Abstract
Introduction: A number of assays have so far been exploited for detection of cancer biomarkers in various malignancies. However, the expression of cancer biomarker(s) appears to be extremely low, therefore accurate detection demands sensitive optical imaging probes. While optical detection using conventional fluorophores often fail due to photobleaching problems, quantum dots (QDs) offer stable optical imaging in vitro and in vivo.
Methods: In this review, we briefly overview the impacts of QDs in biology and its applications in bioimaging of malignancies. We will also delineate the existing obstacles for early detection of cancer and the intensifying use of QDs in advancement of diagnostic devices.
Results: Of the QDs, unlike the II-VI type QDs (e.g., cadmium (Cd), selenium (Se) or tellurium (Te)) that possess inherent cytotoxicity, the I-III-VI 2 type QDs (e.g., AgInS2, CuInS2, ZnS-AgInS2) appear to be less toxic bioimaging agents with better control of band-gap energies. As highly-sensitive bioimaging probes, advanced hybrid QDs (e.g., QD-QD, fluorochrome-QD conjugates used for sensing through fluorescence resonance energy transfer (FRET), quenching, and barcoding techniques) have also been harnessed for the detection of biomarkers and the monitoring of delivery of drugs/genes to the target sites. Antibody-QD (Ab-QD) and aptamer- QD (Ap-QD) bioconjugates, once target the relevant biomarker, can provide highly stable photoluminescence (PL) at the target sites. In addition to their potential as nanobiosensors, the bioconjugates of QDs with homing devices have successfully been used for the development of smart nanosystems (NSs) providing targeted bioimaging and photodynamic therapy (PDT).
Conclusion: Having possessed great deal of photonic characteristics, QDs can be used for development of seamless multifunctional nanomedicines, theranostics and nanobiosensors.
Keywords: Bioimaging, Bioconjugates, Cancer, Multimodal nanomedicines, Quantum dots, Theranostics
Introduction
To date, malignancies have been categorized as one of the leading life-threatening diseases worldwide. This is mainly due to lack of imaging devices for detection of cancers at its early stage of development. As a golden rule, the earlier the diagnosis of the cancer, the higher the chance for the patient’s survival. While over 200 diverse forms of cancers (e.g., breast, lung, prostate and ovarian cancers) are becoming a real menace worldwide,1 currently used cancer diagnosis treatment modalities often fail to provide significant improvements. Thus, emergence of novel effective and specific strategies for cancer detection and therapy continue to become an inevitable need and indispensable scope of oncologists and cancer field researchers, for which implementation of new technologies are required to advance the early detection and management of cancers.2
Among different treatment strategies, use of multifunctional NSs such as polymer-/lipid-based nanoparticles (NPs), gene-based nanomedicines, Ab/Ap bioconjugates with drugs/toxins, monoclonal antibodies (mAbs), Ab scaffolds, Ab fragments (e.g., Fab, scFv), as advanced classes of pharmaceuticals, may provide much more effective means for the targeted therapy of cancer. Nevertheless, the fast growing fields of targeted therapy of cancer using multifunctional NSs and theranostics have yet to attain the final objectives as cancer therapy modalities. In fact, conjugation of homing and imaging devices with therapeutic agents is deemed to significantly improve the efficacy of these NSs.3 Of the conjugation steps, decoration of NSs with optical imaging devices is an important pace because it provides great possibility for concurrent imaging and therapy, however in this process the conjugation moieties (e.g., types of fluorophores and conjugating linkers such as homobifunctional and heterobifuctional linking agents) needs to be carefully selected.4 While the conventional fluorophores suffer from photobleaching, the QDs nanocrystals display stable optical properties necessary for targeted molecular imaging of cancer. Conjugation of organic/inorganic fluorophores to advanced NSs has resulted in emergence of a new class of seamless NSs called theranostics with simultaneous imaging and immunotherapy competencies. From biophotonic viewpoints, QDs are heterogeneous NPs and show unique optical characteristics such as broad absorption and distinct emission bands, upon which they have been nominated for various potential applications ranging from medicine to energy.5 In fact, their multimodal photonic characteristics make them very attractive agents for molecular photoacoustic (MPA) imaging.6 QDs with a size range between 2 and 10 nm in diameter used for bioimaging/biosensing show mobility of charge carriers (e.g. electrons and holes), which are constrained within the nanoscale dimensions. Once conjugated with homing devices such as Abs and aptamers (Aps), the bioconjugated QDs are capable of tracking different targets at molecular and cellular dimensions. Thus, QD-based monitoring of cancer metastasis and cancer development is achievable through monitoring the relocation of cancer cells.
In comparison with other normal organic dyes, QDs have a wide excitation spectrum along with symmetric and narrow emission spectra. Above all, the dissimilar QDs can concurrently emit different fluorescence under a similar excitement. Thus, simultaneous monitoring/tracking several biomarkers may provide a promising platform for more precise diagnosis through such multicolor QD probes.7 Further, at equal excitation photon flux, QDs are capable of taking up 10-50 times more photons than organic dyes. This can grant much brighter fluorescence, while the QDs possess greater tenability than organic dyes for an accurate wavelengths from ultraviolet (UV) to near infrared (NIR).8 Typically several manufactured QDs are able to emit in the range of 700-900 nm (Fig. 1), while NIR QDs display great potential to be exploited for in vivo fluorescence monitoring as well as quantifying a panel of biomarkers on intact cancer cells.9 It should be enunciated that the simultaneous detection of various antigens by means of different emission properties of QDs will be very beneficial in cancer biology, in particular when detection of colocalized biomarkers are required. In fact, molecular diagnostics can become an important element when some procedures for diagnosing are achieved at the point-of-care, in particular for developing of personalized medicines. The QDs immunoconjugates appear to provide a highly stable fluorescence with simple excitation and instrumentation.
Fig. 1 .
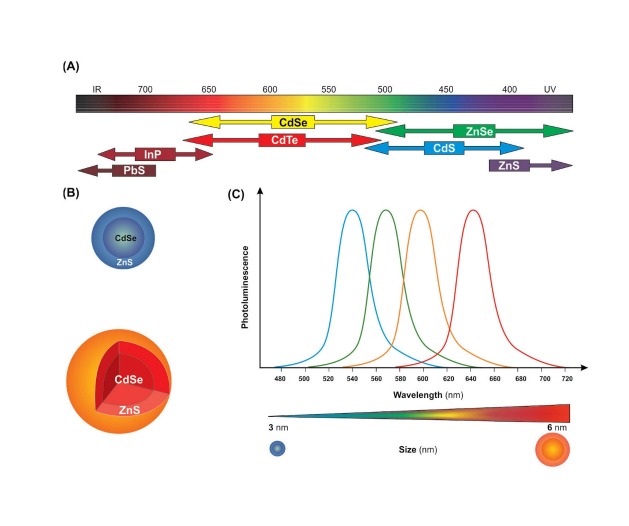
Schematic representation of various quantum dots (QDs). A) Different types of QDs and thier coresponding emissions. B) Anatomy of QDs. C) Size-dependent emission of QDs. Image was adapted with permission from a study published by Barar and Omidi.4 Note: not drawn to scale.
