Insight from Bart Lambrecht (left) and Martin Guilliams (right)
Lambrecht Photo courtesy of www.vib.be
Monocytes and macrophages belong to the mononuclear phagocyte system (MPS). The concept of the MPS is based on the idea that circulating monocytes represent the immediate precursors of tissue macrophages. Recent genetic fate mapping of myeloid cells caused a conceptual frameshift, as it was found that many macrophages are derived from embryonic precursors that seed the tissues before birth and then self-maintain without any significant contribution from circulating monocytes. In this new view, monocytes merely represent an “emergency squad” that can be rapidly recruited to sites of inflammation to provide a transient supplement of inflammatory macrophages to the local tissue-resident macrophage pool of embryonic origin.
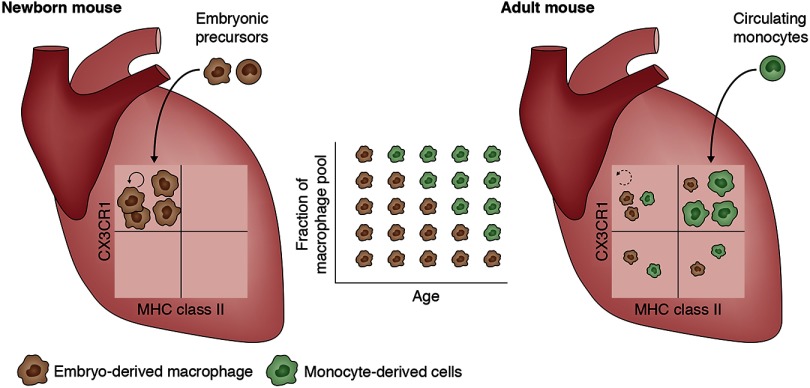
In this issue, Molawi et al. challenge this new concept by reporting that cardiac macrophages are initially of embryonic origin but are progressively replaced by monocyte-derived cells. These newcomer macrophages lack the capacity to self-renew and are slowly but constantly replaced by circulating monocytes. In this regard, the heart resembles the skin and intestine. There, macrophages are also mainly derived from circulating monocytes. Because the skin and the intestine are barrier sites with an important influence of the microbiome, a continuous low-grade inflammation could explain continuous recruitment of circulating monocytes. Molawi et al. report that cardiac macrophages of embryonic origin gradually lose the capacity to self-renew with age and hypothesize that this allows circulating monocytes to occupy the heart niche and join the cardiac macrophage pool. Why embryonic macrophages would lose their self-renewal capacity in the heart but not in the liver, the lung, or the spleen remains to be determined. Another unexplored hypothesis is that continuous microtrauma induced by cardiac contraction is the driver of monocyte influx to the sterile heart.
Cardiac macrophages derived from embryonic progenitors gradually lose their capacity to self-renew and are continually replaced in adulthood by macrophages derived from circulating monocytes, even in the absence of inflammation.
Tissue-resident macrophages are astonishingly diverse in terms of gene expression and are adapted to perform specific functions in their tissue of residence. The unique functions of cardiac macrophages remain largely unknown. Molawi et al. describe four subsets of cardiac macrophages based on the expression of CX3CR1 and MHC class II (see figure). Embryonic macrophages preferentially give rise to MHC class IIlow cells, while most monocyte-derived macrophages express high levels of MHC class II. This implies a different capacity to interact with lymphocytes and distinct functional adaptations for embryonic macrophages. Macrophages play an important role in wound healing, and many cardiovascular diseases are associated with poor or exaggerated tissue repair. It will therefore be interesting to investigate whether embryonic macrophages are better at tissue repair compared with monocyte-derived macrophages, and whether the presence of the embryonic pool explains the superior regeneration capacity of the heart at a young age. Manipulation of macrophage self-renewal might yield important therapeutic applications to increase macrophage populations involved in tissue homeostasis and repair, while avoiding the recruitment of macrophages that fuel inflammation.
With these exciting prospects and new concepts, macrophage biology is going through a revival period, and this study certainly contributes to that. Given the breadth of tissue-specific macrophage heterogeneity, both in terms of functional specialization and cellular origin, we have many years ahead until we fully understand the cell that initiated immunology research more than a century ago.
References
- Molawi, K., et al. 2014. J. Exp. Med. 10.1084/jem.20140639. [DOI] [Google Scholar]