion in Noc2-/- by Noc2 transgene. (a) Interaction of Noc2 and various Rab3 isoforms. Lysate from COS-1 cells transfected with Flag-tagged Rab3 isoforms and Rab5 were evaluated for binding to GST-Noc2 in the presence of GDP-βS or GTP-γS. Rab3 isoforms and Rab5 and GST-Noc2 were detected by immunoblotting with anti-Flag antibody or IgG-purified antibody against rat Noc2. GST-Noc2 binds to all isoforms of Rab3 in the presence of GTP-γS, but does not bind to Rab5. (b) Rab3 effector mRNA expressions. For amplification of rabphilin3, brain cDNA was used as a positive control of PCR, because rabphilin3 is expressed in brain but not in pancreatic islets (41). α-tubulin, a housekeeping gene, was used as a positive control for cDNA synthesis from pancreatic islets. (c) Interaction of Noc2wt or Noc2AAA and GTP forms of various Rab3 isoforms. Noc2AAA was generated by replacing WFY (residues 154–156) with alanines, as described (25). Lysate from COS-1 cells transfected with Flag-tagged Rab3 isoforms was evaluated for binding to GST-Noc2wt or GST-Noc2AAA in the presence of GTP-γS. Mutant Noc2 does not bind to any of the isoforms of Rab3. (c) Rescue of impaired insulin secretion in Noc2-/- mice by Noc2 transgene. Pancreatic islets of Noc2-/- mice were infected with recombinant adenoviruses carrying LacZ, Noc2wt, or Noc2AAA cDNA at the same titers (multiplicity of infection, 100) for 48 h after isolation. Ad-LacZ, Ad-Noc2wt, and Ad-Noc2AAA indicate adenovirus carrying LacZ, Noc2wt, and Noc2AAA, respectively. Defective Ca2+-triggered insulin secretion in Noc2-/- mice is completely restored by Noc2wt gene transfer (Ad-LacZ, 0.69 ± 0.07 ng per islet per 30 min; Ad-Noc2wt, 1.20 ± 0.11 ng per islet for 30 min, n = 9; *, P < 0.01), but the mutant Noc2 has no effect (Ad-Noc2AAA: 0.65 ± 0.11 ng per islet per 30 min, n = 9). Open columns, Noc2+/+ mice; filled columns, Noc2-/- mice. Values are means ± SEM.
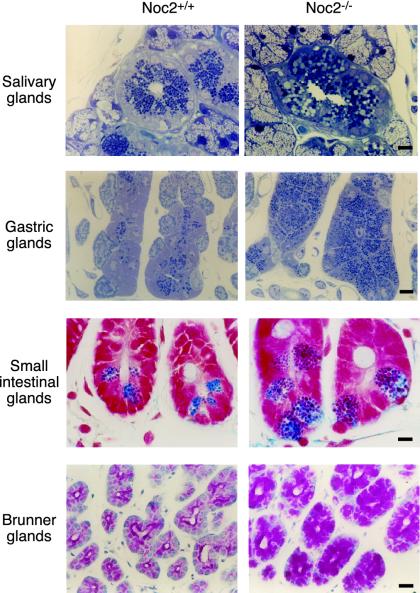
We then performed morphological analysis of the pancreatic islets of Noc2-/- mice. There are no apparent abnormalities in morphology of the pancreatic islets or the insulin secretory granules of Noc2-/- mice by immunohistochemistry for pancreatic hormones or electron microscopic analysis (Fig. 6, which is published as supporting information on the PNAS web site). In addition, insulin content in pancreatic islets is similar in Noc2+/+ and Noc2-/- mice (insulin content: Noc2+/+ mice, 53.5 ± 11.2 ng/mg protein, n = 10; Noc2-/- mice, 57.9 ± 7.5 ng/mg protein, n = 9). Interestingly, however, a striking abnormality appears in exocrine pancreas (Fig. 4a). Light and electron microscopic analyses show acinar cells in exocrine pancreas of Noc2-/- mice to be enlarged due to a remarkable accumulation of secretory granules (zymogen granules) throughout the cytoplasm. To evaluate exocrine pancreatic function, we examined in vitro secretion of amylase, a major secretory protein in zymogen granules (6). In contrast to Noc2+/+ mice, there was no amylase secretion (percent amylase release of total amylase content) from pancreatic acinar cells of Noc2-/- mice in response