Abstract
Diabetes is a pandemic disease characterized by autoimmune, genetic and metabolic abnormalities. While insulin deficiency manifested as hyperglycemia is a common sequel of both Type-1 and Type-2 diabetes (T1DM and T2DM), it does not result from a single genetic defect—rather insulin deficiency results from the functional loss of pancreatic β cells due to multifactorial mechanisms. Since pancreatic β cells of patients with T1DM are destroyed by autoimmune reaction, these patients require daily insulin injections. Insulin resistance followed by β cell dysfunction and β cell loss is the characteristics of T2DM. Therefore, most patients with T2DM will require insulin treatment due to eventual loss of insulin secretion. Despite the evidence of early insulin treatment lowering macrovascular (coronary artery disease, peripheral arterial disease and stroke) and microvascular (diabetic nephropathy, neuropathy and retinopathy) complications of T2DM, controversy exists among physicians on how to initiate and intensify insulin therapy. The slow acting nature of regular human insulin makes its use ineffective in counteracting postprandial hyperglycemia. Instead, recombinant insulin analogs have been generated with a variable degree of specificity and action. Due to the metabolic variability among individuals, optimum blood glucose management is a formidable task to accomplish despite the presence of novel insulin analogs. In this article, we present a recent update on insulin analog structure and function with an overview of the evidence on the various insulin regimens clinically used to treat diabetes.
Keywords: insulin, insulin analogues, genetic engineering, diabetes
Introduction
Diabetes mellitus is characterized by elevated blood glucose levels as a result of insulin deficiency and/or increased hepatic glucose production. The International Diabetes Foundation declared that 371 million people currently have diabetes with a worldwide prevalence of 8.3%.1,2 In addition, 4.6 million deaths (8.2% globally) were attributed to diabetes in 2011.3 According to the most recent CDC reports, diabetes is the third most common disease and the seventh leading cause of death in North America. Intriguingly, half of the patients with diabetes are not even aware they have the disease. There are two main forms of diabetes.4 The most common form in humans is type-2 diabetes (T2DM), which accounts for approximately 90% of all diabetes cases. T2DM generally starts with the loss of insulin sensitivity, but the disease eventually leads to impaired β cell function. At least 50% of T2DM patients will require insulin treatment during the disease course. Type-1 diabetes (T1DM) is the less common form (10%) and is characterized by autoimmune destruction of pancreatic islet β cells resulting in insulin deficiency.5 The molecular pathogenesis of T1DM is not well defined; however, it is generally accepted that environmental and/or genetic factors may predispose individuals to T1DM.6 Because of the complete lack of insulin, treatment with exogenous insulin is the mainstay of treatment in patients with T1DM. While hyperglycemia is a common denominator in both T1DM and T2DM, the clinical features and pathophysiology vary greatly between the two diseases.7
Since prolonged exposure to hyperglycemia can cause vascular complications leading to multiple organ failure, proper control of blood glucose is vital to avert diabetes and limit the development of chronic complications. Insulin is indicated for patients with diabetes, especially when the combination of exercise, diet and oral antiglycemic agents is not adequate to maintain glycemic control. Current guidelines support starting insulin therapy in patients whose glycemic targets are not achieved or for those with glycosylated hemoglobin (HbA1c) >8.5% (Normal: <5.7%; Pre-diabetes: 5.7–6.4%; Diabetes: >6.5%). Control of fasting plasma glucose (FPG) and postprandial glucose (PPG) levels are essential to reduce the risk of diabetes related-complications. The ultimate goal of insulin therapy is to mimic the physiologic insulin secretion profile to control both FPG (to suppress endogenous hepatic glucose production) and PPG associated with increased risk for cardiovascular complications. However, physiologic insulin secretion cannot easily be compensated just by insulin administration into the patients. One possible cure for patients with T1DM is pancreas transplantation, but this is currently not the best option for all T1DM patients due to the paucity of donor organs,8 need for major surgery and life-long immunosuppression.9 Pancreatic islet cell transplantation offers an alternative means to restore insulin expression for prolonging and improving the quality of life in T1DM patients.10 Despite the initial success obtained in islet transplantation, the high frequency of non-functioning grafts and secondary graft failure unfortunately requires many patients to resume insulin administration within 5 years.11 Thus, there is a desperate need to improve insulin therapy including its delivery method for diabetic patients.12 To develop a better acting insulin agent for treatment, it is crucial to understand the molecular structure, biosynthesis and the mechanism of insulin secretion as explained below.13
Structure, Biosynthesis and Secretion of Insulin
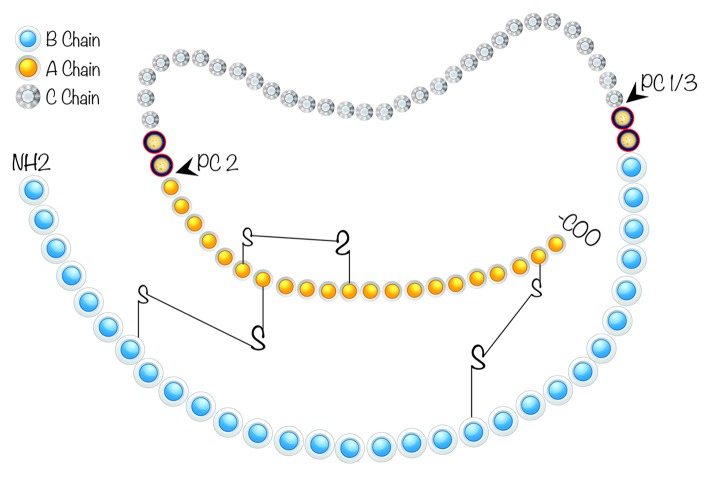
The human insulin gene, which consists of three exons and two introns, is on chromosome 11p15.5.14 While the first exon is the non-coding region, the second exon encodes a signal peptide, the B chain and a part of the connecting peptide (C peptide). The rest of the C peptide and the A chain are encoded in the third exon. A DNA fragment of 350 base pairs located upstream of the transcription start site is required for the cell type specific gene expression of insulin in pancreatic β cells.15 Preproinsulin is encoded as a single-chain precursor from the insulin gene.16 Following translocation to ER, the signal peptide is cleaved to generate proinsulin, which is then reduced and unfolded (Fig. 1). At this point, the C peptide is still present linking the B and A chains. As shown in Figure 1, specific pairing of three disulfide bridges (A6A11, A7B7 and A20B19), which is required for stability and bioactivity of insulin, takes place after the folding of proinsulin in ER. Because proinsulin weakly binds to the insulin receptor its biologic activity is extremely low (5%). The C peptide is excised by specific prohormone convertases during its transit through the Golgi apparatus and entry into the immature secretory granules.17 C peptide removal is necessary for proper folding of insulin, yielding the bioactive hormone.18 Prohormone Convertase 1 (PC1) cleaves proinsulin between residues 32 and 33 (Arg, Arg) while the cleavage site for Prohormone Convertase 2 (PC2) is between residues 65 and 66 (Lys, Arg).19 Then, the C-terminal Arg-Arg residues of the B chain are removed by carboxypeptidase H (a.k.a. carboxypeptidase E). Newly made insulin binds to Zn2+ and forms hexamers within specialized secretory granules for storage. Zn2+ provides insulin with protection against denaturation and misfolding, stabilizing the molecular structure.20 Stored insulin is predominantly released from the pancreatic β cells through a regulated pathway while only about 1% of insulin (and proinsulin) is secreted through the constitutive pathway.21 Hexamers of insulin dissociate into biologically active monomers following secretion into the portal vein. Insulin’s half-life is only about 5 to 7 min in circulation.
Figure 1. Schematic representation of human proinsulin. C-peptide, a 31 amino acid (aa) residue peptide, is depicted between A (21 aa) and B (30 aa) chains. In healthy individuals, both insulin and C-peptide are secreted in equimolar amounts from pancreatic β cells. In patients with diabetes, pancreatic β cells are destroyed by auto-immunity resulting in deficiency of both insulin and C-peptide. Although diabetic patients may routinely receive insulin injections to compensate the insulin deficit, no replacement for C-Peptide is currently administered to diabetic patients. Prohormone Convertase (PC 1/3 and PC 2) cleavage sites necessary for the removal of C-peptide from insulin are also shown.