In this review, we provide some important insights on structural and physicochemical properties as well as surface modifications and impacts of QDs for in vitro and in vivo imaging and sensing in various tumors.
Structural and optical properties of QDs
QDs semiconductors, as one of the most studies nanocrystals, have been used as imaging agents for formulation of anticancer nanomedicines and theranostics because of possessing superior fluorescent properties.10 Once excited by a laser beam, the QDs can emit fluorescent light based on their size, while the band gap energy determines the energy and therefore the color of a particular QD. It should be pointed out that the QDs fluorescent light is inversely proportional to the size of the QD – the smaller the size, the bluer the emission and the larger the size, the redder the emission. The optical spectra of various QDs appear to be different (Fig. 1A), so is the architecture of QD nanocrystals (Fig. 1B). Fig. 1 (panel C) schematically shows the emission spectra of the monodispersed CdSe/ZnS QDs with diameters from 3 to 6 nm. Such potentiality makes them very attractive for detection of various markers using multimodal NSs. They are typically composed of atoms from groups II-VI (e.g., CdSe‚ CdS‚ CdTe, ZnSe), III-V (InP and InAs) and IV-VI (PbSe), for more details reader is directed to see a study conducted by Michalet and coworkers.11
For the production of bulk quantities of these semiconductor nanocrystals, the common method used is the colloidal suspension synthesis under high-temperature conditions in organic solvent with nucleation of semiconductor materials.12 In this method, briefly, an organic solvent (e.g., octadecene) is stirred at constant rate and heated up to over 300°C, and then solutions containing the semiconductor metals are injected. The metals first decompose under high heat, then recombine to form alloys that contain particulated seeds, where a single QD nanocrystal construction contains approximately 200-10,000 atoms.13
Many distinctive characteristics of QDs make them very attractive imaging agents for biomedical applications since they possess:
high photoluminescence (PL) quantum yield,
markedly high molar extinction coefficient values in comparision with tradionally used organic dyes,
broad absorption with narrow and symmetric emission spectra spanning the UV to NIR,
large distinction between the excitation and emission spectra.14
Unlike organic dyes, these inorganic hydrophobic nanostructures are exceedingly stable showing repeatability on cycles of excitation or fluorescence for a long period.11 Optically, QDs are able to emit light with a decay time roughly between the 30–100 nanoseconds, which is remarkably slower than the autofluorescence background decay.15
Specific optical properties of the QDs (e.g., size-tunable and wide absorption, sharp and symmetrical photoluminescence spectra, large two-photon absorption cross-section, and brilliant photostability) make QDs as perfect imaging agent for biomedical approaches.16 QDs are photoactivated through one- or multi-photon excitation by the large two-photon absorption cross-section. Further, in the band-edge state, contribution of the sharp photoluminescence bands of QDs has transpired through carrier recombination.9 Of note, the photoluminescence spectra can be adjusted from UV to NIR regions through fluctuation of the core/shell materials (Fig. 1). Accordingly, based upon the core size as well as core material, the photoluminescence of QDs is utilized for bioimaging and PDT. For instance, some QDs (e.g., CdS, CdSe, InP, CdTe, PbS and PbTe) with 2.5 nm in diameter show near visible to NIR band-edge absorption. However, like all other QDs, in vivo applications of NIR QDs are limited due to their toxicity resultant from the heavy metals.17-19 The broad absorption bands in QDs are advantageous in two/multi-photon excitation, and arbitrarily selecting NIR wavelength and wavelength of two-photon excitation is resolved based on the main energy gap of a QD.20
In sp3-hybridized semiconductors (e.g., InP, GaAs, CdSe), a single electron created by exitment moves very quickly in response to an applied energy. As a result, the excited states decay radiatively in a defect-free direct-gap semiconductor such as CdSe.21 For example, in CdSe nanocrystals, the lowest unoccupied band encompasses Cd 5s orbitals, while the highest occupied band comprises Se 4p orbitals. The energy states of QDs have already been delineated through implementation of particles in a sphere model (e.g. lowest hole-state is signified as 1S3/2 for CsSe QDs), and the electron/emitting state is depicted by the total angular momentum F=L±1/2 21. Larson et al. (2003) determined the two-photon absorption cross-section value as Goeppert–Mayer unit.22
Thus far, several evidences demonstrated that QDs are suitable and efficient probes for bioimaging and PDT. Table 1 lists the optical properties of general QDs.
Table 1 . Optical properties of ordinary QDs .
Once conjugated to certain homing devices/targeting moieties, they can be recruited for detection and sensing of (a) cell surface receptors, (b) biomarkers of a wide range of diseases (e.g., malignancies), and (c) molecular markers of biological fluids. For example, the QD-based western blotting technique is able to detect bio-macromolecules (e.g., proteins) as low as 20 pg per lane).28
Synthesis and preparation of QDs
Huge endeavors have been devoted to achieve safe QDs with strong and stable photo luminescence distributed in the visible and NIR region towards imaging of cancer cells. So far, CdSe and CdTe core-only QDs and CdSe/ZnS, CdSe/ZnCdS and CdTe/CdSe core/shell QDs have wildly been used for cancer research, nevertheless the safety of these nanocrystals has been a big concern for in vivo uses despite great achievements for in vitro cell based applications.29,30
