Abstract
Mindfulness-based stress reduction (MBSR) is an established program shown to reduce symptoms of stress, anxiety, and depression. MBSR is believed to alter emotional responding by modifying cognitive–affective processes. Given that social anxiety disorder (SAD) is characterized by emotional and attentional biases as well as distorted negative self-beliefs, we examined MBSR-related changes in the brain-behavior indices of emotional reactivity and regulation of negative self-beliefs in patients with SAD. Sixteen patients underwent functional MRI while reacting to negative self-beliefs and while regulating negative emotions using 2 types of attention deployment emotion regulation—breath-focused attention and distraction-focused attention. Post-MBSR, 14 patients completed neuroimaging assessments. Compared with baseline, MBSR completers showed improvement in anxiety and depression symptoms and self-esteem. During the breath-focused attention task (but not the distraction-focused attention task), they also showed (a) decreased negative emotion experience, (b) reduced amygdala activity, and (c) increased activity in brain regions implicated in attentional deployment. MBSR training in patients with SAD may reduce emotional reactivity while enhancing emotion regulation. These changes might facilitate reduction in SAD-related avoidance behaviors, clinical symptoms, and automatic emotional reactivity to negative self-beliefs in adults with SAD.
Keywords: social anxiety, neuroimaging, mindfulness, attention, emotion
The concept of mindfulness has attracted attention in the domains of basic emotion research, clinical science, and social–cognitive–affective neuroscience. The most studied form of mindfulness training in the United States is mindfulness-based stress reduction (MBSR), a structured group program of mindfulness training developed by Kabat-Zinn (1990). There is also increasing interest in mindfulness-based exercises in the context of clinical interventions for anxiety and depression disorders, as well as other clinical problems (Allen, Chambers, & Knight, 2006; Carmody, 2009).
At this stage in the field’s development, we believe it is useful to apply Western psychological models of cognitive–affective processes to the study of mindfulness in order to clarify how mindfulness training works (Carmody, 2009). More specifically, we suggest that an emotion regulation framework (Gross, 2007) may help clarify the processes that underlie MBSR, processes that may be distinct from those implicated in other more traditional modalities such as cognitive–behavioral therapy (Hofmann & Asmundson, 2008).
Mindfulness-Based Stress Reduction
MBSR consists of multiple forms of mindfulness practice, including formal and informal meditation practice, as well as hatha yoga (Kabat-Zinn, 1990). The formal practice consists of breath-focused attention, body scan-based attention to the transient nature of sensory experience, shifting attention across sensory modalities, open monitoring of moment-to-moment experience, walking meditation, and eating meditation. Informal practice entails brief pauses involving volitionally shifting attention to present moment awareness. Together, this package of mindfulness practices aims to enhance the ability to observe the immediate content of experience, specifically, the transient nature of thoughts, emotion, memories, mental images, and physical sensation.
Two specific forms of nonelaborative, nonconceptual attention-focusing meditations that are introduced in MBSR are (a) focused attention defined as object-based (e.g., sensations induced during breathing) volitional selective attention in the present moment with ongoing assessment of the quality of attention, and (b) open monitoring defined as settling attention into a state of mere observation or monitoring in the present moment on any experience (thought, emotion, physical sensation) without any explicit focus on an object (Lutz, Slagter, Dunne, & Davidson, 2008).
Although there is no explicit instruction in changing the nature of thinking, or emotional reactivity, MBSR has been shown to diminish the habitual tendency to emotionally react to and ruminate about transitory thoughts and physical sensations (Ramel, Goldin, Carmona, & McQuaid, 2004; Teasdale et al., 2000); reduce stress, depression, and anxiety symptoms (Chiesa & Serretti, 2009; Evans et al., 2008; Segal, Williams, & Teasdale, 2002); modify distorted patterns of self-view (Goldin, Ramel, & Gross, 2009); amplify immune functioning (Davidson et al., 2003); enhance behavioral self-regulation (Lykins & Baer, 2009); and improve volitional orienting of attention (Jha, Krompinger, & Baime, 2007). Recent functional neuroimaging studies of MBSR have provided evidence of reduced narrative and conceptual and increased experiential and sensory self-focus at post-MBSR (Farb et al., 2007) and decreased conceptual–linguistic self-referential processing from pre- to post-MBSR (Goldin, Ramel, et al., 2009).
MBSR and Emotion Regulation
Theorists have suggested that MBSR may reduce symptoms of stress, anxiety, and depression by modifying emotion regulation abilities, but it is not yet clear which specific abilities may be enhanced by MBSR (Chambers, Gullone, & Allen, 2009). This is because emotion regulation refers to a variety of strategies that can be implemented at different points during the emotion-generative process to influence which emotions arise, when and how long they occur, and how these emotions are experienced and expressed (Gross, 2007). Distinct forms of emotion regulation have their own neural circuitry and temporal features (Goldin, McRae, Ramel, & Gross, 2008).
The process model of emotion regulation (Gross, 1998) proposes five families of emotion regulation strategies, including situation selection, situation modification, attentional deployment, cognitive change, and response modulation. There is evidence that MBSR and long-term mindfulness meditation practice may directly influence attentional deployment, specifically the ability to exert cognitive control of negative rumination (Ramel et al., 2004), self-focused attention (Goldin, Ramel, et al., 2009), attention allocation and regulation (Slagter, Lutz, Greischar, Nieuwenhuis, & Davidson, 2008), and orienting to a spatial cue (Jha et al., 2007). Lutz et al. (2008) have proposed that such training of attention is expected to result in “improvement in the capacity to disengage from aversive emotional stimuli … enabling greater emotional flexibility” (p. 4). However, the proposed effects of MBSR on emotional reactivity and attentional deployment require empirical investigation.
