Abstract
Vitamin D appears to have an important role in the modulation of the central nervous system. Vitamin D exerts its biological effects through its interaction with the vitamin D receptor (VDR). Located on chromosome 12 (12q13.1), the VDR gene has many different polymorphisms. Some of them are known to affect the VDR function, such as FokI (rs2228570, T/C) single nucleotide polymorphism. We aimed to explore a potential relationship between FokI VDR polymorphism and impulsiveness in alcohol-dependent (AD) patients. The study population consisted of 148 patients diagnosed with alcohol dependence (DSM-IV criteria) and 212 healthy controls. DNA was extracted from whole blood samples using the standard procedure. Genotypes were analyzed using a real-time PCR method. We found that FokI VDR gene polymorphism was associated with impulsivity [Barratt Impulsiveness Scale (BIS)-11 total score; P = 0.014], and with attentional impulsivity (BIS-11 subscale; P = 0.002) in the male AD patients. Our results suggest that CC FokI genotype of the VDR gene is associated with a higher level of impulsivity in these patients. This finding supports the hypothesis that impulsiveness, which significantly contributes to development of alcohol dependence, has a genetic background.
Keywords: Alcohol dependence, Impulsivity, Vitamin D receptor gene, Genetic polymorphism
Introduction
Alcohol dependence may be defined as loss of control over alcohol consumption, which is continued despite the harmful consequences that follow. Alcoholics are not able to drink alcohol in a controlled manner, even if it threatens their health, work or family. Certain personality features are likely to contribute to the development of alcohol dependence. These can include, for example, high levels of impulsivity or poor impulse control, which are considered both a predictor of the development of alcoholism and an extremely important risk factor for relapse to drinking in individuals attempting to maintain abstinence [1–3].
Studies on alcoholism have shown a contribution of both genetic and environmental components to its development. Specific genes that are associated with brain function and modulating neurotransmission may be important in assessing a person’s risk of developing alcoholism, possibly through their influence on certain personality features [4, 5].
The main neurotransmitter systems involved in developing alcohol dependence are gamma-aminobutyric acid (GABA), glutamate, dopamine, opioid, and serotonin systems. Disturbances in their metabolism and function are considered factors related to an increased risk of both alcohol and drug dependence, which may have a basis in abnormal brain function in the prefrontal cortex, the amygdala and the nucleus accumbens [1].
The role of vitamin D in the human body is more extensive than classically defined and goes beyond calcium homeostasis regulations, preventing rickets and osteomalacia. Vitamin D is involved in maintaining the proper function of the immune system, cardiovascular system, cellular proliferation, energy metabolism, and muscle strength [6]. Moreover, recent data suggest that 1,25(OH)2D3, a most-potent natural vitamin D metabolite, plays a role in the overall modulation of the central nervous system and may play a role in maintaining normal brain function [7, 8]. Vitamin D receptors (VDR) and activating enzymes (e.g., 1α-hydroxylase, the enzyme involved in the formation of the biologically active form of vitamin D) have been found in several areas of adult human brain, including the prefrontal cortex, cingulate gyrus, hippocampus, thalamus, hypothalamus, and substantia nigra [9]. The effect of vitamin D on the nervous system is associated with the regulation of the expression of genes involved with GABA-ergic neurotransmission and neuromediator synthesis [10–12]. In addition, vitamin D3 induces synthesis of neurotrophic factors such as nerve growth factor (NGF) and glial-cell-line-derived neurotrophic factor (GDNF) [13]. Moreover, vitamin D has been shown to be involved in neuroprotection and calcium homeostasis regulation by inducing the synthesis of Ca2+ binding proteins and modulating activity of the L-type calcium channels in neurons [14].
A few variants of the VDR, located on the long arm of chromosome 12 (12q13.1), have been found. Some of them are known to affect the VDR’s function, such as FokI (rs2228570, T/C) single nucleotide polymorphism (SNP). This SNP is located inside a start codon (ATG), and when the C variant is present, an alternative start site is used, leading to the expression of a shorter VDR protein (424 aa), which demonstrates a different activity than the longer one (427 aa) [15]. However, it has not yet been proven whether polymorphic variants of the VDR gene can affect the mental state of alcohol-dependent (AD) individuals by modulating expression of the array of genes related to central nervous system function. To date, VDR polymorphisms have been considered as potential contributing factors for developing schizophrenia as well as multiple sclerosis, but no significant relations were observed [16, 17]. In addition, vitamin D deficiency has been linked to an increased risk of developing major depression in both young adults [18] and elderly subjects [19].