QDs are primarily prepared by means of organometallic and aqueous synthesis (the-so-called orQDs and aqQDs, respctively). Methodologically, the colloidal synthesis technique using organic solvents/phase has been reported to be the most used technique for preparation of high quality core and core/shell QDs.31 Having capitalized on this technique, CdSe QDs were synthesized in classic reaction at 230–300 °C using dimethyl cadmium (CdMe2) dissolved in trioctylphosphine (TOP) and TOPSe.32 Because of toxic and pyrophoric impacts of CdMe2, somewhat safer cadmium precursors such as cadmium oxide and cadmium acetate have been used in the structure of QDs. Further, CdTe QDs has directly been produced in the aqueous phase with quantum yield as high as 80%.33 The CdTe QDs modified with thioglycolic acid (TGA) have been used for in vitro detection of cancer cells as reported for the GSH-TGA co-capped CdTe QDs-antibody probe to label the colorectal cancer cells, CCL187, in vitro.34 Regardless of all the advantages of CdSe and CdTe QDs in the biological science as size-tunable absorption and photoluminescence, unfortunately in vivo applications of these nanocrystals can impose intrinsic toxicity.29,35 To resolve the toxicity concerns, based on band gaps, various nanocrystals based have been synthesized. As a result, it was found that the indium phosphide (InP) QDs can be used for in vivo imaging with less toxicity impacts as compared with Cd-based QDs,14 even though aspiration of InP was shown to produce pleural fibrosis.36 In 1997, Dabbousi et al. prepared the ZnS shells on CdSe core to create CdSe/ZnS QDs. In practice, a solvent mixture which has composed of tri-n-octylphosphine
oxide (TOPO) and TOP was organized by heating TOPO at 190 °C and then cooling to 60 °C and adding TOP.37 Furthermore, hexane was used as a required factor for construction a CdSe QD suspension. The mentioned suspension then relocated into the solvent mixture for purifying hexane. CdSe suspension was added to a solution of hexamethyldisilathiane and diethyl zinc in the TOP and ZnS were grown at 140–220 °C as a shell for CdSe QDs.38 Thereupon, after achieving the required width of ZnS shells, the core/shell QDs were separated by of 1-butanol and methanol in the room temperature. Of various synthesized QDs, CdTe/CdSe, CdSe/ZnS and CdSe/CdS QDs that are able to emit photoluminescence in the NIR range are deemed to be appropriate probes for in vivo imaging and PDT,39 even though they also show toxic effects to some extent. Compared to the organometallic methods, aqueous synthetic approaches appear to be easier, environmental friendly and cost-effective.33,40 The aqQDs are water-dispersed nanosystems that need no further post-modifications due to presence of hydrophilic ligand molecules on their surfaces. The aqQDs appear to possess profoundly smaller hydrodynamic diameter (typically < 5.0 nm) in comparison with orQDs, nevertheless these nanocrystals may display poor optical properties.29
Surface functionalization and decoration of QDs
Surface modification and stabilization of QDs appears to be the most essential steps for the regulation of biological functions (e.g., cytotoxicity) and successful biomedical uses.41 The surface of QDs need to be decorated because (a) most of the synthesized QDs are water-insoluble and must be modified to become hydrophilic, (b) uncoated QDs are very reactive and may inadvertently interact with nonspecific biomacromolecules, (c) QDs are mainly made from heavy metal and impose undesired toxic effects when applied in cell/animal models.42 Fig. 2 shows general approaches for surface modification of QDs.
Fig. 2 .
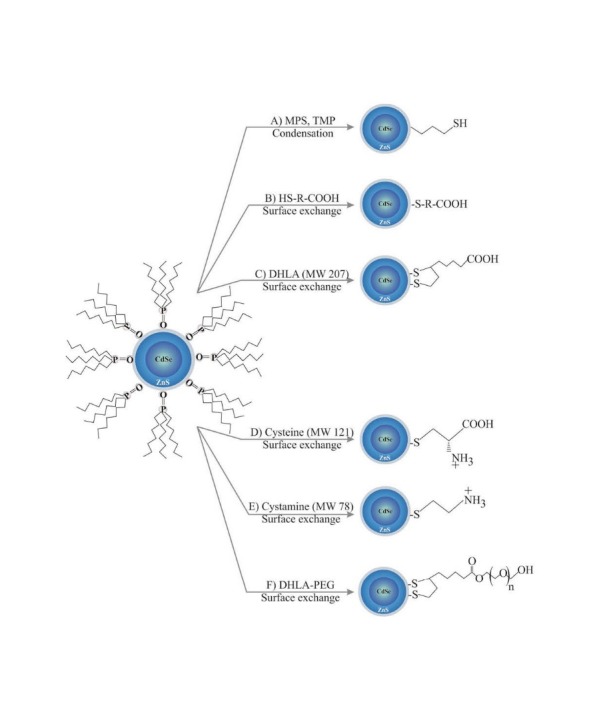
A schematic examples for surface decoration of QDs. Note: not drawn to scale. DHLA: dihydrolipid acid.
Under some circumstances (e.g., exposure to UV), QDs may be oxidized and hence liberate heavy metals (e.g., cadmium ions) into the biological environment.41 This may exacerbate its undesired intrinsic biological impacts on nucleic acids, enzymes and other biomolecules through generation of reactive oxygen species (ROS), while an appropriate coating method seems to efficiently reduce such inadvertent impacts.43
In general, for solubilization of QDs, they can be decorated with hydrophilic polymers such as polyethylene glycol polymer (PEG), an approach so-called PEGylation. In addition, dihydrolipoic acid (DHLA), dendrimers and multidentate phosphine polymers can be attached to the surface of QDs, at which they become more hydrophilic NSs with functionalized groups.44 Other amphiphilic polymers, triblock copolymer and acrylic acid can also be used for solubilization/stabilizing of QDs even though these coating protocols may inevitably enlarge the overall size of these nano-assemblies and consequently interfere with the end point aims.44 While amphiphilic phospholipids, calixarenes and cyclodextrins have been used for coating of QDs,45 the microemulsion strategy with silica-coating method was reported to provide uniform sizes of QDs. Such methodologies can be also used for functionalization of QDs through conjugation with diverse biofunctional molecules.46 Generally speaking, to become a functionalized NS, QDs need to be cross-linked with desired small molecules or ligand of biomolecules (e.g., Ab, Ap) by means of conjugating linkers such as SPDP.4 Such functionalization can be initiated through various functionalized carboxylic, thiol and amine groups. For example, swapping a thiol with molecules containing a sulfhydryl group or proteins with cysteine residue can be used for functionalization.47 Streptavidin modified QDs is deemed to be a specific strategy for linking QDs to biotin-tagged biomolecules (e.g., peptides, Aps, Abs and small molecules), which can be utilized for engineering nano-scaled theranostics, cancer diagnosis probes or even QDs functionalized with cell-penetrating entity as specific ligand for the intracellular delivery of cargos.48 Table 2 represents some selected applications of functionalized QDs.
Table 2 . Well-known targeting molecules used for QDs modification in cancer monitoring .