MBSR, Emotion Regulation, and Social Anxiety Disorder
One clinical context in which MBSR’s effects of emotion regulation might be investigated is social anxiety disorder (SAD). SAD is a very common psychiatric condition that is characterized by intense fear of evaluation in social or performance situations (Jefferys, 1997). Patients with SAD have a strong tendency to focus on both internal cues (e.g., negative thoughts and self-imagery) and external cues (e.g., other’s facial expressions) during social situations (Schultz & Heimberg, 2008). This attentional focus serves to maintain social anxiety symptoms by interfering with habituation processes that lead to corrective learning in vivo and during cognitive-behavioral therapy (Heimberg & Becker, 2002).
Recent electrophysiological studies have demonstrated that adults with SAD demonstrate abnormal attentional processes consisting of early hypervigilance followed by attentional avoidance (i.e., reduced visual processing) of social threat stimuli (Mueller et al., 2008). Studies have shown that adults with SAD show diminished recruitment of brain networks implicated in cognitive regulation (dorsolateral prefrontal cortex [PFC], dorsal anterior cingulate cortex) and in attention regulation (posterior cingulate/precuneus, inferior parietal lobe, supramarginal gyrus) during cognitive reappraisal of emotional reactivity to social threat (Goldin, Manber, Hakimi, Canli, & Gross, 2009) and to negative self-beliefs (Goldin, Manber Ball, Werner, Heimberg, & Gross, 2009).
Two studies have examined the impact of MBSR on SAD. One study found equivalent improvement in patients with generalized SAD on mood, functionality, and quality of life with either 8-week MBSR or 12-week cognitive–behavioral group therapy (CBGT), but significantly lower scores on clinician- and patient-rated measures of social anxiety for CBGT compared with the MBSR group (Koszycki, Benger, Shlik, & Bradwejn, 2007). A recent study of MBSR for adults with generalized SAD showed reduced anxiety, negative self-view, and conceptual-linguistic self-referential processing along with increased self-esteem and positive self-view (Goldin, Ramel, et al., 2009). However, little is yet known about how MBSR influences the neural bases of emotional reactivity and emotion regulation, particularly when someone with SAD is challenged with social anxiety-related negative self-beliefs, which are a core feature of SAD.
The Present Study
To investigate MBSR-related changes in emotion reactivity and regulation of negative self-beliefs in patients with SAD, we assessed clinical symptoms and obtained behavioral and neural measures of emotional reactivity and regulation at baseline and post-MBSR. Clinically, we expected MBSR-related changes, including reduced symptoms of anxiety and depression and enhanced self-esteem in patients with SAD. In the emotion regulation task, we examined two forms of attention deployment: breath-focused attention (the target regulation strategy) and distraction-focused attention (a control regulation strategy). We expected MBSR-related changes in relation to the breath-focused mindful attention, including (a) decreased negative emotion after implementing breath-focused attention, (b) decreased brain activity in emotion-related limbic activity (i.e., amygdala), and (c) increased activity in attention-related brain regions, but (d) no change related to distraction-based attention.
Method
Participants
Sixteen right-handed adult patients (nine women) diagnosed with primary generalized SAD met DSM-IV criteria based on the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV; DiNardo, Brown, & Barlow, 1994). Based on the interview, past comorbid conditions included two patients with obsessive–compulsive disorder, three with dysthymia, and four with major depressive disorder; current conditions included three with generalized anxiety disorder, three with specific phobia, and one with panic disorder without agoraphobia. Patients were on average middle age (M = 35.2 years, SD = 11.9), college educated (M = 16.3 years of education, SD = 3.5), and diverse in race: eight Anglo Americans, five Asian Americans, two Latino Americans, and one Native American. Patients provided informed consent in accordance with Stanford University’s Human Subjects Committee guidelines for ethical research. Two patients (one men and one women) declined the post-MBSR magnetic resonance (MR) assessment because of distress about scanning.
Inclusion and Exclusion Criteria
To be eligible for the study, patients had to pass a MR scanning safety screen on three occasions, as well as not report current use of psychotropic medication, prior meditation training, history of neurological or cardiovascular disorders, or met diagnostic criteria for current Axis I psychiatric disorders other than social anxiety, generalized anxiety, agoraphobia, or specific phobia disorders.
Clinical Assessment
In addition to the clinical diagnostic interview (ADIS-IV), self-report inventories were used to assess social anxiety (Liebowitz Social Anxiety Scale; Liebowitz, 1987), depression (Beck Depression Inventory—II; Beck, Steer, & Brown, 1996), rumination (Rumination Style Questionnaire; Nolen-Hoeksema, 1991), state anxiety (Spielberger State–Trait Anxiety Inventory; Spielberger, Gorsuch, & Lushene, 1970), and self-esteem (Rosenberg Self-Esteem Scale; Rosenberg, 1965).