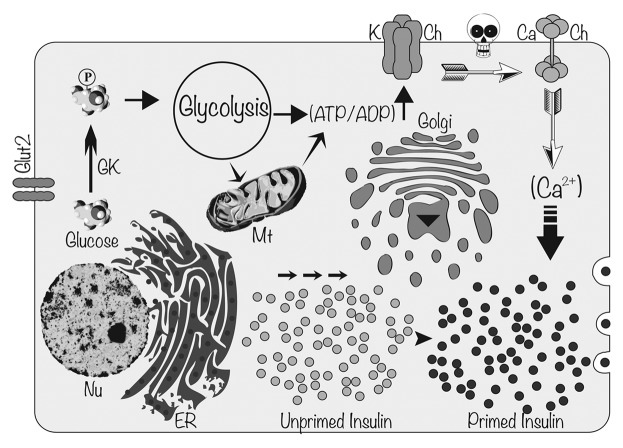
Because insulin is necessary to metabolize carbohydrates, protein and fat, a certain level of insulin secretion is needed to maintain euglycemia not only after meals but also for basal metabolism between meals. An insulin release of 0.5–1.0 unit per hour is sufficient to maintain basal metabolism and limit hepatic glucose production between meals.22 In addition, 1 unit of insulin per 10 g of carbohydrate is released during the postprandial phase of insulin secretion (the meal-stimulated phase) prompting the diffusion of ingested nutrients (mainly glucose) into the periphery.23 In healthy individuals, basal insulin is constantly released at low levels in response to hepatic glucose output, while prandial (bolus) insulin is secreted intermittently in response to elevated glucose levels following a meal. Basal insulin is the background insulin that is secreted by the pancreas and is present throughout the day (24 h), regardless of feeding. Bolus insulin (quick bursts of insulin) refers to the additional amounts of insulin the pancreas would naturally produce in response to glucose absorbed from the food. Therefore, the amount of bolus insulin made depends on the size and content of the meal. Thus, whenever blood glucose concentration rises above 100 mg/dL (5.6 mmol/L), insulin is released from pancreatic β cells (Fig. 2). In normal healthy people, endogenous insulin secretion generally peaks within one hour after a meal (postprandial glycemia). Then, insulin and glucose levels return to basal levels within two hours. Intriguingly, glucose-induced insulin secretion is biphasic.24 The first phase of insulin secretion, which takes place in humans within 5 min after stimulation, primarily results from the release of existing insulin containing vesicles stored in the cytoplasm. This first phase is responsible for suppressing hepatic glucose output, restricting PPG elevations, and inducing phase 2 insulin release of newly manufactured insulin. The second phase (plateau phase), in contrast, requires further processing of newly synthesized insulin and then the priming of insulin secretory vesicles (Fig. 2). This second phase lasts 1 to 2 h until normoglycemia is established. Interestingly, in patients with T2DM the first phase of insulin secretion is entirely absent and the second phase is severely reduced more than 50%.20 Therefore, there is a fundamental defect in postprandial insulin release in patients with T2DM and total absence of insulin in patients with T1DM. For this reason, both short- and intermediate-acting insulin regimens are under development to mimic endogenous insulin response as explained below.
Figure 2. Molecular mechanism of glucose induced insulin secretion. When glucose enters into pancreatic β cells through Glucose Transporter-2 (GLUT-2), it gets phosphorylated to glucose 6-phosphate by GlucoKinase (GK). Phosphorylated glucose enters into glycolytic pathway and electrons are transported through the Electron Transport Chain in mitochondria yielding ATP. Increased ATP/ADP ratio and closure of ATP sensitive K channels (K Ch) lead to membrane depolarization. Change in membrane potential (depicted as a skull) opens up voltage gated Ca2+ channels (Ca Ch) causing influx of Ca2+ into pancreatic β cells. Increased cytosolic Ca2+ concentration facilitates the fusion of insulin-containing secretory vesicles with plasma membrane releasing insulin. Nu, nucleus; Mt, mitochondria; ER, endoplasmic reticulum.
Development of Insulin as a Therapeutic Agent
History of insulin
Studies examining insulin among species suggested significant conservation of the insulin amino acid sequence among vertebrates (bovine insulin is different in three amino acids and porcine insulin is different in one amino acid from human insulin). Since insulin from one species is biologically active in another, cow, pig, horse and fish pancreases were initially used to isolate insulin peptide for human clinical use. Soon after the discovery of insulin in 1921, insulin purified from porcine and bovine pancreases was made commercially available. Since then, insulin has been mixed with additives and even tailored at the molecular level to modify its pharmacokinetic properties to manage diabetes. As some insulin configurations prolonged the pharmacokinetic profile of insulin, others accelerated insulin’s effects in the bloodstream. Furthermore, these insulin preparations were used alone or in combination with other insulin formulations to produce premixed insulin regimens. For example, Protamine Zinc Insulin (PZI) was generated by adding protamine and zinc to porcine/bovine insulin as a means of slowing insulin absorption thereby prolonging insulin’s action. However, PZI insulin could not be combined with other forms of insulin, due to the amount of protamine it contained. Unfortunately, PZI was limited by slow onset of action, resulting in a greater risk of hypoglycemia with reports of sudden and severe attacks. Presently, PZI insulin is primarily for veterinary use, and the only PZI insulin still available for humans is Hypurin Bovine Protamine Zinc exclusively sold by CP Pharma in the UK.
Intermediate-acting neutral protamine Hagedorn (NPH) insulin was produced in 1936 when Nordisk formulated “isophane” porcine insulin by adding neutral protamine to regular insulin, resulting in a suspension of crystalline zinc insulin pooled with the positively charged polypeptide, protamine. The onset of action of NPH insulin is typically 2–4 h, with a peak in activity within 4–10 h and an effective duration of 10–16 h. However, NPH is associated with a higher frequency of hypoglycemia compared with other long acting insulin analogs due to its peaking action. Furthermore, NPH insulin displayed a significant intra-patient variation in absorption causing variations in peak and duration from one injection to another. Contrary to PZI, the NPH insulin can be premixed with intermediate-acting insulin and used alone or in combination with soluble insulin as a once- or twice-daily injection.
The lente insulins were developed in the 1950s by mixing neutral suspensions of insulin with small amounts of zinc ions to prolong insulin action. Lente insulin is an intermediate-acting insulin with an onset of action of 1.5 h after it is injected. The effect is maximal between 4 and 8 h, and the effect may last as long as 24 h after injection. The duration of action of lente insulin family depends on the physical state, size and zinc content of the suspended insulin particles.
Insulin extracted from the pancreases or various animal species were used in human until the early 1980s. Variable rates of absorption and insulin action, allergic skin reactions at injection sites, problems with immunogenicity associated with reduced efficacy, systemic reactions from IgE mediated anaphylaxis and the development of recombinant human insulin resulted in gradual decline in the use of animal insulin in humans with diabetes.25 Even though insulin isolated from animal pancreases has been widely used for decades, only a few pharmaceutical companies are currently producing animal insulin for human use due to cost and safety concerns. Instead, companies are manufacturing biosynthetic human insulin using recombinant DNA technology.