Considering the function of vitamin D and the properties of the VDR FokI polymorphism we aimed the current study at assessing the association between VDR gene variants and impulsiveness in AD patients.
Materials and methods
Participants
The study group consisted of 148 AD patients, 106 males and 42 females. The participants were aged 23–71 years, with a mean age of 43.3 ± 9.8. They had been diagnosed as AD under the current DSM-IV definition [20] and were consequently admitted to residential addiction treatment programs at the Medical University of Warsaw. The median age of onset of drinking problems was 23 (IQR 19–31) years and the median duration of alcohol dependence was 15 (IQR 9–25) years. All patients were unrelated Caucasians, of Polish nationality, and were included in the study after written informed consent. The control group consisted of 212 subjects (112 unrelated healthy blood donors after relevant laboratory tests and consultations with appropriate physicians, and 100 healthy subjects attending a periodic general health checkup without history of alcoholism). The study was approved by the Medical University of Warsaw local bioethics committee and the Institutional Review Board at the University of Michigan.
Measures
The Michigan Alcoholism Screening Test (MAST) was the instrument used to quantify severity of alcohol dependence. The MAST is a self-administered 25-item questionnaire, originally designed to identify probable cases of alcoholism [21]. Patients were also evaluated with the Mini International Neuropsychiatric Interview (M.I.N.I.), a short structured interview for both DSM-IV and ICD-10 psychiatric diagnoses [22].
The level of impulsivity was measured by the Barratt Impulsiveness Scale (BIS-11) [23, 24]. The BIS-11 is a subjective measure of impulsivity. It is a self-administered questionnaire, by which global impulsivity and its different dimensions are assessed. The six basic factors of impulsivity in BIS-11 are: motor impulsivity, attention factor, perseverance, cognitive instability, cognitive complexity and self-control. The three complex factors of impulsivity are combinations of basic factors: motor impulsivity (motor impulsivity as a basic factor and perseverance), non-planning impulsivity (self-control and cognitive complexity), and attentional impulsivity (attention factor and cognitive instability). In this study, the total scores of BIS-11 and scores for the three complex factors of impulsivity were analyzed.
Genotyping
Peripheral blood samples were collected in EDTA tubes from both the patients and the controls. DNA was isolated according to the standard procedure and then stored at −20 °C until use.
The genotypes of the VDR FokI (rs2228570) SNP were analyzed in 148 patients and in 212 controls using a real-time PCR (polymerase chain reaction) method. Genotyping was carried out with the LightSNiP typing assay (TIB-MolBiol, Berlin, Germany) by analyzing the melting curves with the LightCycler® 480 system available from Roche Diagnostics. Real-time PCR reactions were performed in 96-well PCR plates with cycling conditions as optimized by TIB-MolBiol.
Statistical analyses
All genotyping results in AD patients and in controls were tested for Hardy–Weinberg Equilibrium (HWE) applying the HWSIM computer program (available at http://krunch.med.yale.edu/hwsim). All other tests were performed by the Statistica software package, version 9.0. Genotype and allele frequencies were compared between groups by Chi square statistics and Fisher’s exact test. The Kolmogorov–Smirnov test was used to test for normal distribution. Associations of clinical variables with genotypes of the candidate gene were examined with analysis of variance (ANOVA) and Student’s t test. The level of impulsivity was the primary outcome phenotype. Association tests were performed by means of ANOVA with the VDR FokI SNP (rs2228570; CC vs. CT vs. TT) as an independent variable and the BIS-11 scores entered as dependent variables. Tukey’s post hoc test was used for post hoc analysis when differences between groups were indicated by ANOVA. Continuous data are presented as means and standard deviations (SD). The criterion for significance was set at <0.05.
Results
The genotype distribution for the VDR FokI polymorphism among AD subjects (χ2 = 2.79, df = 1, P = 0.09) and controls (χ2 = 3.02, df = 1, P = 0.08) was in Hardy–Weinberg equilibrium.
Comparison of AD patients and controls in respect to CC, CT and TT genotype distribution yielded no significant differences (P = 0.06). Frequencies of the C and T alleles were 0.56 and 0.44 both in patients and controls (Table 1). Also, no significant differences were observed in the comparison of CC and TT genotype frequencies between male and female AD patients (χ2 = 1.61, df = 1, P = 0.205; data not shown).
Table 1.