Procedure
Recruitment strategies consisted of electronic bulletin-board listings and referrals from mental health clinics. Patients completed a phone screen to establish initial eligibility for the study. Next, patients were administered a structured clinical diagnostic interview in the laboratory. After meeting MR scanning and diagnostic criteria, eligible patients completed online questionnaires and a brain imaging session within the following week. At the scanning session, patients were introduced to the emotion regulation task and given two practice trials with negative self-beliefs not used during the fMRI experiment. Participants attended MBSR for 2 months and then returned to the laboratory to complete all assessments again.
Mindfulness-Based Stress Reduction
The standard MBSR protocol developed by Kabat-Zinn (1990) was delivered in an academic setting. MBSR consisted of a 2.5-hr once-weekly small-group (eight members in a group) format for eight sessions plus one half-day meditation retreat. Participants were given meditation CDs created by Kabat-Zinn to support home formal practice. Participants were instructed to complete a self-report daily monitoring form each evening to record both formal and informal meditation practices. Participants attended most MBSR classes (M = 7.07, SD = 0.83) and completed a moderate amount of weekly hours of home meditation practice (M = 2.26 hr, SD = 0.55) separate from the 2.5-hr weekly class. MBSR was delivered by a member of the team (PG) who, while not being board certified in the Western tradition of MBSR, lived and studied in Buddhist monasteries in Nepal and India for 6 years prior to returning to the United States and being trained in and leading MBSR courses in medical and academic settings for 10 years.
Regulation of Negative Self-Beliefs Task
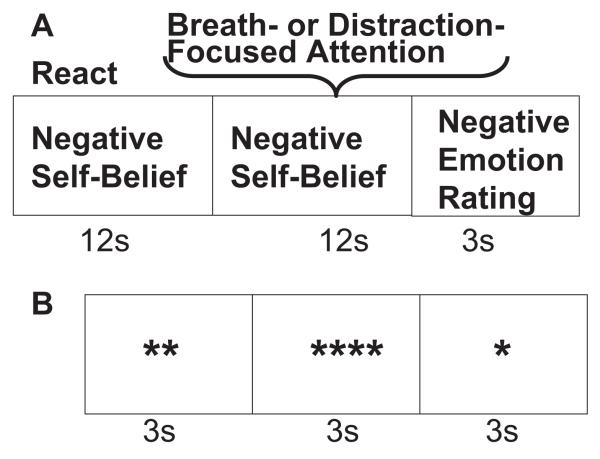
The regulation task consisted of 18 experimenter-selected social anxiety-related negative self-beliefs that refer to self-focused, self-critical personal beliefs (e.g., “I am ashamed of my shyness,” “People always judge me”). Each trial consisted of reacting to a negative self-belief for 12 s, implementation of attention regulation based on a cue to either “Shift attention to the breath” (breath-focused attention; nine trials) or “Count backward from 168” (distraction-focused attention; nine trials) for 12 s (see Figure 1). The two attention regulation conditions were presented in a fixed pseudorandom sequence. After implementing breath-focused attention or distraction-focused attention, participants provided a negative emotion rating (How negative do you feel right now? 1 = not at all, 2 = slight, 3 = moderate, 4 = very much) for 3 s. Ratings were recorded using Eprime software (Version 2; Psychology Software Tools, Inc., Pittsburgh, PA) with a button response pad positioned in the participant’s right hand (3 s).
Figure 1.
Structure for breath- and distraction-focused attention trials and asterisk counting trials. (A) Reacting to a negative self-belief followed by a cue to implement breath-focused or distraction-focused attention regulation while the same negative self-belief remains on the screen. (B) A single block of asterisk counting.
The comparison baseline condition consisted of identifying the number of asterisks on the screen every 3 s (range: 1–5 asterisks) and making a button press to indicate the number of asterisks on the screen at any given time. There were six 9-s blocks of asterisk counting randomly inserted throughout the experiment. Prior to scanning, participants were trained on the regulation task with four negative self-beliefs not used in the experiment. They were instructed to read repeatedly a single negative self-belief presented in white against a black background on a screen mounted on the head coil inside the scanner. When a cue appeared above the belief, participants shifted attention to the physical sensation of their own inhalation and exhalation (breath-focused attention) or began subtracting by ones from a three-digit number projected above the statement (distraction-focused attention). The regulation task was 9 min 12 s (368 time points × 1.5 s = 552 s) in duration.
Image Acquisition
A General Electric 3 Tesla Signa magnet was used to acquire anatomical and functional images. We used a custom-built quadrature “dome” elliptical bird cage head coil and a T2*-weighted gradient echo spiral-in/out pulse sequence to obtain blood oxygenation level-dependent (BOLD) contrast (Glover & Law, 2001). A wax bite bar, padding, and plungers were used to reduce head movement. A single functional run was used to acquire 368 volumes consisting of 22 sequential axial slices each. Scanning parameters also included TR = 1,500 ms, TE = 30 ms, flip angle = 60, field of view = 22 cm, frequency encoding = 64, single shot, voxel resolution = 3.44 mm2 in-plane and 5 mm through-plane. A fast spin-echo spoiled-grass pulse sequence was used to obtain a high-resolution anatomical image (voxel resolution = .862 × 1.2 mm; field of view = 22 cm, frequency encoding = 256).