Recombinant human insulin was developed during the 1960s and 1970s and was approved for pharmaceutical use in 1982. The precursor protein to recombinant human insulin is synthesized by genetically modified organisms and proteolytically cleaved to generate active insulin. Eventually all animal-derived insulin produced by pharmaceutical companies was replaced by synthetic, recombinant human insulin. Recombinant human insulin constitutes 70% of insulin currently sold worldwide. Accordingly, NPH, lente, Ultralente were reformulated using human insulin. Because physicians eventually favored NPH insulin and other basal insulin analogous over Ultralente, the use of Ultralente among patients declined over time, and its manufacture was discontinued by Eli Lilly in 2005. The use of extensively purified recombinant insulin is associated with reduced levels of anti-insulin antibodies, but it is not known to what extent anti-insulin antibodies affect glucose homeostasis in patients with diabetes.26
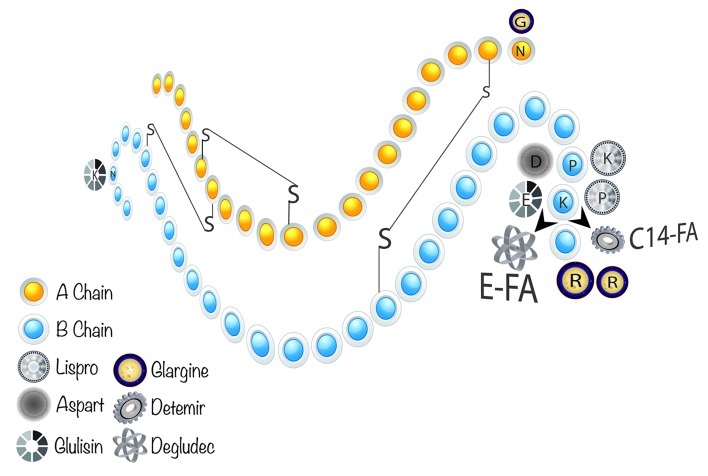
Hydrogen bonding between the C-termini of B chains facilitates insulin monomers to form dimers in solution, while the presence of Zn2+ forces insulin dimers into hexamers. The fact that monomers and dimers can easily disperse in the blood, but hexamers cannot, has an important clinical implication in the treatment of diabetes. Regular insulin was the first available insulin preparation and thus, the first short-acting insulin. Regular insulin has an onset of action 30–60 min after injection, a peak effect in 2–3 h and a total duration of action of 8–10 h. Rapid-acting insulin and short-acting insulin (Regular insulin Humulin R or Novolin R) are called bolus insulin because they are absorbed quickly into the circulation and reduce blood glucose within a few hours. Because regular or short-acting insulin is injected before a meal to compensate the immediate rise in blood glucose arisen mostly from the carbohydrate content of the meal, they are also called prandial insulins. The goal of using bolus insulin therapy is to quickly control hyperglycemia resulting from postprandial blood glucose excursions following a meal. However, it is very difficult to achieve proper glycemic control using regular human insulin (e.g., Humulin R, Novolin R, Velosulin BR, Actrapid) because of the low absorption rate of insulin consisting of a high percentage of hexamers bound to a zinc molecule (Fig. 3). Upon subcutaneous injection, it takes 60–90 min for insulin hexamers to dissociate into dimers and monomers for absorption into the bloodstream.27 Because diabetic patients may not closely follow the timing requirements for insulin injections, a mismatch between blood glucose and insulin concentration can easily occur. Therefore, regular insulin has a tendency of causing postprandial hyperglycemia soon after meals and delayed hypoglycemia several hours after meals. Consequently, improper glucose management eventually predisposes patients to hypoglycemia and diabetes-related complications such as neuropathy, nephropathy and retinopathy.28 A series of recombinant insulin analogs (“designer” insulin) have been developed29 that contain subtle deviations of the human insulin sequence, yielding molecules with somewhat better insulin receptor binding properties and glucose-disposing capabilities (Fig. 3). Recent insulin analogs include additional or substituted amino acid residues or other functional groups modified by genetic engineering or biochemical reaction. These modifications alter the speed of bioavailability through modification of the pharmacokinetic (PK) and pharmacodynamic (PD) properties of the insulin molecule.
Figure 3. Molecular structure of fast and long acting insulin analogs. P(B28) and K(B29) residues at the COO− terminus of B chain are reversed in Insulin Lispro. Insulin Aspart has D residue in place of P at B28. K(B29) residue is changed to E, and N(B3) is substituted with K in Insulin Glulisin. Glargine has an addition of two dibasic aa (RR) at the COO− end of B chain (B31 and B32) in addition to a substitution of N to G at position A21. B29K residue is attached to a fatty acid (myristic acid) in Insulin Detemir, which lacks T(B30) (LysB29 (N-tetradecanoyl)des(B30)). In Insulin Degludec, however, T(B30) is deleted and K(B29) has been coupled to a fatty acid (hexadecanedionic acid) via E (Glu) bridge.
Insulin Analogues from Structure to Function
Fast-acting insulin analogs
The purpose of amino acid substitutions of fast-acting insulin analogs is to endorse monomer stability with rapid dissociation and absorption after subcutaneous injection. The first of the marketed “fast-acting” insulin analogs developed in 1996—called insulin lispro (Humalog)—was bioengineered such that the penultimate lysine and proline residues on the C-terminal end of the B-chain were reversed.30 This change does not modify receptor binding but effectively prevents the formation of insulin dimers and hexamers allowing larger amounts of active monomeric insulin to be immediately available for postprandial injections. Due to its shortened delay of onset, insulin lispro permits a somewhat flexible dosing schedule compared with regular insulin that demands a longer waiting period after injection before starting a meal. Thus, insulin lispro provides faster subcutaneous absorption, an earlier and a greater insulin peak, a shorter duration of action and better control of postprandial glucose excursions compared with regular human insulin.31 However, patients taking insulin lispro may experience hypoglycemia if they do not eat within 15 min after taking the medication. Furthermore, if the meals lack proper amount of carbohydrates postprandial hypoglycemia may occur. Thus, the insulin lispro dose should vary based on the meal composition and size.32
Another fast-acting insulin analog is insulin aspart (marketed by Novo Nordisk as “NovoLog/NovoRapid”). In insulin aspart the amino acid B28 that is normally a proline has been replaced with an aspartic acid residue permitting increased charge repulsion to further prevent hexamer formation.33 The modified insulin sequence was inserted into the Saccharomyces cerevisiae genome, and the insulin was harvested from a bioreactor. In June 2000, the U.S. Food and Drug Administration (FDA) approved insulin aspart for marketing. The onset of action of the drug is ~15 min, the peak action is achieved within 45–90 min, and the duration lasts 3–5 h. Since insulin aspart has a more rapid onset and a shorter duration of efficacy compared with normal human insulin, it should be administered in a regimen with long-acting insulin. Because insulin aspart has a low binding to plasma proteins it is eliminated from the blood faster with an average half-life of 81 min compared with 141 min for regular human insulin.
The newest addition to the class of rapid acting recombinant insulin analogs is insulin glulisine (Apidra® sold by Sanofi-Aventis).34 Insulin glulisine is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12). Insulin glulisine (3BLys29BGlu-human insulin) differs from human insulin such that the asparagine at position B3 has been replaced by a lysine and the lysine in position B29 replaced with glutamic acid.35 While zinc is required for stabilization in hexameric forms to achieve a practical shelf life for aspart and lispro,36 the oligomeric molecules of glulisine are stable without the addition of zinc apparently due to the unaltered proline at position B28 leading to molecular dimerization.37 When injected subcutaneously, insulin glulisine appears earlier in the blood than human insulin. Insulin glulisine given by subcutaneous injection is usually administered with longer-acting insulin. However, for subcutaneous injection insulin glulisine should not be mixed with insulin preparations other than NPH insulin. Insulin glulisine has a flexible administration period, as it can be administered immediately before or after meals. It is usually injected up to 15 min before a meal or within 20 min after starting a meal.
In summary, the fast-acting insulin analogs are absorbed within 10–15 min of a subcutaneous injection, and they peak within 30–90 min and have a duration of action of 4–6 h mimicking normal physiological prandial insulin release. They can mirror endogenous insulin action profile more closely than regular human insulin. They can be injected within 15 min of a meal, unlike regular human insulin, which must be administered 30–45 min before a meal. Thus, patients can inject immediately before eating a meal or even right after the meal, and this feature provides more flexibility and convenient use. Rapid-acting insulin analogs all have similar action profiles, and they are all equally effective in managing postprandial hyperglycemia. Compared with regular human insulin, they exhibit better control of postprandial glucose excursions and have a lower occurrence of postprandial hypoglycemia. Nevertheless, fast-acting insulin analogs are prescribed in combination with longer-acting insulin to maintain proper glycemic control.
Most patients with T1DM, as well as those with T2DM, require a combination of rapid-acting insulin with each meal and basal insulin (namely basal bolus therapy). Most of the premixed insulin analogs include a rapid-acting analog for prandial coverage and a protaminated analog for basal needs. Premixed insulin preparations are useful in eliminating self-mixing and minimizing the number of injections. Insulin pumps can also be handy to continually infuse rapid-acting insulin to supply basal needs and deliver bolus doses at mealtimes as an alternative to multiple daily injections.