| Type of QD | Target molecule | Conjugation/modification method | Cell type | References |
| CdSe/ZnCdS (cysteine) | GPI/cRGD | NHS-EDC reaction | Prostate cancer cells | 49 |
|
CdSe/CdS/ZnS (N-(2-aminoethyl)-6, 8-dimercaptooctanamide, amine-DHLA) |
Hyaluronic acid | Electrostatic interaction | HeLa cells | 50 |
| CdTe/CdS (-COOH) | Carbohydrate | NHS-EDC reaction | HeLa (intracellular) | 51 |
| QDs 605,655,705 (-COOH) | MUC-1, AS1411, TTA1 | EDC reaction | PC-3, NPA, HeLa cells | 52 |
| CdSe (-COOH) | Lectin | NHS-EDC reaction | Leukemia cells | 53 |
| ITK QD 525/655 (NH2-PEG) | Dendrotoxin-1 (DTX-1) | N-succinimidyl iodoacetate and 2-iminothiolane reaction | C6 glioma cells | 54 |
| CdSe/ZnSe (-COOH) | β-CD-L-Arg | Electrostatic interaction | ECV304 cells | 55 |
To accomplish the anticipated optical properties of affinity-conjugated QDs, surface modification(s) of QDs as a key step seems to be necessary for controlling the undesired aggregation and non-specific binding.56 Having capitalized on such strategies, QDs-based assays such as Ab-QD and Ap-QD bioconjugates have been designed and used, as cost effective and more stable platform, for specific detection of biomarkers involved in various diseases,57,58 in particular different types of malignancies.59-61 Of various methods used in detection of biomarkers, detection of nucleic acids through hybridization methodology using QDs conjugated-oligonucleotides seems to be one of the most promising approaches.62,63 For example, the Ab-QD conjugates have successfully been used for the detection of insulin-like growth factor receptor in human breast cancer MCF7 cells,64 and targeted imaging of BxPC3 human pancreatic cancer cells using near-IR CdTeSe/CdS QDs armed with single-domain antibody (sdAb) 2A3.65
QDs bioconjugates
Conjugation of QDs with other macromolecules/small molecules can be accomplished using different conjugation methods such as streptavidin-biotin complex,66 and 1-ethyl-3-(3-dimethylamino- propyl)carbodiimide hydrochloride (EDC) together with N-Hydroxysuccinimide (NHS) chemistry.67 Further, to use the bisarsenical affinity probes (e.g., FlAsH, ReAsH,5 and AsCy3) as tool for assessing protein location/function, smart nanohybrids have recently been developed through conjugation of CrAsH (a FlAsH analogue) to hydro-soluble and biocompatible QDs, which was used to target proteins by selectively binding to cystein-tagged proteins 68. These researchers used amino polyethylene glycol (PEG) phospholipids in the micelle QD for covalent linkage of CrAsH to the QD by EDC-mediated coupling method. The resultant nano-hybrids showed efficient and selective binding potential to 4Cys-tagged proteins with high resistance to photobleaching.
QDs are considered as attractive tools for detection of various antigens mainly due to their wide emission properties.69, 70 Such characteristics have been exploited for the in situ hybridization (FISH) assay with higher detection sensitivity and also QD-FISH technique. The latter approach has successfully been used for identifying the mRNA expression of neurons in the midbrain region of mouse.69 Perhaps, the best example for their clinical application is their NIR emission properties, which has successfully been implemented for the sentinel lymph node (SLN) mapping in lung cancer,71 and in vivo imaging of oral squamous cell carcinoma through targeting epidermal growth factor receptor (EGFR).72
To be conjugated with desired bioelements, the surface properties of QDs need to be modified towards better aqueous solubility.7 To this end, QDs have been functionalized using bifunctional linkers such as N-succinimidyl S-acetylthioacetate (SATA) and N-Succinimidyl 3-(2-pyridyldithio)propionate (SPDP).73 Besides, amphiphilic molecules (e.g., octylamine-modified poly-acrylic acid) can be used for modifying the hydrophobic surface of QDs,74 which seem to pose no/less interference with the structure/nature of the surface and optical properties of QDs. Fig. 3 represents schematic illustration of a general methodology for conjugation of QDs to various biomolecules such as mAbs, siRNAs and/or small molecules.
Fig. 3 .
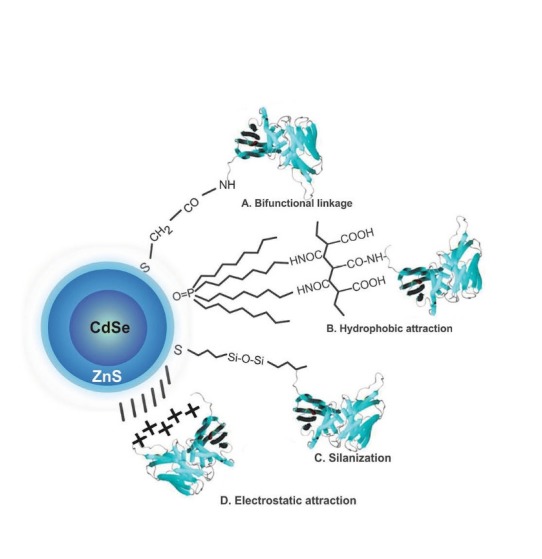
Schematic representation of a general methodology for the conjugation of QDs to various biomolecules such as mAbs, siRNAs and/or small molecules. Seme selected paradigms of QD bioconjugations are shown as A, B, C and D approaches. Note: not drawn to scale.
It should be also stated that surface oxidation and pH are two important factors which are able to influence QDs surface modification strategies.75 Using EDC/NHs chemistry, the JT95 IgM Ab, specific to thyroid carcinoma associated antigen, was conjugated to the CdSe carboxyl QDs to form QD-JT95 NSs that have successfully been used for immunoblot and immunoquantitive assays.76 Recently, a site-specific covalent conjugation of QDs with target proteins in vivo has been reported using an intein-based method, which possessed key steps including (a) fusion of Pleckstrin-homology (PH) domains with the N-terminus half of a split intein (IN), (b) conjugation of the C-terminal (IC) intein-derived peptide to streptavidin-coated QDs in vitro, and (c) in vivo expression of PH–IN following microinjection of PH–IN RNA and IC –QDs into Xenopus embryos.77 These QD–PH based NSs provided a real time monitoring possibility within live embryos, in which NIR-emitting QDs allowed monitoring of the QD conjugates within depths where the enhanced green fluorescent protein (EGFP) was not detectable. Anti-HER2 monoclonal antibody (mAb) armed CdTe QDs decorated with RNase A (HER2-RQDs) were harnessed in gastric cancer nude mouse models. These HER2-RQDs nanoprobes were found to be able to selectively target the gastric cancer MGC803 cells and inhibit the growth of the gastric cancer tissues, resulting in extended survival time of the tumor bearing mice.78 For engineering such nanoprobes, the ribonuclease-A-conjugated CdTe QD Clusters (RQDs) were first synthesized and then the N-succinimidyl iodoacetate (SIA) molecules were used for coupling of RNase A with the amine group and grafting of the thiolated HER2 mAb with the iodoacetyl group.
Taken together, QDs are characterized with high levels of brightness and photostability (e.g., in drug delivery and in vitro imaging), broad absorption spectra, size- and composition-tunable (e.g., in multicolor imaging) and narrow fluorescence emission (e.g., in vivo/ in vitro diagnosis).79 Fig. 4 epitomizes functionalization of QDs by heterobifunctional cross-linkers such as N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) or N-succinimidyl S-acetylthioacetate (SATA), reader is directed to see our recent review in surface modified multifunctional nanomedicines.4
Fig. 4 .