fMRI Data Preprocessing
BOLD signal preprocessing and statistical analysis was conducted with Analysis of Functional NeuroImages: AFNI version 2008_07_18_1710, Nov 18 2008 (Cox, 1996). Visual and computational examination of each volume yielded no signal artifacts or movement outliers greater than 1.0 mm motion correction in the x, y, or z directions. Thus, no scans were omitted. The first 6 s of images obtained while the magnet field was gaining stabilization were eliminated. Volume registration, realignment, and calculation of six motion parameters (three translations and three rotations) were conducted on an empirically determined optimal base image derived from an automated recursive analysis of the root-mean-square adjustment for motion correction at each time point. There was no evidence of stimulus-correlated motion for any of the task conditions. A high-pass temporal filter (0.011 Hz) was used to remove low-frequency oscillations in the BOLD signal time series in each voxel. BOLD signal was converted to percentage signal deviation from the mean signal per voxel.
fMRI Statistical Analysis
The AFNI 3dDeconvolve program was used to implement a single multiple regression model that included baseline parameters to remove nuisance variance in each voxel’s time series related to mean, linear, and quadratic drifts and the six motion correction parameters. Reference vectors for each condition (asterisk counting, react negative self-beliefs, breath-focused attention, distraction-focused attention) were convolved with a gamma variate model (Cohen, 1997) of the hemodynamic response function to account for the hemodynamic delay to peak BOLD responses. Resultant statistical parametric maps were then subjected to spatial smoothing with a 4-mm3 isotropic Gaussian kernel to enhance signal-to-noise. The through-plane dimension of each voxel was resampled to 3.438 mm to create isotropic voxels. Maps were transformed to the standard Talairach space (Talairach & Tournoux, 1988). Second-level t tests were conducted according to a random-effects model. Neural results are reported for the contrast of react negative self-belief versus asterisk counting, breath-focused attention regulation versus react negative self-belief, and distraction-focused attention versus react negative self-belief.
Correction for the multiple comparisons obtained in fMRI data analysis was applied. The AFNI AlphaSim program, a Monte Carlo simulation bootstrapping procedure, was employed to identify a joint-probability threshold consisting of a voxel-wise threshold of p < .005 and minimum cluster-volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3) that resulted in protection against false positive cluster detection at p < .01 in the whole-brain analyses.
Results
Clinical Results
Paired t tests showed that from baseline to post-MBSR patients had decreased social anxiety, depression, rumination, and state anxiety, as well as increased self-esteem (see Table 1). There were no missing self-report responses.
Table 1. Clinical Measures.
| Dependent variable |
Baseline M ± SD |
Post–MBSR M ± SD |
t(15) | |
|---|---|---|---|---|
| Participants, n | 16 | 14 | ||
| LSAS | 68.7 ± 21.2 | 49.3 ± 17.0 | 4.3*** | .59 |
| BDI-II | 8.7 ± 9.1 | 3.4 ± 3.2 | 2.2* | .27 |
| RSQ | 26.4 ± 6.5 | 19.3 ± 95.7 | 3.8** | .53 |
| STAI-State | 41.5 ± 9.3 | 29.6 ± 6.4 | 8.4*** | .84 |
| RSE | 22.7 ± 4.6 | 27.2 ± 4.7 | 3.7* | .51 |
Note. LSAS = Liebowitz Social Anxiety Inventory; BDI–II = Beck Depression Inventory—II; RSQ = Rumination Style Questionnaire; STAI = Spielberg State–Trait Anxiety Inventory; RSE = Rosenberg Self-Esteem Scale.
p < .05.
p < .01.
p < .001.
Behavioral Results
Baseline
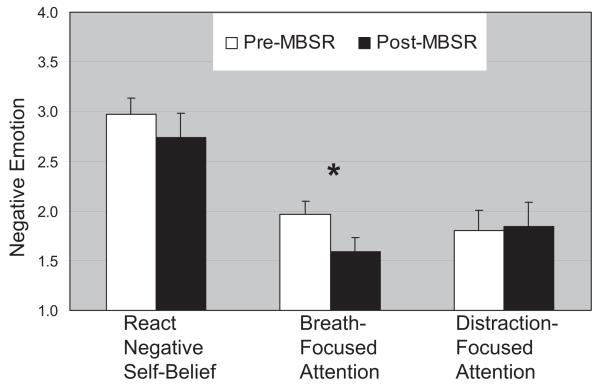
Compared with reacting to negative self-beliefs, both breath-focused attention, t(15) = 6.28, p < .001, and distraction-focused attention, t(15) = 7.48, p < .001, resulted in reduced negative emotion (see Figure 2). Negative emotion did not differ between breath- and distraction-focused attention regulation (p > .11).
Figure 2.
Negative emotion experience ratings pre- and post-mindfulness-based stress reduction (MBSR). The negative emotion ratings (How negative? 1 = not at all, 2 = slight, 3 = moderate, 4 = very much) when reacting to negative self-beliefs and when regulating using breath-focused attention and distraction-focused attention during the fMRI experimental task pre- and again post-MBSR. Ratings for reacting to negative self-beliefs were collected post-fMRI. * p < .01. Error bars represent standard error of the mean.
MBSR-related changes
Paired t tests showed that from pre- to post-MBSR patients had no changes in self-reported negative emotion when reacting to negative self-beliefs, t(14) = 1.21, p > .31, and when using distraction-focused attention, t(14) = 0.41, p > .71. However, there was a reduction in negative emotion when implementing breath-focused attention from pre- to post-MBSR, t(14) = 3.25, p < .01.