Long-acting insulin analogs
Despite the emphasis on developing recombinant forms of insulin that resist hexamer formation, insulin hexamers are desirable when the duration of action is to be prolonged. For example, insulin glargine (LANTUS® marketed by Sanofi-Aventis) was developed in 2000 and is a long-acting form of basal insulin injected once a day for patients with T1DM or T2DM. A glycine substitution for asparagine at A21 and the addition of two arginine residues at the carboxyl terminal of the B chain allow glargine to form a precipitate (hexamer-micro crystals) upon injection. The arginine amino acids shift the isoelectric point from pH 5.4 to 6.7, making the molecule water-soluble at an acidic pH. After subcutaneous injection, which can cause discomfort and a stinging sensation due to acidic solute, the increase in pH (to ~7.4) causes the insulin to come out of solution generating aggregates of insulin hexamers. The end result is a slower dissociation of the insulin hexamers into monomers. In the neutral pH subcutaneous area, protein aggregates form resulting in a slow, peakless dissolution and absorption of insulin from the site of injection, providing a long duration of action lasting 24–26 h. The prolonged action of insulin glargine reduces the peaking effect lowering the risk for hypoglycemia. Compared with NPH insulin, insulin glargine manifests a lower incidence of severe hypoglycemia.38 However, in the absence of endogenous insulin, insulin glargine should be administered in combination with a fast acting insulin taken with meals to reduce postprandial hyperglycemia. Insulin glargine cannot be mixed with other forms of insulin, and it is not approved for use in children under 6 years old. Intriguingly, it is the only 24 h insulin approved exclusively for once-a-day use.
T2DM is a major risk factor for cardiovascular disease (CVD), and CVD represents the leading cause of death in patients with T2DM. The ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention: NCT00069784) was designed and conducted to determine the extent to which basal insulin glargine treatment could reduce cardiovascular events in diabetic patients when fasting glucose levels are targeted.39 Although the results of the trial demonstrated no significant differences between insulin glargine vs. conventional treatment in terms of cardiovascular outcomes,40 there is still a debate going on how to interpret the results of the ORIGIN Trial among the scientists.41
Insulin detemir is a long-acting (up to 24-h duration of action) recombinant human insulin analog (Levemir, Novo Nordisk), which has the last amino acid in the B chain (threonine) deleted and a fatty acid (myristic acid) covalently linked to the B29 allowing it to bind to albumin in the circulation.42Insulin detemir is produced by expression of recombinant DNA in Saccharomyces cerevisiae followed by a chemical modification. Although detemir is quickly absorbed after injection, dissociation from albumin in blood and distribution to peripheral tissues is relatively slow prolonging its efficacy. The mean duration of action of insulin detemir ranges from 5.7 h at the lowest dose to 23.2 h at the highest dose. Insulin glargine and detemir have shown similar efficacies for glycemic control, but a higher proportion of patients need twice-daily dosing with detemir. Generally, the recommended dose is once or twice daily for insulin detemir and once daily for insulin glargine. In a clinical study, insulin detemir reduced Hemoglobin A1C (HgA1c) to target levels of 7.0% for 70% of patients similar to human basal insulin NPH, and it was associated with a lower risk of hypoglycemia in patients with T1DM or T2DM.42 Moreover, additional clinical data has suggested that long-acting insulin analogs caused less weight gain than NPH insulin.43
As mentioned previously, hexamer formation slows the absorption of insulin while it increases its duration of efficacy. Not surprisingly, soluble multihexamers were formulated to generate ultralong-acting insulin analogs—one of which is insulin degludec developed by Novo Nordisk.44 Insulin degludec is basal insulin that forms soluble multihexamers after subcutaneous injection, resulting in an ultralong action.45 It was developed as an alternative to insulin glargine as part of a basal-bolus regimen.46 The addition of hexadecanedioic acid to the lysine at the B29 position results in the formation of multi-hexamers in subcutaneous tissues. This allows for the formation of a subcutaneous depot that results in slow insulin release into the systemic circulation.47 Similar to insulin glargine and insulin detemir, insulin degludec has an onset of action of 30–90 min. There is no peak in activity due to the slow release into systemic circulation.48 It has a half-life twice as long as currently available basal insulin products, with a 42-h duration of effect. Because its effect may last as long as 40 h unlike 18–26 h achieved with insulin glargine and insulin detemir, three subcutaneous injections are sufficient to control blood glucose levels of diabetic patients up to a week.49 The efficacy and safety of varying the daily injection time of insulin degludec was investigated in a 26-week randomized, open-label, parallel-group, treat-to-target trial in individuals with T2DM.50 Based on the results from this study, the daily injection time of insulin degludec (extreme dosing intervals of 8–40 h) could be varied without compromising glycemic control or safety. Furthermore, insulin degludec by itself is associated with less pharmacodynamic and within-subject variability compared with insulin glargine, decreasing the possibility of inducing hypoglycemia in diabetic patients.48 Patients need to take significantly lower doses of basal insulin degludec than those taking insulin glargine to achieve similar blood glucose levels. Insulin degludec can be mixed with other insulins, thereby could provide better glycemic control.
Nocturnal hypoglycemia has been associated with poor quality of sleep, decreased sense of well-being, fatigue and reduced productivity. Not surprisingly, in a recent clinical trial (BEGIN Basal-Bolus Type 1 trial), insulin degludec provided effective glycemic control while lowering the risk of nocturnal hypoglycemia, which is a major limitation of insulin therapy.51 The Committee for Medicinal Products for Human Use (CHMP) in Europe adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product insulin degludec under the name Tresiba in October 2012.
In conclusion, long-acting insulin (basal insulins-insulin glargine, insulin detemir and NPH insulin) provides a basal concentration of insulin for the purpose of controlling fasting hyperglycemia and blood glucose concentrations before meals throughout the day. Almost all T2DM patients starting insulin therapy begin with basal insulin alone or in combination with metformin or other oral antidiabetes agents.
Current hurdles of using insulin and insulin analogues
Because T1DM develops as a result of absolute insulin deficiency, all patients require insulin treatment at the time of diagnosis and intensive insulin therapy is the preferred approach to manage patients with T1DM. A basal-bolus insulin regimen (long acting basal insulin once-daily plus rapid-acting prandial bolus insulin) is now the standard approach in most patients with T1DM, but intensive insulin therapy may pose patients with an increased risk for atherosclerosis. In contrast, patients with T2DM require insulin therapy later in the disease course since the disease develops slowly as a result of decline in pancreatic β-cell function and the loss of β cell mass. For that reason, the timing of insulin treatment in patients with T2DM caused some controversy among physicians. The assessment of HbA1c levels has become both a standard measure for assessing the efficacy of an individual treatment and compliance, in addition to being a laboratory test to establish treatment goals. HbA1c is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. According to the American Diabetes Association (ADA) HbA1c levels should be maintained < 7%, while the American Association of Clinical Endocrinologists and the International Diabetes Federation suggest a more aggressive target value of < 6.5%. The management of T2DM requires a three-step approach, as suggested by American Diabetes Association (ADA) and European Association for the Study of Diabetes.52,53 Metformin and lifestyle modifications are advised as a first line treatment. If HbA1c is still higher than 7% then, basal insulin, a sulfonylurea or a thiazolidinedione [or Glucagon Like Peptide-1 (GLP1) receptor agonist- Dipeptidyl peptidase-4 (DPP4) inhibitor] is added to the treatment regimen.54 Finally, intensified insulin therapy is advised as a last resort. Therefore, maintaining HbA1c levels ≤ 7%, in accordance with professional guidelines, is essential to restrict the progression of diabetes-related complications. Early initiation of insulin is advised only when patients present with weight loss and more severe symptoms, random glucose levels consistently > 300 mg/dL or FPG levels > 250 mg/dL. Thus, for patients with T2DM, insulin is usually given to patients only after combinations of two or even three oral antidiabetic medications have failed to provide proper glycemic control. The fact that only 11% of patients with T2DM who are using oral antidiabetics are prescribed insulin therapy55 demonstrates that insulin is used as a last resort in the management of T2DM.56 Many physicians including endocrinologists choose to delay insulin therapy until absolutely necessary.57 Patients’ inability to properly comply with the therapy, the risk of hypoglycemia and weight gain associated with insulin administration are among the major concerns raised by the physicians.58 Nevertheless, patients with HbA1c levels > 10% require a basal-bolus or premixed insulin regimen.
One of the main problems associated with insulin replacement therapy is that it is difficult to deliver exogenous insulin in way that mimics β cell function. One reason for this difficulty is that exogenously administered insulin has to travel the venous and pulmonary circulation before reaching the liver via the hepatic artery. In contrast, insulin released from pancreatic β cells travels directly to the liver by way of portal vein. Thus, the liver is exposed to relatively high insulin concentrations and is responsible for the uptake of 60% of the pancreatic insulin output. When exogenous insulin is delivered subcutaneously, it is distributed throughout the body and the liver may be “under-insulinized.”