to either CCK or carbachol, both of which are known to be potent stimuli of amylase secretion. These results demonstrate that Noc2 is an essential molecule for regulated exocytosis of zymogen granules in exocrine pancreas. The acinar cells of Noc2-/- mice resemble those of mice lacking transcription factor NeuroD (NeuroD-/- mice) (37). In NeuroD-/- mice, the overabundance of zymogen granules in acinar cells is thought to be secondary to a developmental defect in CCK-secreting intestinal cells. To determine whether the overabundance of zymogen granules in pancreatic acinar cells of Noc2-/- mice is due to a primary defect in the acinar cells or to a secondary defect in the CCK-secreting intestinal cells, we generated chimeric mice between wild-type GFP-Tg mice and Noc2-/- mice by aggregating four cell-stage-fertilized eggs of mice. If disruption of Noc2 in pancreatic acinar cells is directly responsible for the morphological abnormality, exocrine pancreas of the chimeric mice should show a mosaic pattern of mixed populations of both GFP-positive acinar cells with normal appearance (originating from GFP-Tg) and GFP-negative acinar cells with an overabundance of zymogen granules (originating from Noc2-/- mice). Histological analysis of the chimeric mice shows a mosaic pattern (Fig. 3c), indicating that the overabundance of zymogen granules in acinar cells of Noc2-/- mice is due primarily to lack of Noc2. Rab3D has been shown to be expressed in pancreatic acinar cells (38). Overexpression of wild-type Rab3D and its dominant-negative form in pancreatic acinar cells has suggested that Rab3D regulates terminal steps of exocytosis of zymogen granules (12, 29). A study of Rab3D knockout mice has shown that Rab3D is not required for exocytosis of zymogen granules, but rather for the maintenance of granule maturation (39). However, the possibility cannot be ruled out that other Rab3 isoforms compensate for the Rab3D-deficient state. We reported previously, as assessed by short exposure (36 h) in autoradiography, that Noc2 mRNA is expressed predominantly in endocrine tissues, but we also found, as assessed by longer exposure (1 week), low level of expression in many tissues. Therefore, we examined histological changes in other exocrine tissues of Noc2-/- mice in which Rab3D is expressed (38), including salivary glands, in which acinar cells secrete amylase and various growth factors, gastric glands, in which chief cells secrete pepsinogen, and small intestinal glands, in which Paneth cells secrete antibacterial lysozymes. An accumulation of secretory granules of increased size and irregular shape is remarkable in all exocrine cells examined in Noc2-/- mice (Fig. 5). In contrast, there are no morphological changes in the secretory cells of Noc2-/- mice in which only constitutive exocytosis occurs, including surface mucous cells of stomach and goblet cells of duodenal epithelium (data not shown). These morphological data suggest that Noc2 is required in regulated exocytosis in the various types of exocrine cells expressing Rab3D.
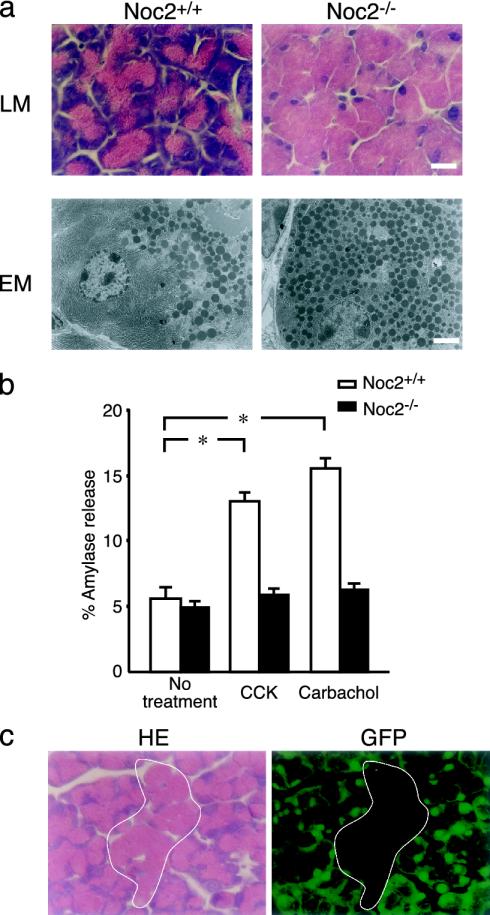
Fig. 4.