Neural Results
Baseline
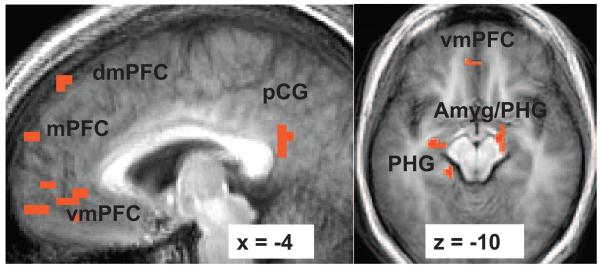
At baseline, a one-sample t test for the contrast of react negative self-belief versus asterisk counting yielded greater BOLD responses in brain regions implicated in self-referential processing (ventromedial and dorsomedial PFC and posterior cingulate/precuneus), emotion (right dorsal amygdala), dorsal and ventral visual processing (bilateral middle and inferior temporal lobes, cuneus, precuneus, angular gyrus, lingual gyrus, inferior and superior parietal cortex), and memory (bilateral parahippocampal gyrus). Greater BOLD responses for asterisk counting versus react negative self-belief included posterior cingulate and lingual gyrus (see Figure 3 and Table 2).
Figure 3.
Greater blood oxygenation level-dependent (BOLD) contrast responses at baseline for the contrast of reacting to negative self-beliefs versus asterisk counting. Thresholded at t ≥ 3.69, voxel p < .005, cluster volume ≥ 163 mm3, cluster p < .01. dmPFC = dorsomedial prefrontal cortex; mPFC = medial prefrontal cortex; vmPFC = ventromedial prefrontal cortex; pCG = posterior cingulate gyrus; Amyg = amygdala; PHG = parahippocampal gyrus. x refers to the location of the sagittal slice (−= left; + = right). z refers to the location of the axial slice (−= inferior; + = superior).
Table 2.
Baseline BOLD Responses for React to Negative Self-Beliefs Versus Asterisk Counting
| Brain region | Talairach coordinates x y z |
% Signal change |
Vol (mm3) | t |
|---|---|---|---|---|
| React > Asterisk | ||||
| Frontal cortex | ||||
| Medial PFC | −6 67 28 | .48 | 610 | 3.80 |
| Medial PFC | −3 59 25 | .53 | 163 | 5.25 |
| Dorsomedial PFC | 0 45 56 | .34 | 326 | 4.84 |
| Ventromedial PFC | −7 52 1 | .83 | 1,221 | 3.77 |
| Ventromedial PFC | −3 62 −5 | .71 | 244 | 5.39 |
| L superior frontal gyrus | −31 28 53 | .28 | 1,018 | 5.37 |
| L middle frontal gyrus | − 31 11 63 | .81 | 977 | 5.70 |
| R precental gyrus | 48 − 10 56 | .24 | 610 | 3.76 |
| Temporal cortex | ||||
| R middle temporal gyrus | 58 − 61 5 | .58 | 326 | 4.02 |
| R middle temporal gyrus | 55 −68 19 | .36 | 163 | 3.72 |
| R inferior temporal lobe | 45 − 3 − 30 | .21 | 204 | 3.78 |
| L inferior temporal lobe | −58 −55 −5 | .27 | 163 | 4.19 |
| R parahippocampal gyrus | 14 −10 −23 | .50 | 204 | 4.88 |
| L parahippocampal gyrus | −21 −17 −12 | .28 | 570 | 4.33 |
| L parahippocampal gyrus | −17 −27 −12 | .48 | 488 | 4.26 |
| Parietal cortex | ||||
| Posterior cingulate | −3 −48 22 | .67 | 326 | 4.11 |
| R inferior parietal lobule | 65 − 41 25 | .55 | 204 | 4.66 |
| L superior parietal lobule | −31 −72 50 | .64 | 244 | 3.59 |
| Occipital cortex | ||||
| L superior occipital gyrus | −38 −79 36 | .49 | 15,413 | 4.42 |
| R precuneus | 45 − 72 36 | .36 | 488 | 3.70 |
| R angular gyrus | 52 − 68 29 | .66 | 366 | 4.87 |
| R lingual gyrus | 10 −96 −5 | .92 | 285 | 3.77 |
| R cuneus | 28 −65 12 | .84 | 285 | 3.58 |
| Subcortical | ||||
| R amygdala/parahippocampal gyrus | 14 −17 −16 | .39 | 1,180 | 4.14 |
| Culmen | −3 −37 −23 | .28 | 163 | 4.97 |
|
| ||||
| Asterisk > React | ||||
| R posterior cingulate cortex | 14 −55 8 | .65 | 285 | 7.69 |
| L lingual gyrus | −10 −72 −2 | .75 | 204 | 4.18 |
| Posterior cingulate cortex | 3 −65 12 | .73 | 204 | 4.32 |
Note. ACC = anterior cingulate cortex; BOLD = blood oxygen dependent; L = left; MBSR = mindfulness-based stress reduction; PFC = prefrontal cortex; R = right; Vol = volume. t-value threshold ≥ 3.69, df = 13, voxel p < .005, minimum cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), cluster p < .01.