The main goal of insulin treatment is to mimic the physiological pattern of insulin secretion to provide better glycemic control. Since endogenous insulin secretion consists of basal secretion and meal-related short bursts, basal-bolus regimens appear to be preferred choice of treatment. This regimen consists of one or two injections of intermediate- or long-acting insulin analogs to provide basal insulin requirements and meal-related injections of short acting soluble insulin/insulin analogs. Therefore, the basal-bolus regimen provides the best glycemic control in reducing diabetes related complications particularly in patients with irregular eating habits. Glycemic control is measured by analyzing glycosylated hemoglobin (HbA1c) concentrations in blood and tight glycemic control (an optimal target of 6.5% for HbA1c based on NICE clinical guideline) is a prerequisite to prevent and reduce diabetes related complications. Unfortunately, insulin-induced improvements in glycemic control are often accompanied by undesirable increases in body weight.59 Despite the fact that initial insulin-induced weight gain has been attributed to decreased urinary glucose excretion and reduced metabolic rate due to the improved glycemic control, the inherent anabolic activity of insulin on both adipose and muscle tissue is blamed for the prolonged weight gain.60 Knowing that 80–90% of patients with T2DM are already overweight, further weight gain is particularly undesirable in these patients. Weight gain had been assumed to be an almost unavoidable consequence of insulin therapy, but the long-acting insulin analog insulin detemir exhibited a unique weight-sparing effect.61 The use of basal insulin analogs may possess some advantages over conventional human insulin preparations in terms of physiologic action profiles, reduced risk of hypoglycemia and reduced weight gain. It is important to note that weight gain with antidiabetic agents is not limited to insulin, as several insulin secretagogues (e.g., sulfonylureas, meglitinides) and insulin sensitizers (e.g., thiazolidinediones) also cause weight gain as a side effect of the treatment.62
Although insulin analogs are either absorbed quickly to mimic insulin action (as in the case of lispro, aspart and glulisine) or gradually (as with insulin detemir, glargine and degludec), these agents lower serum glucose levels by enhancing glucose uptake into skeletal muscle and adipose tissue and preventing gluconeogenesis, glycogenolysis and lipolysis. Despite these advantages, currently available insulin analogs fail to mimic the endogenous insulin secretion profile especially when used at high doses.63 When these agents are used at low doses, diabetic patients would not have 24-h insulin coverage.64 In addition, metabolic variability among individuals further complicates management of diabetes based on insulin analogs. In these patients, insulin-induced recurrent hypoglycemia can be a limiting factor preventing the achievement of an ideal glycemic control.65 If not recognized and treated properly, hypoglycemic coma can lead to severe brain damage. Symptoms of low blood sugar include headache, nausea, hunger, confusion, drowsiness, weakness, dizziness, blurred vision, fast heartbeat, sweating, tremor, trouble concentrating, confusion or seizure (convulsions).
Short acting human insulin needs to be injected at least half an hour before a meal for optimal effect, since it has a delayed onset of action of 20–30 min. The prolonged duration of action of 6–8 h and variability in absorption of short acting human insulin produces a higher risk of hypoglycemia. Although short acting insulin analogs are beneficial in lowering postprandial hyperglycemia in diabetic patients compared with regular insulin, only a small beneficial effect for glycemic control (magnitude of difference in HbA1c) was observed in T1DM patients whereas no such improvements were detected in T2DM patients.66 In addition to hypoglycemia and weight gain, fast-acting insulin analogs have several other side effects like hypokalemia, allergic reactions (particularly at the site of injection), lipodystrophy and constipation.
Insulin glargine has a greater affinity for the insulin-like growth factor receptor (IGF-1R) than native insulin. Activation of the IGF-1R by high levels of insulin results in mitogenic signaling and has been associated with increased tumor cell proliferation.67 Since circulating IGF-1 is linked to the development of solid tumors and leukemia, patients using some of the new insulin analogs might be posed to an increased risk for cancer.68Insulin glargine undergoes a sequential cleavage of the COOH terminus of the B-chain—yielding metabolites M1 and M2 after subcutaneous injection. Although, M1 and M2 fully retain the same metabolic properties as human insulin, contrary to insulin glargine, their affinity for IGF-1R is similar to that of human insulin.69 Unlike glargine, M1 and M2 displayed lower potency for the stimulation of insulin/IGF-1 hybrid receptors in MCF-7 cells compared with human insulin.70 Therefore, it is highly unlikely that there is an increased risk for cancer in humans following administration of insulin glargine via IGF-1R stimulation since glargine is rapidly converted to M1 in vivo.
A possible link between insulin glargine (Lantus) and increased risk of developing cancer (in particular, breast cancer) was evaluated in four large-scale registry studies from Sweden, Germany, Scotland and the rest of the UK. Higher doses of Lantus were translated into higher risk for cancer in the German study involving 127,000 insulin-treated patients from an insurance database.67 Because of this result, European Association for the Study of Diabetes (EASD) issued an urgent call for more research into a possible link between insulin glargine and increased risk for cancer. After carefully reviewing all the information available, European Medicines Agency (EMEA) stated in a final report that available data did not provide a cause for concern and changes to the prescribing advice were not necessary. Moreover, the American Diabetes Association (ADA) described the published registry studies as “conflicting and confusing” and “inconclusive” and advised patients to continue using Lantus without any worry. The first long-term study to assess the relationship between glargine, and cancer was conducted as the ORIGIN study demonstrating a neutral effect of glargine after more than 6 years of use.40
Insulin degludec is a long-acting basal insulin that forms soluble multihexamers on subcutaneous injection. The European Union licensed the product in 2 forms known as Tresiba (insulin degludec) and Ryzodeg (insulin degludec/insulin aspart combination), but the U.S. FDA declined to approve Novo Nordisk's insulin degludec, requesting additional cardiovascular-outcomes data from a dedicated trial. Apart from the European Union, insulin degludec is also approved in Japan and Mexico. Approval for use of insulin degludec from the U.S. FDA may come in the near future.
Future Direction of Insulin Therapy and the Management of Diabetes
Eventually all patients with T1DM and most patients with T2DM require insulin therapy. Since insulin regimens in diabetes are no longer considered “one type and size fit all,” personalized-care based approaches have been suggested to achieve glycemic targets.71,72 Intensive insulin therapy, which requires close supervision, has proven long-term benefits, but the risk of severe hypoglycemia has a major impact on quality of life.73 Despite the presence of various fast-acting and/or long-acting insulin analogs, it is very difficult to achieve an optimal glycemic control in diabetic patients. Consequently, these patients are at increased risk of developing diabetes-related complications over time. Substantial research effort has been spent developing alternate routes of insulin delivery that are safe, effective and without injection concerns. Various alternatives to injectable insulin include (but are not limited to) oral tablets designed to resist insulin digestion in the gastrointestinal tract, oral sprays for buccal insulin delivery, inhaled insulin, SmartInsulin, insulin patches, insulin pumps and artificial pancreas.
Oral administration of exogenous insulin would deliver the drug directly into the liver through portal circulation, mimicking endogenously secreted insulin. Insulin delivered to the liver is expected to control hepatic glucose production, a major contributor to hyperglycemia in T2DM. In addition, orally administered insulin has the potential benefit of enhancing patient compliance. Thus, many laboratories have explored methods of delivering enough intact insulin from the gut to the portal vein to manage blood glucose.74 A clinical trial (NCT01597713) involving 83 participants and investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of an oral insulin analog (NN1954) was successfully completed by Novo Nordisk in conjunction with Danish manufacturer Merrion Pharmaceuticals Plc. in October 2012. On January 16, 2013, the Israel-based Oramed Company applied to the U.S. FDA for permission to enter Phase 2 trials for its oral insulin capsule (ORMD-0801). The U.S. FDA approved Oramed plans to conduct a 12-month trial in 10 different locations across the U.S. involving 147 patients with T2DM. IN-105, an oral insulin product of Biocon (a biopharmaceutical company based in Bangalore, India), recently entered Phase 3 clinical trials. Even though a considerable amount of oral insulin will have to be taken for a prolonged period of time to see an effect (which raise some safety concerns about the side-effects of oral insulins), the potential market for oral insulin is predicted to be enormous. Therefore, it is expected that more companies will develop oral insulin regiments in the future.