Comparison of genotype and allele frequencies of VDR FokI polymorphism (rs2228570) in alcohol-dependent patients (AD) and control subjects
| N | Genotype frequencies | Allele frequencies | ||||
|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | ||
| AD patients | 148 | 51 (34 %) | 63 (43 %) | 34 (23 %) | 165 (0.56) | 131 (0.44) |
| Controls | 342 | 101 (29 %) | 181 (53 %) | 60 (18 %) | 383 (0.56) | 301 (0.44) |
| Statistics | χ2 = 4.62, df = 2, P = 0.099 | χ2 = 0.01, df = 1, P = 0.94 | ||||
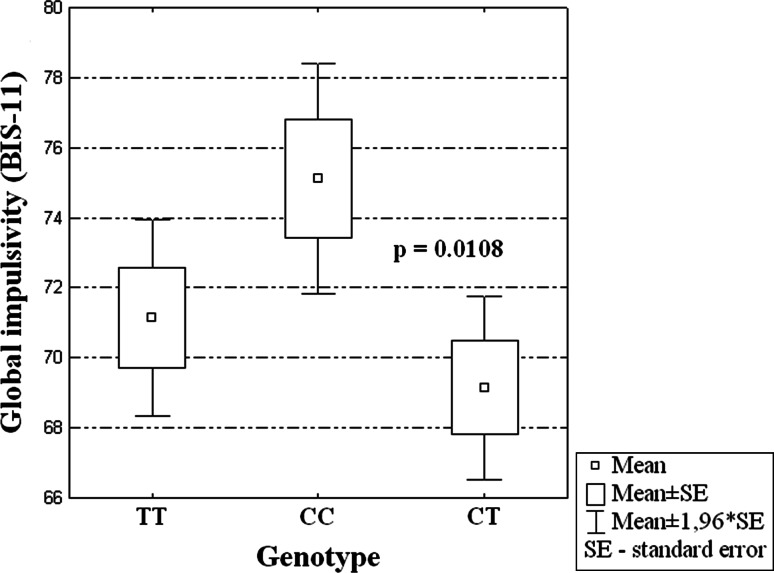
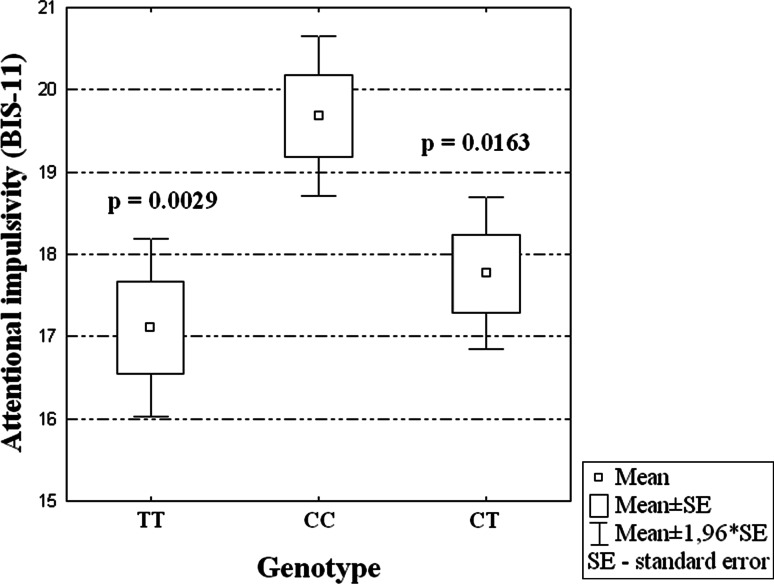
In the group of male AD patients, the VDR FokI (rs2228570) polymorphism was associated with a significantly higher levels of both general (P = 0.014) and attentional impulsiveness (P = 0.002) based on BIS-11 scores (Table 2). After correction for multiple comparisons, in the subgroup of male patients it was found that subjects with CC genotype of the VDR gene had higher global impulsivity evaluated by the BIS-11 than patients with CT genotype (see Fig. 1). Moreover, male AD subjects with CC genotype had higher attentional impulsiveness scores than the patients with the other two genotypes (TC or TT) (see Fig. 2).
Table 2.
Relationships between different types of impulsivity and VDR gene FokI polymorphism in male alcohol-dependent patients
| FokI VDR polymorphism | Global impulsivity (BIS-11) | Attentional impulsivity (BIS-11) | Motor impulsivity (BIS-11) | Nonplanning impulsivity (BIS-11) |
|---|---|---|---|---|
| CC (n = 35) | 75.11 ± 9.92 | 19.69 ± 2.94 | 26.06 ± 4.72 | 29.37 ± 4.58 |
| CT (n = 43) | 69.14 ± 8.77 | 17.77 ± 3.08 | 23.98 ± 3.49 | 27.39 ± 4.59 |
| TT (n = 28) | 71.14 ± 7.59 | 17.11 ± 2.94 | 24.64 ± 3.78 | 29.39 ± 2.85 |
| ANOVA | F = 4.424, df = 2 P = 0.014 | F = 6.600, df = 2 P = 0.002 | F = 2.647, df = 2 P = 0.076 | F = 2.850, df = 2 P = 0.062 |
The values presented are arithmetic means and standard deviations (mean ± SD)
BIS-11 Barratt’s impulsiveness scale, ANOVA analysis of variance
Fig. 1.