MBSR-related changes
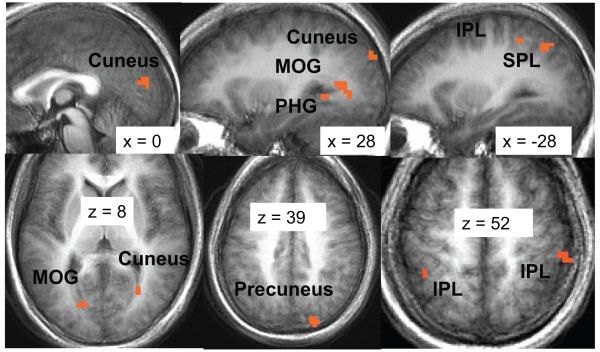
From pre- to post-MBSR, there were no results for the contrast of distraction-focused attention versus react negative self-beliefs. For breath-focused attention versus react negative self-belief, however, there were greater BOLD responses at post-MBSR versus pre-MBSR in brain regions implicated in visual attention (inferior and superior parietal lobule, cuneus, precuneus, middle occipital gyrus), as well as parahippocampal gyrus. There were no areas of brain activity greater for react versus breath-focused attention (see Figure 4 and Table 3).
Figure 4.
Greater blood oxygenation level-dependent (BOLD) contrast responses for post-versus pre-mindfulness-based stress reduction (MBSR) for the contrast of breath-focused attention versus reacting to negative self-beliefs. Thresholded at t ≥ 3.21, voxel p < .005, cluster volume ≥ 163 mm3, cluster p < .01. MOG = middle occipital gyrus; PHG = parahippocampal gyrus; IPL = inferior parietal lobule; SPL = superior parietal lobule. x refers to the location of the sagittal slice (−= left; + = right). z refers to the location of the axial slice (−= inferior; + = superior).
Table 3. Changes in BOLD Responses From Pre- to Post-MBSR for Breath-Focused Attention Versus React Negative Self-Beliefs.
| Brain region | Talairach coordinates x y z |
% Signal change |
Vol (mm3) | t |
|---|---|---|---|---|
| Post-MBSR > Baseline | ||||
| Breath > React | ||||
| Temporal cortex | ||||
| R parahippocampal gyrus 7 | 28 −51 1 | .41 | 163 | 3.36 |
| Parietal cortex | ||||
| L inferior parietal lobule 3 | −38 −44 56 | .40 | 651 | 3.78 |
| R inferior parietal lobule 11 | 55 −34 53 | .66 | 163 | 3.32 |
| L superior parietal lobule 4 | −28 −65 46 | .61 | 366 | 3.45 |
| Precentral gyrus 5 | −31 −24 70 | .32 | 366 | 3.77 |
| Occipital cortex | ||||
| Medial cuneus 1 | 0 −79 22 | .37 | 2,646 | 4.01 |
| L cuneus 8 | −17 −75 8 | .67 | 163 | 4.29 |
| R cuneus 10 | 28 −85 32 | .26 | 163 | 3.51 |
| R middle occipital gyrus 2 | 28 − 61 8 | .64 | 1,017 | 3.22 |
| R precuneus 6 | 17 −85 39 | .39 | 326 | 3.48 |
| R precuneus 9 | 14 −68 36 | .37 | 163 | 3.39 |
|
| ||||
| React > Breath | ||||
| None | ||||
Note. BOLD = blood oxygen dependent; L = left; MBSR = mindfulness-based stress reduction; PFC = prefrontal cortex; Vol = volume. t-value threshold ≥ 3.21, df = 13, voxel p < .005, minimum cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), cluster p < .01.
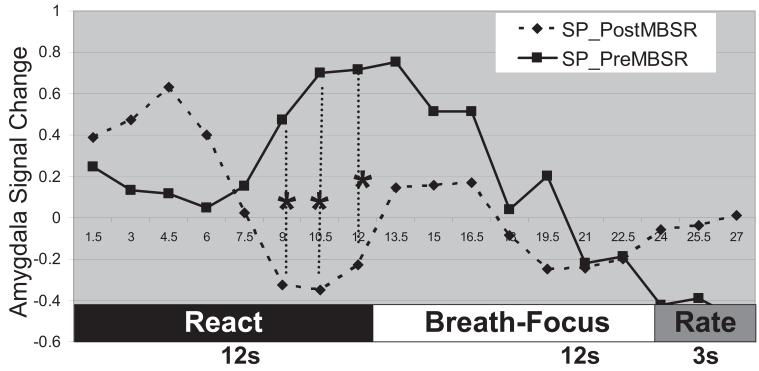
To better understand how MBSR training influences the effect of breath-focused attention on a neural index of emotional reactivity to negative self-beliefs, we investigated the BOLD signal time series of the right dorsal amygdala activation observed at baseline in response to react negative self-beliefs (see Figure 5). At baseline, there was a delay of approximately 6 s before amygdala activity began to ramp up toward a peak response at the end of the 12-s react negative self-belief component of the block. Compared with pre-MBSR, at post-MBSR patients with SAD demonstrated a significant decrease of right amygdala response prior to the cue to shift attentional focus to breath sensation.
Figure 5.
Right dorsal amygdala blood oxygenation level-dependent (BOLD) contrast signal time series during reacting to negative self-beliefs and breath-focused attention in social phobics (SP) at both pre- and post-mindfulness-based stress reduction (MBSR). * p < .05. Rate = negative emotion rating; React = reacting to the negative self-belief; Breath-Focus = instruction to focus attention on breath sensation.