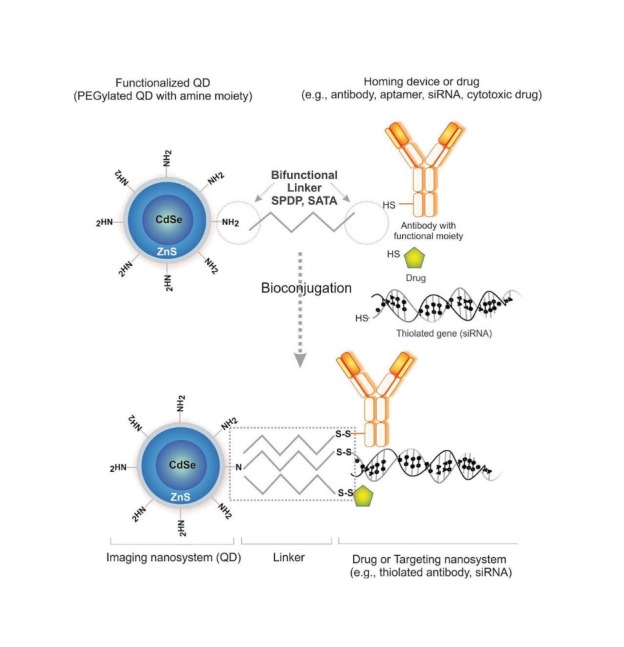
Functionalization of QDs by heterobifunctional cross-linkers. SPDP: N-succinimidyl 3-(2-pyridyldithio) propionate. SATA: N-succinimidyl S-acetylthioacetate. Note: not drawn to scale.
Toxicity of QDs
Most of the orQDs have been reported to impose cytotoxicity to some extent.80 Such cytotoxicity appear to be largely dependent upon various factors such as size, capping materials, dose of QDs, surface chemistry, coating bioactivity and route of exposure,81 while the residual organic molecules can also induce toxic impacts in the target cells/tissue.80 For example, in a study, male Wistar rats were head-nose exposed to 0.52 mg Cd/m3 for 5 days (6 h/day). Histological examination, clinical factors in blood, bronchoalveolar lavage fluid and lung tissue were examined 3 days after the last exposure. It was found that the Cd-based QDs were able to cause local neutrophil inflammation in the lungs, while no CNS toxicity was reported.82
Su et al. (2011) studied the short- and long-term in vivo biodistribution, pharmacokinetics, and toxicity of the aqQDs in mice. These researchers showed that the aqQDs could initially accumulate in the liver after 0.5-4 h post-injection, then in kidney and blood circulation in long term (15-80 days). They also reported the size-dependency of the biodistribution of aqQDs, in which accumulation of larger aqQDs was observed in the spleen. Their findings highlighted that aqQDs impose negligible toxicity in mice even at long-term exposure.83 These researchers have previously reported that use of a series of water-dispersed CdTe QDs, CdTe/CdS core–shell QDs, and CdTe/CdS/ZnS core–shell–shell QDs can be well tolerated even at very high concentration and incubation for long-time by various cell lines, perhaps due to the protective impacts of the ZnS shell impeding the release of Cd ions from the inner side.80 To study the genotoxicity impacts of QDs in vivo, the long-term toxicity of CdSe-ZnS QDs with different surface coatings was investigated in Drosophila melanogaster.84 The results revealed that all differently coated QDs could significantly affect the lifespan of treated group, inducing a significant escalation in ROS levels and enhanced genotoxicity with increased rate of apoptosis in haemocytes. These researchers concluded that the in vivo degradation of QDs with consequent release of Cd+2 ions may be the main reason for such toxic effects as the coated QDs displayed decreased overall toxicity.
Mechanistically, desorption of Cd and creation of free radical may result in inadvertent interaction with intracellular components. It has been reported that surface oxidation of QDs can lead to the formation of reduced Cd that can be released from QDs causing cell death within primary hepatocytes isolated from rats, which was largely dependent upon processing conditions and QDs dose.85 Derfus et al. (2004) showed that, capitalizing on standard conditions of synthesis with solvent TOPO under an inert atmosphere and water-solubilization with mercaptoacetic acid (MAA), the CdSe QDs were not cytotoxic (Fig. 5A). However, once exposed to air for 30 min, TOPO-capped CdSe QDs can become oxidized and very toxic to cells (Fig. 5B) in dose-dependent manner. Such toxic impacts appear to be from generation of free radicals.86 Hepatocytes co-cultured with non-parenchymal 3T3 fibroblasts cells to support in vitro liver-specific functions were exposed to the EGF-coated red QDs and examined by “micropatterning” techniques (Figs. 5C, D, F). As examined through cell viability, migration, and differentiated function for up to 2 weeks in culture, it was found that organically coated, ZnS-capped CdSe QDs could be considered as biocompatible NSs with hepatic tissue.85
Fig. 5 .
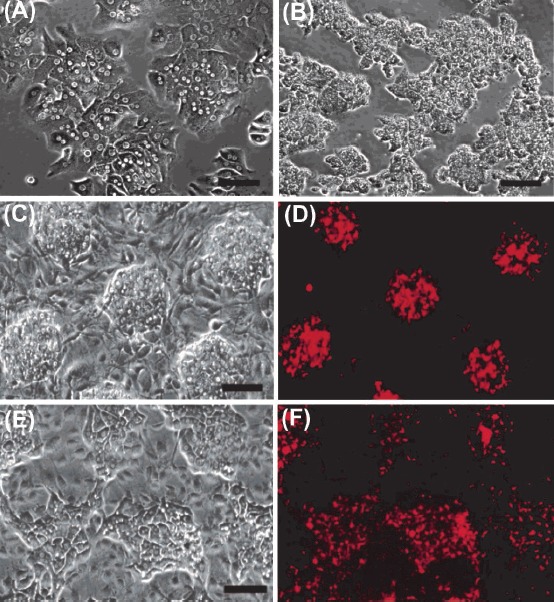
Cytotoxicity of CdSe quantum dots (QDs) in vitro. Toxicity of CdSe QDs in liver culture model is dependent on the processing conditions and the dose of QDs. Panels A and B respectively represent the phase contrast microscopies of the control hepatocyte cultures with well-defined intercellular boundaries and nuclei (A), and nonviable cultures exposed to cytotoxic QDs with granular cytoplasm and undefined intercellular boundaries and nuclei (B). C) Phase contrast image on day 1 showing co-culture of hepatocyte colonies surrounded by fibroblasts. D) Fluorescence image of QD-labeled hepatocytes. E) Phase contrast micrograph of reorganization of hepatocyte colonies. F) Fluorescence image of QD-labeled hepatocytes after 7 days of co-culture. Data were adapted with permission from a study reported by Derfus et al.85
To further elucidate the mechanisms underlying CdSe-core QD-induced apoptosis, Chan et al. studied the mitochondrial membrane potentials and mitochondrial release of cytochrome c in human neuroblastoma cells. They showed that these nanocrystals are able to elicit loss of mitochondrial membrane potential and mitochondrial release of cytochrome c in a concentration dependent manner.87 Having looked at signaling pathway, these researchers showed that CdSe-core QDs, but not ZnS-coated CdSe QDs, can induce apoptosis via various signaling pathways such as ROS-, JNK-, caspase-9- and caspase-3-mediated apoptotic pathways in IMR-32 cells, with attendant under-expressed survival signaling molecules including HSP90, Ras, Raf-1 and ERK-1/2.87
Further, it should be noted that the commonly used materials for solubilization such as MAA and MPA may also induce cytotoxic effects as previously reported for cysteamine and TOPO causing DNA damage.19 Nevertheless, PEGylation of QDs appears to reduce cytotoxicity of QDs via slowing the uptake of QDs by the reticuloendothelial system (RES), liver and spleen.73 In fact the physicochemical properties of QDs (e.g., size, morphology, shell coating and surface characteristics) and their diverse intracellular destinies may determine their endpoint cytotoxicity, therefore approximation of the factual extent of QD cytotoxicity seems to be a difficult issue even though it can be somewhat predicted. In short, among numerous types of QDs, groups III–V QDs have been reported to display lower cytotoxicity than groups II–VI QDs.88, 89 Biocompatible and less toxic Znx S-Agy In1-y S2 (ZAIS) QDs appear to offer greater plausibleness as the optical probes in vivo even though synthesis of these QDs may encounter with some shortcomings, including: (a) high reaction temperatures, (b) poorly-controlled growth rates, (c) long-reaction times, (d) difficulties in high throughput synthesis, and (e) requirement for intricate synthetic approaches to engineer QDs with different emissions profiles.89 Such limitations have been resolved through a novel sonochemical approach for the synthesis of a library of biocompatible ZAIS QDs.89
Having highlighted all these studies, it should be pointed out that, to fully understand mechanisms of QDs toxicities in target cells/tissues, high throughput genomics, proteomics and metabolomics studies need to be conducted similar to the investigations previously reported for non-viral gene delivery systems by Omidi and coworkers.90-100