Oral-lyn™, an investigational liquid formulation (oral spray) of human regular insulin, is administered with a spray propellant (RapidMist device) using advanced buccal drug delivery technology of Generex (Canada) for prandial insulin therapy.75 The buccal mucosa is richly vascularized, which permits faster absorption of insulin. The drug has a shorter total duration of activity compared with subcutaneously injected insulin. To date, Oral-lyn™ has been administered to a large number of subjects in over 30 clinical studies. Oral-lyn™ is rapidly absorbed in the mouth (within 15 min) reaching maximum insulin concentration in the blood by ~30 min and returning to baseline in ~2 h. It is easy to use, portable, convenient and needle-free drug administration, which can be conveniently taken immediately prior to meals with less risk of hypoglycemia. Oral-lyn™ is currently approved for commercial sale in several countries, but it is still considered an investigational drug in the U.S. Thus, it is not approved for commercial use and is only available to qualified individuals through healthcare professionals registered in the treatment IND, which is an expanded access program granted by the U.S. FDA.
Inhaled insulin delivery systems can provide a noninvasive alternative for patients with diabetes, without the fear of injections. Considering nasal, buccal and pulmonary alternative routes for systemic delivery of insulin, the lung represented the best option since it is highly vascularized and possess relatively permeable large alveolar surface area. Phase 3 clinical trials were conducted for many inhaled insulin products Exubera® (Pfizer/Nektar), AERx® iDMS (Novo Nordisk), Technosphere® (MannKind) and AIR® Inhaled Insulin (Alkermes/Eli Lilly).76 Among these, only Exubera® had initially been granted marketing authorization by the U.S. FDA in 2006.76 As of October 2007, however, Pfizer declared that it would no longer manufacture or market Exubera due to the lack of support among physicians and patients.77 Interestingly, in early 2008 Novo Nordisk (AERx® iDMS) and Eli Lilly (AIR®) both ended their efforts to develop an inhaled form of insulin. MannKind Corp. remains optimistic about their Technosphere® inhaled insulin product.
Diabetes patients currently use pens and traditional syringes to inject a single dose of insulin or use insulin pumps delivering continuous low doses of insulin during the day. A glucose-responsive insulin formulation called SmartInsulin (SmartCells Inc.) was developed as an alternative to multiple insulin injections. In this method, insulin is chemically modified so the active hormone is released only in the presence of a certain concentration of glucose. Below that level, insulin remains bound and insoluble until the next blood-sugar excursion. Thus, insulin can only be released in response to a specific blood glucose concentration providing better basal and prandial insulin levels with lower risk of hypoglycemia.78
Short-acting or rapid-acting insulin preparations have been delivered as continuous subcutaneous insulin infusion (CSII) using insulin pumps for several decades despite the expense and inconvenience of traditional insulin pump technology.79 Patients with diabetes have been increasingly using insulin pumps for CSII and continuous glucose monitoring systems (CGMS) to improve glycemic control and quality of life. Insulin pumps provide users a tool to administer insulin more effectively, accurately and with some flexibility compared with injections. Use of insulin pumps reduces the incidence of severe hypoglycemic events compared with multiple daily injections (MDI). Several patch pumps systems (small, lightweight and completely or partially disposable units that readily adhere to the body), which consist of an insulin reservoir, delivery system and a cannula have been developed and some have been approved for marketing by the U.S. FDA to simplify the delivery process.80 To avoid frequent insulin dosage titration to maintain glycemic control, Hygieia Inc. has developed the Diabetes Insulin Guidance System (DIGS) that measures a patient’s blood glucose, analyzes patterns in these measurements and then automates insulin dosage titration based on these values. As demonstrated in a Phase 1 study, DIGS successfully automated weekly dose adjustments for diabetic patients.81 Although Hygeia Inc. intends to embed DIGS in a handheld device in the near future, DIGS has not yet received regulatory clearance from the U.S. FDA.
A fully automated closed-loop dual sensor bi-hormonal artificial pancreas system that does not require human interaction delivering insulin and glucagon via the subcutaneous route has recently been described.82 The U.S. FDA released new guidelines in December 2011 to speed up the development of an artificial pancreas system, and soon after approved the first outpatient clinical trial for an artificial pancreas (March 2012) that could potentially automate insulin care for millions of T1DM patients living in the U.S. The artificial pancreas is an external hand-held device with a continuous glucose monitor (CGM) and an insulin pump, intended to provide the right amount of insulin at the right time.
Because regular human insulin and insulin analogs do not contain the C-peptide, there is a great debate on whether the C-peptide should be included in insulin preparations since it provided some beneficial effects to diabetic patients in recent studies.83 Skepticism arose because early stage T2DM patients unexpectedly displayed high levels of C-peptide in their blood. It is now clear that the C-peptide is not simply an insulin marker, but it also has some beneficial effects for diabetics in terms of improving kidney function, nerve function and blood flow to vital organs.84 One proposed mechanism of C-peptide function involves the activation of the GLUT1 transporter leading to glucose clearance by way of adenosine triphosphate (ATP) release from red blood cells, a known stimulus for the blood vessel dilator nitric oxide.85
Insulin therapy does not include amylin replacement. Amylin, also known as Islet Amyloid Polypeptide (IAPP), is a peptide needed for optimum glycemic control since it slows down gastric emptying and blocks glucagon production. Pramlintide, a synthetic analog of human amylin, was approved for adult use in conjunction with insulin in patients with both T1DM and T2DM before a meal to better manage postprandial glucose excursion.86 While pramlintide and exenatide have been approved for use in the U.S. as adjunctive therapy, they are not incorporated into the ADA treatment algorithm due to their relatively low effectiveness, scarcity of clinical data and relative expense. Finally, there is also a strong argument favoring leptin use as an adjunctive therapy to insulin injection in patients with T1DM.87
Concluding Remarks
There is no question that insulin and insulin analogs are more efficacious in reducing HbA1c levels (and managing T1DM and T2DM) compared with oral antidiabetics. Because of the limited efficacy of noninsulin diabetic medications, insulin analogs are expected to facilitate the transition between oral agents and insulin therapy more successfully compared with human insulins.88 Despite the improvements in efficacy, safety, mechanisms of delivery and even patient flexibility and convenience, some clinical challenges still exist. In terms of total direct healthcare costs, insulin analogs and older insulin treatments are equally expensive, as the treatment with insulin analogs is associated with increase in quality of life and life expectancy. In addition, reduced risk of hypoglycemia, greater convenience and, in some instances, less weight gain are among the many benefits offered by insulin analogs compared with human insulins. Accordingly, fast-acting insulin analogs can mirror endogenous insulin action profile more closely exhibiting better postprandial glucose management properties than regular human insulin. Moreover, long-acting insulin analogs possess relatively flat time-action profiles lasting up to 24 h. This mode of action mimics endogenous basal insulin profile better than NPH insulin causing less nocturnal (bedtime) hypoglycemia. Expectedly, if/when insulin degludec is approved by the U.S. FDA, it may soon overtake the long-acting basal insulin market currently dominated by insulin glargine (Lantus),89 since it confers a more flexible dosing schedule and reduced risk of hypoglycemia.90 Ultimately, more scientific data on the clinical efficacy of insulin degludec including cardiovascular outcome and the safety profile is needed to determine its potential impact in diabetes treatment.