Relations Between Clinical and Neural Domains
We examined whether MBSR-related changes in social anxiety symptom severity were associated with MBSR-related changes in BOLD responses for the breath-focused attention versus react negative self-belief contrast. Larger reductions in social anxiety symptoms over the course of MBSR were associated with greater BOLD responses during breath-focused attention versus reactivity to negative self-beliefs in visual attention brain regions, including medial cuneus (r = .68, p = .03), left cuneus (r = .65, p = .04), and right middle occipital gyrus (r = .65, p = .01).
Discussion
The goal of this study was to investigate MBSR-related changes in patients with SAD on behavioral and neural bases of emotional reactivity and regulation of negative self-beliefs. MBSR was hypothesized to reduce clinical symptoms and negative emotional reactivity to negative self-beliefs via reductions in brain activity related to emotion reactivity with increases in attention-related brain networks.
Clinical Measures
MBSR-related changes included reduction in symptoms of social anxiety, depression, rumination, state anxiety and increased self-esteem in adults with SAD. MBSR-related reductions in social anxiety symptoms have been observed previously in adults with SAD (Koszycki et al., 2007). MBSR-related changes for anxiety, depression, and self-esteem in SAD are similar to the moderate effect sizes found in a recent meta-analysis of behavioral and pharmacological interventions (38 studies) for SAD (Alcazar, Meca, Rodriguez, & Saura, 2002).
Behavioral Measures
From pre- to post-MBSR, patients with SAD reported reduced negative emotion experience when implementing breath-focused attention during the fMRI experiment, but not for attention distraction. This suggests MBSR-related down-regulation of emotional reactivity to negative self-beliefs when redirecting attention to breath sensation. The lack of change from pre- to post-MBSR in negative emotion experience during the react negative self-belief condition may be due to overlearned responses (e.g., an automatic tendency to perceive statements about the self as threatening) to negative self-beliefs in patients with SAD. Similarly, the lack of change from pre- to post-MBSR in attention distraction may be due to its not having been a treatment focus.
Neural Measures
Baseline neural responses showed that reacting to negative self-beliefs resulted in activation of the midline cortical regions implicated in self-referential process, including ventromedial PFC, dorsomedial PFC, and posterior cingulate/precuneus (Northoff et al., 2006), as well as emotion (amygdala) and memory (parahippocampal gyrus) processes.
The absence of any significant MBSR-related changes in neural responses during attention distraction is not surprising primarily because this form of attention regulation is not trained during the course. In contrast, there were robust MBSR-related changes associated with breath-focused attention regulation in visual attention-related parietal and occipital brain regions, perhaps more specifically related to alerting to a stimulus (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). In addition, MBSR-related reductions in social anxiety symptom severity were associated with increased MBSR-related neural responses in cuneus and middle occipital brain regions implicated in visual attention. This suggests two possible interpretations. First, MBSR may have helped adults with SAD be more visually engaged in (i.e., less avoidant of) negative self-beliefs. Alternatively, greater neural recruitment of attention-related brain regions may be due to more refined visualization of the movement of the breath at the nostrils, which also involves better allocation of attention to the task.
Numerous studies have shown exaggerated amygdala response in adults with SAD in response to social anxiety-related stimuli, including harsh faces (Phan, Fitzgerald, Nathan, & Tancer, 2006; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002) and critical comments (Blair et al., 2008). Furthermore, one study has demonstrated that adults with SAD who were classified as responders to either citalopram medication or CGBT had a reduced amygdala activity from baseline to posttreatment (Furmark et al., 2002).
The MBSR-related change in amygdala BOLD signal time series during react and regulate components suggests enhanced initial emotion reactivity or detection of emotional salience of negative self-beliefs as suggested by the initial amygdala spike at the post-MSBR assessment. The enhanced initial emotion response indexed by amygdala activity is transient as evidenced by a significant decrease in amygdala activity at the end of the react trials well before the onset of the cue to shift attention to the breath. This might reflect an effortful attempt to implement breath-focused attention emotion regulation at baseline and a MBSR-related shift to a more automatic implementation of breath-focused attentional regulation even before being cued to do so. This highlights the possibility that one potential outcome of MBSR is to change specific aspects of attention regulation from explicit (i.e., more effortful) to an implicit (i.e., more automatic) process.
Clinical Implications
These results suggest that MBSR-related changes in attention processes may modify habitual reactivity in the context of negative self-beliefs. In patients with SAD who would normally show attentional avoidance of threat stimuli, MBSR might help attenuate avoidance and increase attentional allocation, as evidenced by the association of reduced SAD symptoms with increased neural response in visual attention-related brain regions. MBSR may be associated with facilitating the ability to implement attentional deployment, a specific emotion regulation strategy that may be aberrant in adults with SAD specifically when encountering social threat stimuli (Schultz & Heimberg, 2008). The ability to redirect attention to thoughts, emotions, and physical sensations, a key feature of MBSR, may be an important skill for adults with SAD to develop because it may enhance the efficacy of exposure therapy for SAD.