Association of CC genotype in FokI VDR SNP and level of global impulsivity in AD male patients (F = 4.424, df = 2, P = 0.014)
Fig. 2.
Association of CC genotype in FokI VDR SNP and level of attentional impulsivity in AD male patients (F = 6.600, df = 2, P = 0.002)
In the female AD subjects, no significant relationship between the genotype distribution and total (F = 0.402, df = 2, P = 0.67) or attentional impulsiveness (BIS-11; F = 0.450, df = 2; P = 0.64) were found (data not shown).
Discussion
The main finding of this study is that genetic variance in the VDR gene is related to differences in impulsivity of male AD patients. It has been found that the VDR FokI (rs2228570) polymorphism is associated to a significant degree with the general and attentional impulsiveness of the AD males. In both cases, individuals with the CC genotype had the highest impulsivity scores.
On the other hand, other studies have reported that, in a rat model of vitamin D deficiency offspring showed severe deficits in brain development and function [11, 25]. In addition, vitamin D deficiency-induced behavioral changes in prenatally depleted animals (DVD-deficient rats) include impaired attention on a latent inhibition task, altered learning and hyperlocomotion compared to control animals [26–28].
In AD patients, low plasma concentrations of vitamin D may be expected as reported by González-Reimers et al. [29, 30]. A low vitamin D level, along with the occurrence of particular variants of the VDR gene resulting in differences in VDR receptor structure and activity, may jointly determine levels of impulsivity and in the present study may account for the higher levels of both global and attentional impulsivity in male AD patients with CC FokI genotype.
Although our study suggests an association between FokI VDR gene polymorphism and levels of impulsivity in male alcoholic individuals, the results need to be replicated on a larger sample. Alcohol is a toxic compound leading to neurodegeneration. A better knowledge of genetic determinants and more accurate concept of what effects a vitamin D deficiency has are fundamental for understanding the homeostatic mechanisms in the central nervous system; and perhaps, in the future, this concept may make it possible to assess the processes that prevent brain damage in AD patients.
In a study by Saunders et al. [31] it was shown that patients who themselves were not alcoholics but had AD family members displayed greater impulsive behavior than those without such a familial background. This relationship applies primarily to men, which is consistent with the differences in heritability of the disorder between males and females as reported in the literature. In the present study, there was no effect of the FokI genotype on impulsivity levels in alcoholic women. Hence, there may exist a gender-dependent effect, which has been also suggested to the relationship between VDR gene polymorphism and development of diabetes type I [32]. The reasons for these differences can probably result from the particular effects of the gender-specific hormones (particularly estrogen in women) on gene expression. Estrogens influence the extent of the VDR gene expression [33], and use of estrogens by women leads to increased expression of the genes of the receptors for active form of vitamin D [34]. However, small number of women in the present study limits the conclusions that can be drawn; our results can only suggest that genetic background might have a stronger influence on impulsivity on men than on women. Therefore, further studies are needed to make a final conclusion.
An additional limitation of the present study is that plasma vitamin D concentration was not measured, which could have added stronger support to the final conclusions and could have been correlated with the VDR genotype-dependent differences in activity and its influence on the level of impulsivity. However, it is well known that genetics—including genetic polymorphism—determines the diversity between individuals and thus also each person’s temperament, especially impulsivity. Neurotransmission is based on Ca2+ flow, which regulation involves vitamin D. The capability of this regulation may depend on molecular differences in the receptor’s structure.
To the best of our knowledge, the present study is the first to report an association between a polymorphism in the VDR gene and a psychometrically-derived impulsiveness trait. Our data suggest that common variants in VDR contribute to high levels of impulsivity, but await verification by independent research. Replication of our analysis in other studies would strengthen the putative link between FokI polymorphism and impulsivity, and would establish VDR as a CNS modulator gene. In conclusion, we are reporting for the first time that genetic variation in VDR is associated with impulsivity in male AD subjects.