Histological and functional analysis of exocrine pancreas. (a) Histological analysis of exocrine pancreas. (Upper) Light microscopic (LM) analysis of acinar cells of exocrine pancreas stained with hematoxylin/eosin. (Lower) Electron microscopic (EM) analysis of acinar cells. Acinar cells in Noc2-/- mice are enlarged due to a remarkable accumulation of secretory granules throughout the cytoplasm. (Scale bars: 10 μmin Upper and 3 μmin Lower.) (b) Amylase secretion from exocrine pancreas in vitro. Amylase secretion was determined by the amount released into medium relative to total cellular content (expressed as percent amylase release) of pancreatic acinar cells. Open columns, Noc2+/+ mice; filled columns, Noc2-/- mice. Both CCK and carbachol stimulate amylase secretion in Noc2+/+ mice significantly, but neither stimulates amylase secretion in Noc2-/- mice [Noc2+/+ mice: 5.6 ± 0.9% (basal level), 13.0 ± 0.7% (CCK-stimulated amylase secretion), and 15.6 ± 0.8% (carbachol-stimulated amylase secretion), n = 12 for each; *, P < 0.0001, respectively; Noc2-/- mice: 4.9 ± 0.5% (basal level), 5.8 ± 0.5% (CCK-stimulated amylase secretion), and 6.2 ± 0.5% (carbachol-stimulated amylase secretion), n = 12]. Values are means ± SEM. (c) Histological analysis of exocrine pancreas of chimeric mice between GFP-Tg and Noc2-/- mice. Exocrine pancreas of the chimeric mice shows a mosaic pattern of mixed populations of GFP-positive acinar cells of normal appearance and GFP-negative acinar cells (circled by white line) having an overabundance of zymogen granules. GFP-positive and -negative cells originate from GFP-Tg and Noc2-/- mice, respectively. (Left)A section stained with hematoxylin/eosin. (Right) A section viewed under a fluorescent microscope. (Scale bar: 10 μm.)
Fig. 5.
Histological analysis of exocrine tissues. (Top) Salivary glands (toluidine blue staining). (Upper Middle) Gastric glands (toluidine blue staining). (Lower Middle) Small intestinal glands (Azan staining). (Bottom) Brunner glands in the duodenum (periodic acid Schiff staining). The accumulation of secretory granules with increased size and irregular shape is remarkable in acinar cells and granulated duct of submandibular glands, gastric chief cells, and Paneth cells of jejunum of Noc2-/- mice. Similar abnormalities also occur in Brunner glands of the duodenum, in which regulated secretion of alkaline mucous occurs. (Scale bars, 10 μm.)
It has been suggested that the mode of regulated exocytosis differs in exocrine and endocrine cells (40), so disruption of Noc2 could well result in distinct abnormalities of exocytosis in exocrine and endocrine cells. Determination of the physiological roles of Rab3 effectors is an important step toward clarification of the mechanism of Rab3-mediated exocytosis. Overexpression or microinjection of the N or C terminus of rabphilin3 has been shown to inhibit Ca2+-triggered exocytosis in different systems (21). However, a recent study (22) of rabphilin3 knockout mice reports that no abnormalities of synaptic transmission were found in these mice, suggesting that rabphilin3 is not required for Rab3A-mediated exocytosis in neurons. Studies of Rim1-/- mice suggest that Rim1, as a scaffolding protein, regulates neurotransmitter release by priming synaptic vesicles in mossy fibers (18–21). Noc2-/- mice exhibit abnormalities that clearly differ from those of knockout mice. Although Rab3 has been shown to participate in a late stage of regulated exocytosis, no intracellular signal that couples to Rab3-mediated exocytosis has been identified. The present study demonstrates that Noc2, interacting with Rab3, inhibits the Gi/o signaling that leads to suppression of Ca2+-triggered insulin secretion from endocrine pancreas, and that Noc2 is required for amylase secretion from exocrine pancreas. Accordingly, Noc2 is a critical molecule in normal regulation of exocytosis in both endocrine and exocrine cells.