Limitations and Directions for Future Research
This study is limited by the lack of a control group or active comparison clinical intervention that would provide a basis for making a stronger inference about how MBSR might modify the behavioral and neural bases of different types of emotion regulation. We examined the effect of attentional emotion regulation on a small set of experimenter-selected negative self-belief stimuli. Although the use of experimenter-selected emotional probes confers greater experimental control, use of participant-generated negative self-beliefs may result in more robust brain–behavioral responses in SAD, which could support a more ecologically valid test of the effects of MBSR on emotion regulation. Using idiographic stimuli can often provide greater fidelity to the actual clinical phenomenology under investigation. However, this can also provide greater variability in the specific targets of emotion regulation.
This study examined only breath-focused attention on sensation at the nostrils. There are many other important mindfulness practices taught in MBSR that deserve empirical investigation, for example, the effects of body scan meditation and mere observation of the changing or transient nature of experience. Thus, results from this study cannot be generalized to other forms of mindful attention such as mindful attention of taste, sound, mental states and other bodily sensations. To begin to specify how MBSR works, it may be instructive to compare the effects of clinical interventions with different mechanisms of change (e.g., cognitive disputation, acceptance, attention training) on the neural bases of mindful attention.
Future studies will benefit from using a randomized clinical trail methodology with at least two groups that undergo two types of stress reduction courses in order to delineate factors that might contribute to changes in attentional deployment, attention brain networks, and specificity of changes in clinical symptoms as well as address other potential confounds such as practice effects and habituation to the scanner environment. Using experimental paradigms that present a variety of linguistic and nonlinguistic emotional probes and assess the effectiveness of several types of emotion regulation strategies may help delineate underlying mechanisms in MBSR. Furthermore, future studies should consider using self-report measures of mindfulness pre-, during, and post-MSBR to examine change during and post-MBSR compared with baseline trait mindfulness.
Conducting an analysis of individual differences at baseline to enhance effective treatment matching to different types of meditation practices (not just mindfulness) and to different combinations or doses of clinical therapies (e.g., pharmacological, cognitive-behavioral, meditation-based interventions) would be a major contribution. Finally, understanding how mindfulness practice in the health care provider interacts with mindfulness practice of the individual receiving health care is a domain of inquiry that has not yet been investigated.
Acknowledgments
This research was supported by National Center for Complementary and Alternative Medicine Grant R21 AT003644-01 awarded to James J. Gross, as well as an National Institute of Mental Health postdoctoral fellowship, and a Mind and Life Summer Research Institute grant awarded to Philippe R. Goldin.
References
- Alcazar AIR, Meca JS, Rodriguez JO, Saura CJI. Treatments for social phobia and their influence on clinical and personality variables: A meta-analysis. Analisis y Modificacion de Conducta. 2002;28:749–777. [Google Scholar]
- Allen NB, Chambers R, Knight W. Mindfulness-based psychotherapies: A review of conceptual foundations, empirical evidence and practical considerations. Australian and New Zealand Journal of Psychiatry. 2006;40:285–294. doi: 10.1080/j.1440-1614.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory—II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J. Evolving conceptions of mindfulness in clinical settings. Journal of Cognitive Psychotherapy. 2009;23:270–280. [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29:560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: A review and meta-analysis. Journal of Alternative and Complementary Medicine. 2009;15:593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) Oxford University Press; New York: 1994. [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. Journal of Anxiety Disorders. 2008;22:716–721. doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive–behavioral therapy. Archives of General Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber Ball T, Werner K, Heimberg RG, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P, Ramel W, Gross JJ. Mindfulness meditation training and self-referential processing in social anxiety disorder: Behavioral and neural effects. Journal of Cognitive Psychotherapy. 2009;23:242–257. doi: 10.1891/0889-8391.23.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- Gross JJ, editor. The handbook of emotion regulation. Guilford Press; New York: 2007. [Google Scholar]
- Heimberg RG, Becker RE. Cognitive-behavioral group therapy for social phobia: Basic mechanisms and clinical strategies. Guilford Press; New York: 2002. [Google Scholar]
- Hofmann SG, Asmundson GJ. Acceptance and mindfulness-based therapy: New wave or old hat? Clinical Psychology Review. 2008;28:1–16. doi: 10.1016/j.cpr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jefferys D. Social phobia: The most common anxiety disorder. Australian Family Physician. 1997;26:1064–1067. [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive Affective and Behavioral Neuroscience. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Dell Publishing; New York: 1990. [Google Scholar]
- Koszycki D, Benger M, Shlik J, Bradwejn J. Randomized trial of a meditation-based stress reduction program and cognitive–behavior therapy in generalized social anxiety disorder. Behavior Research and Therapy. 2007;45:2518–2526. doi: 10.1016/j.brat.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykins ELB, Baer RA. Psychological functioning in a sample of long-term practitioners of mindfulness meditation. Journal of Cognitive Psychotherapy. 2009;23:226–241. [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine. 2008;39:1–12. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Carmona PE, McQuaid JR. The effects of mindfulness meditation on cognitive processes and affect in patients with past depression. Cognitive Therapy and Research. 2004;28:433–455. [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Princeton University Press; Princeton, NJ: 1965. [Google Scholar]
- Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: Potential for interactive processes. Clinical Psychology Review. 2008;28:1206–1221. doi: 10.1016/j.cpr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. Guilford Press; New York: 2002. [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, Davidson RJ. Theta phase synchrony and conscious target perception: Impact of intensive mental training. Journal of Cognitive Neuroscience. 2008;21:1536–1549. doi: 10.1162/jocn.2009.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Teasdale JT, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]