QDs-based paradigms for imaging of cancer
By far, QDs have widely been used for in vitro biological applications due to their unique characteristics such as broad absorption bands with sharp and symmetrical characteristics, size-tunable absorption and photoluminescence spectra, large two-photon absorption cross-section, photoluminescence spectra and excellent photo-stability.
It should be highlighted that the sharp and size-tunable photoluminescence of QDs favors multiplexed bioimaging. Owing to the tunable absorption and photoluminescence spectra (from UV to NIR regions) by varying the core material, one single laser beam can excite several QDs with different sizes. Further, a designated size of a single QD can display near visible to NIR band-edge absorption and photoluminescence based upon nature of alloy (i.e., CdS, CdSe, InP, CdTe, PbS, PbSe and PbTe QDs). Thus, core size or the core material can bestow multicolor flexibility in terms of absorption spectrum and photoluminescence color for biological imaging and PDT even though blinking of QDs continues to be a limitation for single-molecule imaging. Fundamentally, due to poor tissue penetration and robust tissue autofluorescence, the NIR excitation appears to be a better option over visible excitation for in vivo bioimaging and PDT. Besides, the NIR lights (~1000 nm) can induce vibrational excitation in particular within photoacoustic NSs such as QDs in cells, resulting in the generation of heat. This phenomenon is the basis of the photothermal and PDT of cancer using QDs despite their toxicity concerns that have limited in vivo applications. Further, during a non-linear process of two-photon absorption, QDs display absorption cross-section values significantly larger than organic dyes, upon which they are considered as attractive probes for two-photon imaging.101 Of note, the lower toxicity of aqueous QDs in vivo has accelerated their translations into clinical applications. For example, it has been reported that encapsulation of QDs in PEGylated phospholipid nanomicelles can result in reduced toxicity of the PbS QDs, which may be used as an imaging tool.102 In fact, multispectral fluorescence imaging (MSFI) potential of QDs make them very attractive and promising tool for sensitive detection of cancer.
Targeted imaging of cancer
Targeted imaging of cancer as MSFI can revolutionize detection and consecutive therapy of cancer. Recently, Han et al. (2010) reported on the improvement of photoluminescence and biocompatibility for the NIR gold-doped CdHgTe (Au:CdHgTe) QDs through an aqueous solution route with L-glutathione and L-cysteine as stabilizers.103 In this investigation, the Au:CdHgTe QDs were covalently conjugated to the series of targeting molecules such as arginine-glycine-aspartic acid (RGD) peptide, anti-EGFR mAb, and anti-carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) mAb. These researchers assessed the cytotoxicity of Au:CdHgTe QDs in both A549 cells and mice and showed IC50 of 158.63 μg/mL and 84.169 μg/mL respectively for Au:CdHgTe QDs and CdHgTe QDs in A549 cells, while the LD50 values of Au:CdHgTe QDs and CdHgTe QDs were respectively 34.919 mg/kg and 29.928 mg/kg body mass in mice. The data show a better tolerance of Au:CdHgTe QDs in both in vitro and in vivo experiments. They successfully implemented these bioconjugates (i.e., QD800-RGD, QD820-anti-CEACAM1, and QD840-anti-EGFR) for in vivo targeted MSFI in tumor bearing xenografts. Similarly, cyclic-RGD-peptide-conjugated type II CdTe/CdS QDs have successfully been implemented for recognition of cancer cells in mice xenografted with pancreatic tumor cells.104
As ideal targets for imaging and treating markers, overexpressed tumor-specific markers (TSMs) or tumor-associated markers (TAMs) have widely been used for in vitro and in vivo applications in various cancers. Of note, QDs conjugated with homing agents have commonly been exploited for targeting and sustained fluorescence visualization of cancer cells. Perhaps, one of the best classic examples for the cancer detection using QDs was demonstrated by Gao et al. (2004), whose work on labeling the human prostate cancer cells (C4-2) via conjugation of QDs with Abs specific to the prostate specific membrane antigen (PSMA). The PSMA positive C4-2 cells were efficiently detected by the QD-Ab conjugate, but not the PSMA-negative PC-3 cells.5
Likewise, Her2 receptor that is a recognized cancer marker molecule up-regulated in many breast cancers has widely been used for detection and therapy of such malignancies. QD conjugated with Trastuzumab (Herceptin™), an anti-Her2 Ab was used for specific imaging of the breast cancer cells. Wu et al. (2003) targeted the human breast cancer cells (SK-BR-3) and mouse mammary tumor sections using these conjugates. These researchers labeled SK-BR-3 cells by means of QD-streptavidin conjugate via targeting the cells with a primary humanized anti-Her2 Ab and secondary biotinylated goat anti-human IgG. Later on in 2007, Yezhelyev et al. exploited such approach and labeled MCF-7 and BT-474 breast cancer cells selectively with visible/NIR QDs conjugated with Abs specific to Her2, EGFR, estrogen receptor (ER), progesterone receptor (PR) and mammalian target of rapamycin (m-TOR).105 Similarly, they harnessed such QDs for multiplexed and quantitative immunohistochemistry.106
In 2010, Kawashima et al. exploited CdSe/ZnS QDs conjugated with EGF and anti EGFR Ab to target the EGFR-overexpressing human epidermoid carcinoma A431 cells.107 Interestingly, Ren et al. used multiplexed single-cell array staining approach, in which QDs were coated with water-soluble thioglycolic aid (TGA) to become biocompatible multi-wavelength bioprobes conjugated with Abs specific to some selected antigens.108 Likewise, the KPL-4 breast cancer cells were selectively labeled using NIR QDs conjugated with Herceptin™. Labeling cancer cells with QDs was further carried on by Weng et al. (2008) who utilized a multimodal method through targeting cancer cells using an Ab, drug delivery using immunoliposomes (ILs), and imaging cells using QDs.109 They exploited carboxylic acid functionalized CdSe/ZnS QDs to conjugate them to a primary amino group in a liposome using EDC chemistry (Fig. 6A). Nude mice xenografted with MCF-7/HER2 in the lower back after i.v. injection with anti-HER2 QD-ILs revealed significant accumulation of QD-armed ILS in treated mice (Fig. 6B). Also, confocal microscopy analysis of sections of frozen tumor tissues (5 μm) harvested at 48 h post-injection revealed significant accumulation of ILs on the HER2-overexpressed tissue analyzed in two-color scanning mode for nuclei stained by DAPI and QD-ILs, shown as blue and red colors, respectively (Fig. 6C), for detailed information, reader is directed to see following work.109
Fig. 6 .