Acknowledgments
We thank M. Okabe (Osaka University, Osaka) and T. Matozaki (Gunma University, Gunma, Japan) for providing GFP-Tg mice and for helpful suggestions in the amylase secretion experiments, respectively; and K. Kotake (Chiba University, Chiba, Japan) for involvement in the initial stage of generation of Noc2-/- mice. This work was supported by a Grant-in-Aid for Specially Promoted Research and Scientific Research Grants from the Ministry of Education, Science, Sports, Culture, and Technology.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PTX, pertussis toxin; CCK, cholecystokinin; GFP-Tg mice, Noc2+/+ mice expressing GFP.
References
- 1.Jahn, R. & Südhof, T. C. (1999) Annu. Rev. Biochem. 68, 863-911. [DOI] [PubMed] [Google Scholar]
- 2.Rettig, J. & Neher, E. (2002) Science 298, 781-785. [DOI] [PubMed] [Google Scholar]
- 3.Burgoyne, R. D. & Morgan, A. (2003) Physiol. Rev. 83, 581-632. [DOI] [PubMed] [Google Scholar]
- 4.Burgoyne, R. D. & Morgan, A. (1998) BioEssays 20, 328-335. [DOI] [PubMed] [Google Scholar]
- 5.Lang, J. (1999) Eur. J. Biochem. 259, 3-17. [DOI] [PubMed] [Google Scholar]
- 6.Williams, J. A. (2001) Annu. Rev. Physiol. 63, 77-97. [DOI] [PubMed] [Google Scholar]
- 7.Takai, Y., Sasaki, T. & Matozaki, T. (2001) Physiol Rev. 81, 153-208. [DOI] [PubMed] [Google Scholar]
- 8.Zerial, M. & McBride, H. (2001) Nat. Rev. Mol. Cell Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
- 9.Castillo, P. E., Janz, R., Südhof, T. C., Tzounopoulos, T., Malenka, R. C. & Nicoll, R. A. (1997) Nature 388, 590-593. [DOI] [PubMed] [Google Scholar]
- 10.Lledo, P. M., Vernier, P., Vincent, J. D., Mason, W. T. & Zorec, R. (1993) Nature 364, 540-544. [DOI] [PubMed] [Google Scholar]
- 11.Fischer von Mollard, G., Stahl, B., Khokhlatchev, A., Sudhof, T. C. & Jahn, R. (1994) J. Biol. Chem. 269, 10971-10974. [PubMed] [Google Scholar]
- 12.Chen, X., Edwards, J. A., Logsdon, C. D., Ernst, S. A. & Williams, J. A. (2002) J. Biol. Chem. 277, 18002-18009. [DOI] [PubMed] [Google Scholar]
- 13.Shirataki, H., Kaibuchi, K., Sakoda, T., Kishida, S., Yamaguchi, T., Wada, K., Miyazaki, M. & Takai, Y. (1993) Mol. Cell. Biol. 13, 2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, Y., Okamoto, M., Schmitz, F., Hofmann, K. & Sudhof, T. C. (1997) Nature 388, 593-598. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki, N., Shibasaki, T., Kashima, Y., Miki, T., Takahashi, K., Ueno, H., Sunaga, Y., Yano, H., Matsuura, Y., Iwanaga, T., et al. (2000) Nat. Cell Biol. 2, 805-811. [DOI] [PubMed] [Google Scholar]
- 16.Wang, J., Takeuchi, T., Yokota, H. & Izumi, T. (1999) J. Biol. Chem. 274, 28542-28548. [DOI] [PubMed] [Google Scholar]
- 17.Kotake, K., Ozaki, N., Mizuta, M., Sekiya, S., Inagaki, N. & Seino, S. (1997) J. Biol. Chem. 272, 29407-29410. [DOI] [PubMed] [Google Scholar]
- 18.Schoch, S., Castillo, P. E., Jo, T., Mukherjee, K., Geppert, M., Wang, Y., Schmitz, F., Malenka, R. C. & Sudhof, T. C. (2002) Nature 415, 321-326. [DOI] [PubMed] [Google Scholar]
- 19.Castillo, P. E., Schoch, S., Schmitz, F., Sudhof, T. C. & Malenka, R. C. (2002) Nature 415, 327-330. [DOI] [PubMed] [Google Scholar]