Acknowledgments
We would like to thank members of the research team in Poland (especially, Izabela Nowosad, MD; Katarzyna Kositorna, MS; Anna Wnorowska, MD; Aleksandra Konopa, MD) as well as the medical staff and patients at “Kolska”, “Pruszkow”, “Petra” and “Solec” Addiction Treatment Centers in Warsaw for their support of this research. This study was supported by the Polish Ministry of Science and Higher Education Grant 2P05D 004 29, the Polish Ministry of Science and Higher Education Grant NN405357239, the Fogarty International Center/NIDA International Substance Abuse Research Program Grant D43-TW05818, the Fogarty International Center/NIAAA International Collaborative Alcohol & Injury Research Training Program Grant D43- TW007569 and NIAAA Grant R21 AA01610.
References
- 1.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1513. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 2.Rubio G, Jimenez M, Rodriguez-Jimenez R, et al. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year followup study. Alcohol Clin Exp Res. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- 3.Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, deWit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res. 2003;27:868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- 5.Kimura M, Higuchi S. Genetics of alcohol dependence. Psychiatry Clin Neurosci. 2011;65:213–225. doi: 10.1111/j.1440-1819.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- 6.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.3945/ajcn.2008.27049. [DOI] [PubMed] [Google Scholar]
- 7.Eyles D, Burne T, McGrath J. Vitamin D in fetal brain development. Semin Cell Dev Biol. 2011;22:629–636. doi: 10.1016/j.semcdb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Harms LR, Burne TH, Eyles DW, McGrath JJ. Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab. 2011;25:657–669. doi: 10.1016/j.beem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/S1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 11.Féron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65:141–148. doi: 10.1016/j.brainresbull.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez B, Relova JL, Gallego R, Ben-Batalla I, Perez-Fernandez R. 1,25-Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J Neurosci Res. 2009;87:723–732. doi: 10.1002/jnr.21878. [DOI] [PubMed] [Google Scholar]
- 13.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by amyloid-β and preventing the amyloid-β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes de Abreu DA, Eyles D, Féron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34:265–277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Handoko HY, Nancarrow DJ, Mowry BJ, McGrath JJ. Polymorphisms in the vitamin D receptor and their associations with risk of schizophrenia and selected anthropometric measures. Am J Hum Biol. 2006;18:415–417. doi: 10.1002/ajhb.20504. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Xie ZF. Polymorphisms in the vitamin D receptor gene and multiple sclerosis risk: a meta-analysis of case–control studies. J Neurol Sci. 2012;313:79–85. doi: 10.1016/j.jns.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;11:3–29. doi: 10.1186/1755-7682-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–512. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4. Washington, DC (Text Revision): American Psychiatric Association; 2000. [Google Scholar]
- 21.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20):22–33, quiz 34–57 [PubMed]
- 23.Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Percept Motor Skills. 1959;9:191–198. doi: 10.2466/pms.1959.9.3.191. [DOI] [Google Scholar]
- 24.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/S0306-4522(03)00040-X. [DOI] [PubMed] [Google Scholar]
- 26.Burne TH, Becker A, Brown J, Eyles DW, Mackay-Sim A, McGrath JJ. Transient prenatal Vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav Brain Res. 2004;154:549–555. doi: 10.1016/j.bbr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005;161:306–312. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes de Abreu DA, Nivet E, Baril N, Khrestchatisky M, Roman F, Féron F. Developmental vitamin D deficiency alters learning in C57Bl/6 J mice. Behav Brain Res. 2010;208:603–608. doi: 10.1016/j.bbr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 29.González-Reimers E, Alvisa-Negrín J, Santolaria-Fernández F, et al. Vitamin D and nutritional status are related to bone fractures in alcoholics. Alcohol Alcohol. 2011;46:148–155. doi: 10.1093/alcalc/agq098. [DOI] [PubMed] [Google Scholar]
- 30.González-Reimers E, Alvisa-Negrín J, Santolaria-Fernández F, et al. Prognosis of osteopenia in chronic alcoholics. Alcohol. 2011;45:227–238. doi: 10.1016/j.alcohol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Györffy B, Vásárhelyi B, Krikovszky D, Madácsy L, Tordai A, Tulassay T, Szabó A. Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol. 2002;147:803–808. doi: 10.1530/eje.0.1470803. [DOI] [PubMed] [Google Scholar]
- 33.Tang S, Han H, Bajic VB. ERGDB: estrogen responsive genes database. Nucleic Acids Res. 2004;32:533–536. doi: 10.1093/nar/gkh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Protiva P, Cross HS, Hopkins ME, et al. Chemoprevention of colorectal neoplasia by estrogen: potential role of vitamin D activity. Cancer Prev Res (Phila) 2009;2:43–51. doi: 10.1158/1940-6207.CAPR-08-0103. [DOI] [PubMed] [Google Scholar]