Acknowledgements
This work is supported by grants from Akdeniz University Scientific Research Administration Division and the Scientific and Technological Research Council of Turkey (TUBITAK-112S114).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Guariguata L. By the numbers: new estimates from the IDF Diabetes Atlas Update for 2012. Diabetes Res Clin Pract. 2012;98:524–5. doi: 10.1016/j.diabres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ginter E, Simko V. Global prevalence and future of diabetes mellitus. Adv Exp Med Biol. 2012;771:35–41. doi: 10.1007/978-1-4614-5441-0_5. [DOI] [PubMed] [Google Scholar]
- 3.DF Diabetes Atlas Group Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2011. Diabetes Res Clin Pract. 2013 doi: 10.1016/j.diabres.2013.02.005. In press. [DOI] [PubMed] [Google Scholar]
- 4.Marchetti P, Dotta F, Lauro D, Purrello F. An overview of pancreatic beta-cell defects in human type 2 diabetes: implications for treatment. Regul Pept. 2008;146:4–11. doi: 10.1016/j.regpep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Sanlioglu AD, Dirice E, Elpek O, Korcum AF, Balci MK, Omer A, et al. High levels of endogenous tumor necrosis factor-related apoptosis-inducing ligand expression correlate with increased cell death in human pancreas. Pancreas. 2008;36:385–93. doi: 10.1097/MPA.0b013e318158a4e5. [DOI] [PubMed] [Google Scholar]
- 6.Fsadni P, Fsadni C, Fava S, Montefort S. Correlation of worldwide incidence of type 1 diabetes (DiaMond) with prevalence of asthma and atopic eczema (ISAAC) Clin Respir J. 2012;6:18–25. doi: 10.1111/j.1752-699X.2011.00239.x. [DOI] [PubMed] [Google Scholar]
- 7.Glaser B. Type 2 diabetes: hypoinsulinism, hyperinsulinism, or both? PLoS Med. 2007;4:e148. doi: 10.1371/journal.pmed.0040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanlioglu AD, Griffith TS, Omer A, Dirice E, Sari R, Altunbas HA, et al. Molecular mechanisms of death ligand-mediated immune modulation: a gene therapy model to prolong islet survival in type 1 diabetes. J Cell Biochem. 2008;104:710–20. doi: 10.1002/jcb.21677. [DOI] [PubMed] [Google Scholar]
- 9.Gruessner RW, Sutherland DE, Najarian JS, Dunn DL, Gruessner AC. Solitary pancreas transplantation for nonuremic patients with labile insulin-dependent diabetes mellitus. Transplantation. 1997;64:1572–7. doi: 10.1097/00007890-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman S, Dirice E, Hapil FZ, Ertosun MG, Ozturk S, Griffith TS, et al. Tracing of islet graft survival by way of in vivo fluorescence imaging. Diabetes Metab Res Rev. 2011;27:575–83. doi: 10.1002/dmrr.1216. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro AM, Lakey JR, Paty BW, Senior PA, Bigam DL, Ryan EA. Strategic opportunities in clinical islet transplantation. Transplantation. 2005;79:1304–7. doi: 10.1097/01.TP.0000157300.53976.2A. [DOI] [PubMed] [Google Scholar]
- 12.Sanlioglu AD, Karacay B, Balci MK, Griffith TS, Sanlioglu S. Therapeutic potential of VIP vs PACAP in diabetes. J Mol Endocrinol. 2012;49:R157–67. doi: 10.1530/JME-12-0156. [DOI] [PubMed] [Google Scholar]
- 13.Sanlioglu AD, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. Insulin gene therapy from design to beta cell generation. Expert Rev Mol Med. 2012;14:e18. doi: 10.1017/erm.2012.12. [DOI] [PubMed] [Google Scholar]
- 14.Owerbach D, Bell GI, Rutter WJ, Brown JA, Shows TB. The insulin gene is located on the short arm of chromosome 11 in humans. Diabetes. 1981;30:267–70. doi: 10.2337/diabetes.30.3.267. [DOI] [PubMed] [Google Scholar]
- 15.Walker MD, Edlund T, Boulet AM, Rutter WJ. Cell-specific expression controlled by the 5′-flanking region of insulin and chymotrypsin genes. Nature. 1983;306:557–61. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- 16.Weiss MA. Proinsulin and the genetics of diabetes mellitus. J Biol Chem. 2009;284:19159–63. doi: 10.1074/jbc.R109.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner DF, Rouillé Y, Gong Q, Martin S, Carroll R, Chan SJ. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996;22:94–104. [PubMed] [Google Scholar]
- 18.Liu M, Ramos-Castañeda J, Arvan P. Role of the connecting peptide in insulin biosynthesis. J Biol Chem. 2003;278:14798–805. doi: 10.1074/jbc.M212070200. [DOI] [PubMed] [Google Scholar]
- 19.O’Rahilly S, Gray H, Humphreys PJ, Krook A, Polonsky KS, White A, et al. Brief report: impaired processing of prohormones associated with abnormalities of glucose homeostasis and adrenal function. N Engl J Med. 1995;333:1386–90. doi: 10.1056/NEJM199511233332104. [DOI] [PubMed] [Google Scholar]
- 20.Doyle ME, Egan JM. Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev. 2003;55:105–31. doi: 10.1124/pr.55.1.7. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987;105:145–53. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell RK, Campbell LK, White JR. Insulin lispro: its role in the treatment of diabetes mellitus. Ann Pharmacother. 1996;30:1263–71. doi: 10.1177/106002809603001111. [DOI] [PubMed] [Google Scholar]
- 23.Galloway JA. New directions in drug development: mixtures, analogues, and modeling. Diabetes Care. 1993;16(Suppl 3):16–23. doi: 10.2337/diacare.16.3.16. [DOI] [PubMed] [Google Scholar]
- 24.Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002;51(Suppl 1):S53–9. doi: 10.2337/diabetes.51.2007.S53. [DOI] [PubMed] [Google Scholar]
- 25.Grammer L. Insulin allergy. Clin Rev Allergy. 1986;4:189–200. doi: 10.1007/BF02991108. [DOI] [PubMed] [Google Scholar]
- 26.Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL, Krasner AS. Immunological responses to exogenous insulin. Endocr Rev. 2007;28:625–52. doi: 10.1210/er.2007-0002. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg BH. The role of technology in diabetes therapy. Diabetes Care. 1994;17(Suppl 1):50–5. [PubMed] [Google Scholar]
- 28.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–83. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 30.Zinman B, Tildesley H, Chiasson JL, Tsui E, Strack T. Insulin lispro in CSII: results of a double-blind crossover study. Diabetes. 1997;46:440–3. doi: 10.2337/diabetes.46.3.440. [DOI] [PubMed] [Google Scholar]
- 31.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43:396–402. doi: 10.2337/diabetes.43.3.396. [DOI] [PubMed] [Google Scholar]
- 32.Burge MR, Castillo KR, Schade DS. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care. 1997;20:152–5. doi: 10.2337/diacare.20.2.152. [DOI] [PubMed] [Google Scholar]
- 33.Rys P, Pankiewicz O, Łach K, Kwaskowski A, Skrzekowska-Baran I, Malecki MT. Efficacy and safety comparison of rapid-acting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: a systematic review. Diabetes Metab. 2011;37:190–200. doi: 10.1016/j.diabet.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Lih A, Hibbert E, Wong T, Girgis CM, Garg N, Carter JN. The role of insulin glulisine to improve glycemic control in children with diabetes mellitus. Diabetes Metab Syndr Obes. 2010;3:403–12. doi: 10.2147/DMSOTT.S5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garnock-Jones KP, Plosker GL. Insulin glulisine: a review of its use in the management of diabetes mellitus. Drugs. 2009;69:1035–57. doi: 10.2165/00003495-200969080-00006. [DOI] [PubMed] [Google Scholar]
- 36.Richards JP, Stickelmeyer MP, Flora DB, Chance RE, Frank BH, DeFelippis MR. Self-association properties of monomeric insulin analogs under formulation conditions. Pharm Res. 1998;15:1434–41. doi: 10.1023/A:1011961923870. [DOI] [PubMed] [Google Scholar]
- 37.Zoete V, Meuwly M, Karplus M. Study of the insulin dimerization: binding free energy calculations and per-residue free energy decomposition. Proteins. 2005;61:79–93. doi: 10.1002/prot.20528. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Fonseca V, McGill JB, Riddle M, Hallé JP, Hramiak I, et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: a long-term, randomised, open-label study. Diabetologia. 2009;52:1778–88. doi: 10.1007/s00125-009-1415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J, Origin Trial Investigators Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention) Am Heart J. 2008;155:26–32, e1-6. doi: 10.1016/j.ahj.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 41.Bolli GB, Consoli A, Giaccari A. Early insulin treatment in type 2 diabetes: ORIGINal sin or valuable choice as ORIGINal treatment? An open debate on the ORIGIN study results. Nutr Metab Cardiovasc Dis. 2012;22:1007–12. doi: 10.1016/j.numecd.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–74. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 43.McFarlane SI. Insulin therapy and type 2 diabetes: management of weight gain. J Clin Hypertens (Greenwich) 2009;11:601–7. doi: 10.1111/j.1751-7176.2009.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinman B, Fulcher G, Rao PV, Thomas N, Endahl LA, Johansen T, et al. Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomised, open-label, phase 2 trial. Lancet. 2011;377:924–31. doi: 10.1016/S0140-6736(10)62305-7. [DOI] [PubMed] [Google Scholar]