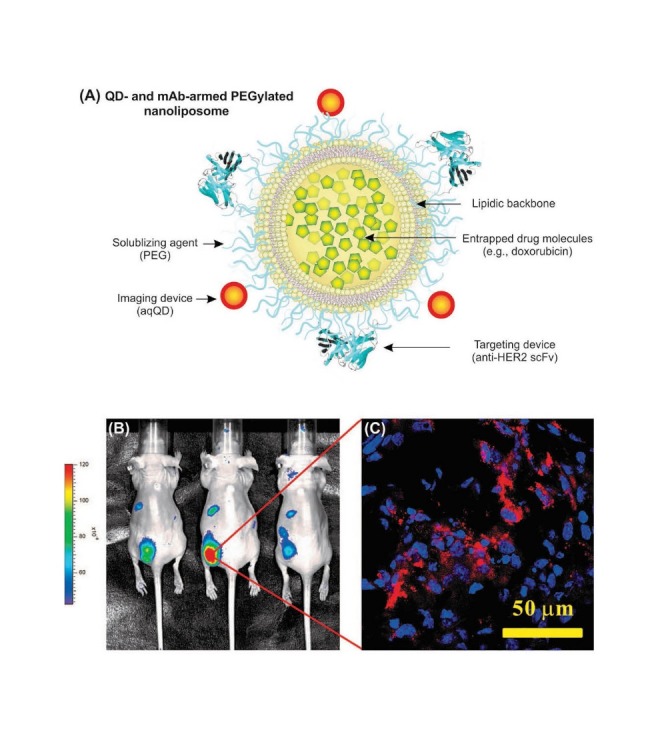
Impacts of targeted multimodal ILs conjugated with QDs. A) Functionalized CdSe/ZnS QDs conjugated ILs armed with scFv. B) Fluorescence image of nude mice bearing MCF-7/HER2 xenografts 30 h after i.v. injection with anti-HER2 QD-ILs. C) Confocal microscopy analysis of section of HER2-overexpressed tissue (5 μm) harvested at 48 h postinjection analyzed using two-color scanning mode for nuclei stained by DAPI (blue) and QD-ILs (red). Data were adapted with permission from a study published by Weng et al.109 QDs: Quantum dots. ILs: immunoliposomes.
Benefits of the multifunctional ILs appear to be (a) selective labeling of the target cancerous cells, (b) bioimaging with high-contrast fluorescence, (c) encapsulating anticancer agents such as doxorubicin (DOX), and (d) intracellular drug delivery. In 2009, in a study, Zhang et al. proposed QDs conjugated with anti-type 1 insulin-like growth factor receptor (IGF1R) as promising multimodal NS for simultaneous targeting and imaging breast cancer cells. The key idea in their approach appeared to be the detection of up-regulated IGF1 R in MCF-7 breast cancer cells by QD-anti-IGFR1 conjugate.64 QDs have also been tailored to single wall carbon nanotubes, resulting in a multifunctional hybrid nanoconstruct for cellular imaging and targeted photothermal therapy,110 even though the safety issues of such approach are unclear. Yong et al. (2009) detected human pancreatic cancer cells selectively by means of QDs conjugated with anti-C1audin-4 Ab and anti-prostate stem cell antigen (anti-PSCA). These NSs were shown to be recognized by the membrane proteins C1audin-4 and PSCA over-expressed metastatic pancreatic cancer.111 Given the successful in vitro detection of cancer cells using QDs conjugated with anticancer antibodies, Kaul et al. devised an antibody-conjugated internalizing QDs for long-term live imaging of cells.112 QDs has been used for multiplexed and quantitative immunohistochemistry.106 Fig. 7 represents multiplexed and quantitative immunohistochemistry of prostate tissue specimens stained with traditional IHC and bioconjugated QDs.
Fig. 7 .