- 20.Koushika, S. P., Richmond, J. E., Hadwiger, G., Weimer, R. M., Jorgensen, E. M. & Nonet, M. L. (2001) Nat. Neurosci. 4, 997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns, M. E., Sasaki, T., Takai, Y. & Augustine, G. J. (1998) J. Gen. Physiol. 111, 243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schluter, O. M., Schnell, E., Verhage, M., Tzonopoulos, T., Nicoll, R. A., Janz, R., Malenka, R. C., Geppert, M. & Sudhof, T. C. (1999) J. Neurosci. 19, 5834-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashima, Y., Miki, T., Shibasaki, T., Ozaki, N., Miyazaki, M., Yano, H. & Seino, S. (2001) J. Biol. Chem. 276, 46046-46053. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto, K., Shibasaki, T., Yokoi, N., Kashima, Y., Matsumoto, M., Sasaki, T., Tajima, N., Iwanaga, T. & Seino, S. (2002) J. Biol. Chem. 277, 50497-50502. [DOI] [PubMed] [Google Scholar]
- 25.Haynes, L. P., Evans, G. J., Morgan, A. & Burgoyne, R. D. (2001) J. Biol. Chem. 276, 9726-9732. [DOI] [PubMed] [Google Scholar]
- 26.De Boer, S. F., Koopmans, S. J., Slangen, J. L. & Van der Gugten, J. (1990) Physiol. Behav. 47, 1117-1124. [DOI] [PubMed] [Google Scholar]
- 27.Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. (1997) FEBS Lett. 407, 313-319. [DOI] [PubMed] [Google Scholar]
- 28.Miki, T., Nagashima, K., Tashiro, F., Kotake, K., Yoshitomi, H., Tamamoto, A., Gonoi, T., Iwanaga, T., Miyazaki, J. & Seino, S. (1998) Proc. Natl. Acad. Sci. USA 95, 10402-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohnishi, H., Samuelson, L. C., Yule, D. I., Ernst, S. A. & Williams, J. A. (1997) J. Clin. Invest. 100, 3044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrasco, G. A. & Van de Kar, L. D. (2003) Eur. J. Pharmacol. 463, 235-272. [DOI] [PubMed] [Google Scholar]
- 31.Lang, J., Nishimoto, I., Okamoto, T., Regazzi, R., Kiraly, C., Weller, U. & Wollheim, C. B. (1995) EMBO J. 14, 3635-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renstrom, E., Ding, W. G., Bokvist, K. & Rorsman, P. (1996) Neuron 17, 513-522. [DOI] [PubMed] [Google Scholar]
- 33.Sharp, G. W. (1996) Am. J. Physiol. 271, 1781-1799. [Google Scholar]
- 34.Katada, T. & Ui, M. (1977) Endocrinology 101, 1247-1255. [DOI] [PubMed] [Google Scholar]
- 35.Henquin, J. C. (2000) Diabetes 49, 1751-1760. [DOI] [PubMed] [Google Scholar]
- 36.Yaekura, K., Julyan, R., Wicksteed, B. L., Hays, L. B., Alarcon, C., Sommers, S., Poitout, V., Baskin, D. G., Wang, Y., Philipson, L. H., et al. (2003) J. Biol. Chem. 278, 9715-9721. [DOI] [PubMed] [Google Scholar]
- 37.Naya, F. J., Huang, H. P., Qiu, Y., Mutoh, H., DeMayo, F. J., Leiter, A. B. & Tsai, M. J. (1997) Genes Dev. 11, 2323-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnishi, H., Ernst, S. A., Wys, N., McNiven, M. & Williams, J. A. (1996) Am. J. Physiol. 271, G531-G538. [DOI] [PubMed] [Google Scholar]
- 39.Riedel, D., Antonin, W., Fernandez-Chacon, R., Alvarez de Toledo, G., Jo, T., Geppert, M., Valentijn, J. A., Valentijn, K., Jamieson, J. D., Sudhof, T. C., et al. (2002) Mol. Cell. Biol. 22, 6487-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemoto, T., Kimura, R., Ito, K., Tachikawa, A., Miyashita, Y., Iino, M. & Kasai, H. (2001) Nat. Cell Biol. 3, 253-258. [DOI] [PubMed] [Google Scholar]
- 41.Inagaki, N., Mizuta, M. & Seino, S. (1994) J. Biochem. (Tokyo) 116, 239-242. [DOI] [PubMed] [Google Scholar]