- 45.Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care. 2011;34:661–5. doi: 10.2337/dc10-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F, Surh J, Kaur M. Insulin degludec as an ultralong-acting basal insulin once a day: a systematic review. Diabetes Metab Syndr Obes. 2012;5:191–204. doi: 10.2147/DMSO.S21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson JD, Neumiller JJ, Campbell RK. Can a new ultra-long-acting insulin analogue improve patient care? Investigating the potential role of insulin degludec. Drugs. 2012;72:2319–25. doi: 10.2165/11642240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Nasrallah SN, Reynolds LR. Insulin Degludec, The New Generation Basal Insulin or Just another Basal Insulin? Clin Med Insights Endocrinol Diabetes. 2012;5:31–7. doi: 10.4137/CMED.S9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heise T, Tack CJ, Cuddihy R, Davidson J, Gouet D, Liebl A, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes: a randomized, controlled trial. Diabetes Care. 2011;34:669–74. doi: 10.2337/dc10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meneghini L, Atkin SL, Gough SC, Raz I, Blonde L, Shestakova M, et al. NN1250-3668 (BEGIN FLEX) Trial Investigators The Efficacy and Safety of Insulin Degludec Given in Variable Once-Daily Dosing Intervals Compared With Insulin Glargine and Insulin Degludec Dosed at the Same Time Daily: A 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–64. doi: 10.2337/dc12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, et al. BEGIN Basal-Bolus Type 1 Trial Investigators Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–97. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 52.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–72. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 53.American Diabetes Association Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 54.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davila EP, Florez H, Trepka MJ, Fleming LE, Niyonsenga T, Lee DJ, et al. Strict glycemic control and mortality risk among US adults with type 2 diabetes. J Diabetes Complications. 2011;25:289–91. doi: 10.1016/j.jdiacomp.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meece J. Dispelling myths and removing barriers about insulin in type 2 diabetes. Diabetes Educ. 2006;32(Suppl):9S–18S. doi: 10.1177/0145721705285638. [DOI] [PubMed] [Google Scholar]
- 57.Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes. 2010;4(Suppl 1):S11–8. doi: 10.1016/S1751-9918(10)60004-6. [DOI] [PubMed] [Google Scholar]
- 58.Grunberger G. The need for better insulin therapy. Diabetes Obes Metab. 2013;15(Suppl 1):1–5. doi: 10.1111/dom.12061. [DOI] [PubMed] [Google Scholar]
- 59.Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin Med Res. 2008;6:54–67. doi: 10.3121/cmr.2008.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sallé A, Ryan M, Guilloteau G, Bouhanick B, Berrut G, Ritz P. ‘Glucose control-related’ and ‘non-glucose control-related’ effects of insulin on weight gain in newly insulin-treated type 2 diabetic patients. Br J Nutr. 2005;94:931–7. doi: 10.1079/BJN20051592. [DOI] [PubMed] [Google Scholar]
- 61.Khunti K, Caputo S, Damci T, Dzida GJ, Ji Q, Kaiser M, et al. on behalf of the SOLVE Study Group The safety and efficacy of adding once-daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012 doi: 10.1111/j.1463-1326.2012.01665.x. In press. [DOI] [PubMed] [Google Scholar]
- 62.Nichols GA, Gomez-Caminero A. Weight changes following the initiation of new anti-hyperglycaemic therapies. Diabetes Obes Metab. 2007;9:96–102. doi: 10.1111/j.1463-1326.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 63.Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9:648–59. doi: 10.1111/j.1463-1326.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 64.Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with Type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23:879–86. doi: 10.1111/j.1464-5491.2006.01913.x. [DOI] [PubMed] [Google Scholar]
- 65.Mohn A, Marcovecchio M, Chiarelli F. Insulin analogues. N Engl J Med. 2005;352:1822–4, author reply 1822-4. doi: 10.1056/NEJM200504283521721. [DOI] [PubMed] [Google Scholar]
- 66.Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med. 2005;165:1337–44. doi: 10.1001/archinte.165.12.1337. [DOI] [PubMed] [Google Scholar]
- 67.Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–44. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varewijck AJ, Janssen JA. Insulin and its analogues and their affinities for the IGF1 receptor. Endocr Relat Cancer. 2012;19:F63–75. doi: 10.1530/ERC-12-0026. [DOI] [PubMed] [Google Scholar]
- 69.Lucidi P, Porcellati F, Rossetti P, Candeloro P, Andreoli AM, Cioli P, et al. Metabolism of insulin glargine after repeated daily subcutaneous injections in subjects with type 2 diabetes. Diabetes Care. 2012;35:2647–9. doi: 10.2337/dc12-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pierre-Eugene C, Pagesy P, Nguyen TT, Neuillé M, Tschank G, Tennagels N, et al. Effect of insulin analogues on insulin/IGF1 hybrid receptors: increased activation by glargine but not by its metabolites M1 and M2. PLoS One. 2012;7:e41992. doi: 10.1371/journal.pone.0041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. American Diabetes Association Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 72.Peters A, Laffel L, American Diabetes Association Transitions Working Group Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society) Diabetes Care. 2011;34:2477–85. doi: 10.2337/dc11-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crasto W, Jarvis J, Khunti K, Davies MJ. New insulins and new insulin regimens: a review of their role in improving glycaemic control in patients with diabetes. Postgrad Med J. 2009;85:257–67. doi: 10.1136/pgmj.2008.067926. [DOI] [PubMed] [Google Scholar]
- 74.Sung HW, Sonaje K, Liao ZX, Hsu LW, Chuang EY. pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin: from mechanism to therapeutic applications. Acc Chem Res. 2012;45:619–29. doi: 10.1021/ar200234q. [DOI] [PubMed] [Google Scholar]
- 75.Heinemann L, Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3:568–84. doi: 10.1177/193229680900300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silverman BL, Barnes CJ, Campaigne BN, Muchmore DB. Inhaled insulin for controlling blood glucose in patients with diabetes. Vasc Health Risk Manag. 2007;3:947–58. [PMC free article] [PubMed] [Google Scholar]
- 77.Siekmeier R, Scheuch G. Inhaled insulin--does it become reality? J Physiol Pharmacol. 2008;59(Suppl 6):81–113. [PubMed] [Google Scholar]
- 78.Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Ther. 2011;13(Suppl 1):S5–14. doi: 10.1089/dia.2011.0068. [DOI] [PubMed] [Google Scholar]
- 79.Carchidi C, Holland C, Minnock P, Boyle D. New technologies in pediatric diabetes care. MCN Am J Matern Child Nurs. 2011;36:32–9, quiz 40-1. doi: 10.1097/NMC.0b013e3181fb8c3a. [DOI] [PubMed] [Google Scholar]
- 80.Anhalt H, Bohannon NJ. Insulin patch pumps: their development and future in closed-loop systems. Diabetes Technol Ther. 2010;12(Suppl 1):S51–8. doi: 10.1089/dia.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a tool that automates insulin titration be a key to diabetes management? Diabetes Technol Ther. 2012;14:675–82. doi: 10.1089/dia.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacobs PG, El Youssef J, Castle JR, Engle JM, Branigan DL, Johnson P, et al. Development of a fully automated closed loop artificial pancreas control system with dual pump delivery of insulin and glucagon. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:397–400. doi: 10.1109/IEMBS.2011.6090127. [DOI] [PubMed] [Google Scholar]
- 83.Wahren J, Kallas A, Sima AA. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes. 2012;61:761–72. doi: 10.2337/db11-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamiya H, Zhang W, Sima AA. The beneficial effects of C-Peptide on diabetic polyneuropathy. Rev Diabet Stud. 2009;6:187–202. doi: 10.1900/RDS.2009.6.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer JA, Froelich JM, Reid GE, Karunarathne WK, Spence DM. Metal-activated C-peptide facilitates glucose clearance and the release of a nitric oxide stimulus via the GLUT1 transporter. Diabetologia. 2008;51:175–82. doi: 10.1007/s00125-007-0853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan A, Raza S, Khan Y, Aksoy T, Khan M, Weinberger Y, et al. Current updates in the medical management of obesity. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:117–28. doi: 10.2174/187221412800604644. [DOI] [PubMed] [Google Scholar]
- 87.Oral EA. Leptin for type 1 diabetes: coming onto stage to be (or not?) Pediatr Diabetes. 2012;13:68–73. doi: 10.1111/j.1399-5448.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- 88.Freeman JS. Insulin analog therapy: improving the match with physiologic insulin secretion. J Am Osteopath Assoc. 2009;109:26–36. [PubMed] [Google Scholar]
- 89.Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, et al. NN1250-3582 (BEGIN BB T2D) Trial Investigators Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- 90.Tahrani AA, Bailey CJ, Barnett AH. Insulin degludec: a new ultra-longacting insulin. Lancet. 2012;379:1465–7. doi: 10.1016/S0140-6736(12)60527-3. [DOI] [PubMed] [Google Scholar]