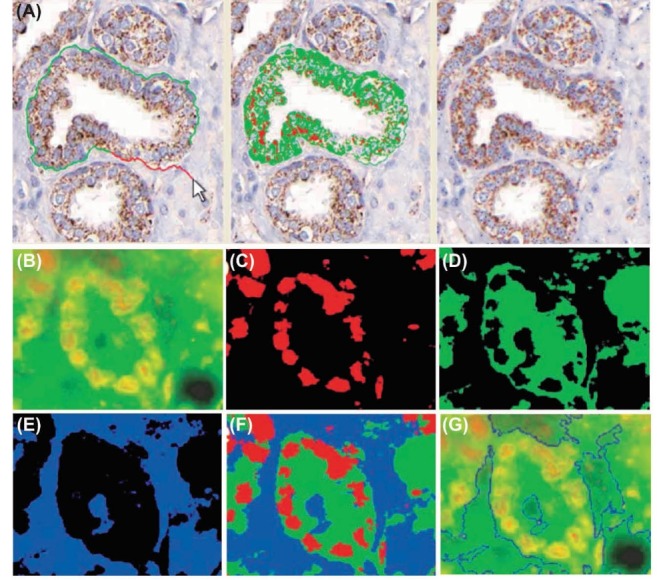
Multiplexed and quantitative IHC using bioconjugated QDs. A) Prostate tissue specimens stained with traditional IHC and bioconjugated QDs. K-means clustering to segment QD-stained tissue image is highlighted by light green and light red colors. Panels B to G represent multiplexed QD-based IHC of the formalin fixed, paraffin embedded (FFPE) prostate tissue samples, and quantitative analysis of cancer biomarkers p53 and EGR-1. The blue color shows the tissue background. B) Original multicolor image. C) p53 protein stained red with QD655. D) EGR-1 protein stained green with QD565. E) Tissue background. F) Superimposed map of dominant markers and background. G) Automated boundary segmentation using level-set algorithms. IHC: immunohistochemistry. Data were adapted with permission from a study published by Xing et al.106
Further, self-assembled nanoscale biosensors have been engineered based on QD FRET donors.113 FRET comprises the transfer of fluorescence energy from a donor particle to a targeted acceptor particle but occasionally it is known as the Forster radius, if the distance between them is smaller than a critical radius.113 FRET causes donor’s emission reduction and increasing in acceptor’s emission intensity. It is suitable for measurement of protein conformational/interaction changes and enzyme activity assay. For example, QD-FRET has been utilized for monitoring protein interactions in the Holliday Junction, immunoassay and play a role as an intermediate in the in the recombination of DNA.114 Detection of DNA arm motion is promising by varying in emission of QD585 as a donor on one arm of the DNA, and Cy5 on a perpendicular arm as an acceptor. In a recent research, maltose binding protein (MBP) has been conjugated to QDs and concentration-dependent upsurge in luminescence was detected, owing to binding affinity similarity of the quenching molecule which readily replaced on addition of maltose.113 For QD-FRET application in imaging activity of proteases, QD-probe via a peptide sequence is bound to a quencher probe that is familiar to a protease. Consequently Emission is resulted after cleavage of the two molecules by protease.115,116 Following studies in bioimaging filed, have evinced the potential of QD-FRET to detect activity of caspase-1, thrombin, trypsin and b-lactamase and discrimination between normal and cancerous breast cells are conceivable by means of the QD-FRET assay of collagenase.47,115
Different environmental factors such as pH and ionic strength of the solution and condition have an efficacious effect on FRET variations.117 In ovarian tumors for identification of Kras oncogene point mutations, a specific nanosensor with producing of FRET was developed and target DNA was acknowledged by a biotinylated capture oligonucleotide and reporter oligonucleotide which labeled with Cy5 fluorophore. Thereupon, through the connection between the QD-streptavidin and biotin, FRET would then transpire and Cy5 fluorescence would be detected because QD and Cy5 were in adjacent proximity. It should be noted that by a mismatch in base pairing fluorescence has not been produced and Cy5 was incapable to accept energy from the QD.118 Fig. 8 shows schematic representation of FRET.
Fig. 8 .
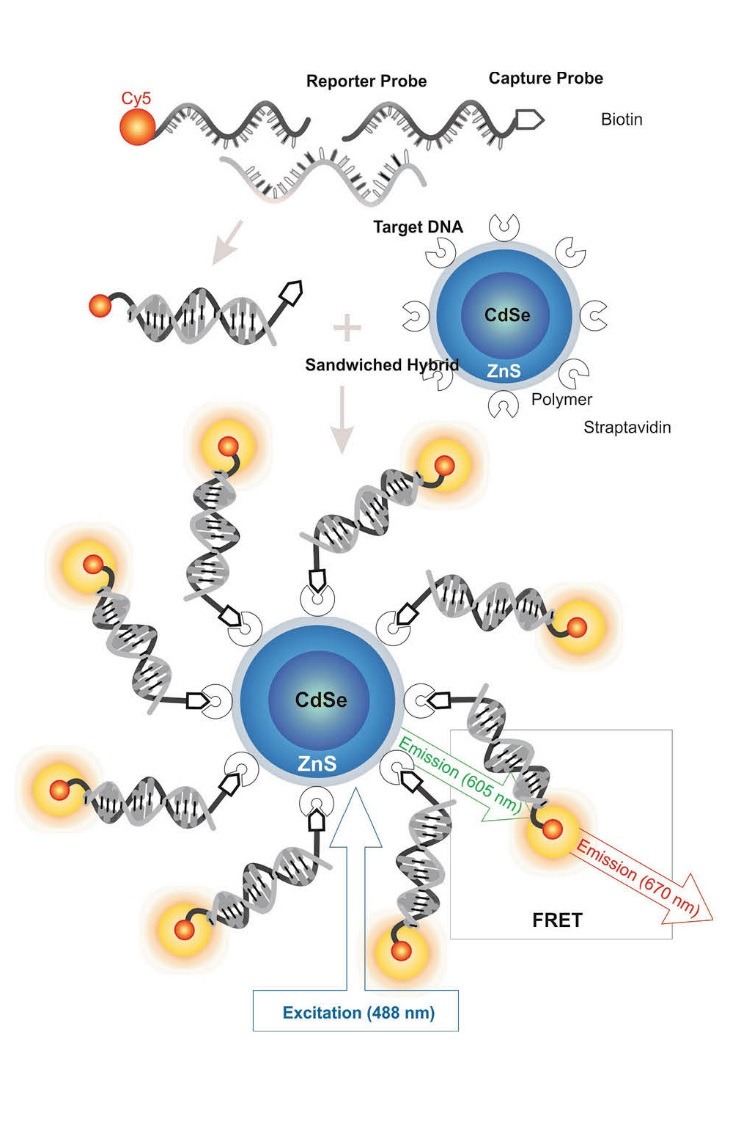
Schematic representation of fluorescence resonance energy transfer (FRET). Image was adapted with permission from a study published by Barar and Omidi.4 Note: not drawn to scale.
Methylation-specific QD fluorescence resonance energy transfer” (MS-qFRET) technique has been advanced to quantify the extent of DNA methylation that can be altered in malignancies and hence can be used as an epigenetic biomarker sensing tool.119 This interesting approach has successfully exploited for determination of methylation in CDKN2A, PYCARD and CDKN2B tumor suppressor genes.119 Cheng et al. devised a FRET-based method to target the glycoprotein mucin 1 (MUC1) in epithelial adenocarcinoma biomarkers using anti-MUC1 aptamer, in which the aptamers fold up and avoid involvement with the DNA conjugated to the QD in the absence of MUC1 and DNA hybridization occurs to make a QD-DNA resulting in placement of the quencher strand in close proximity to the QD.61 This unique bioassay encompassed a dynamic range between 0.25 to 2 µmol/L with the limit of detection (LOD) of 250 nmol/L. In a protease assay using QDs, a peptide sequence for a particular protease tethers a QD to a specific fluorescent dye/protein or quencher dye and gold NPs, and expanding of the QD fluorescence emission was shown by the cleavage of the peptide layer via protease.120
Cellular tracking and fluorescent labelling
Intracellular delivery of QDs are difficult topic, so many methods have been designed for delivering of QDs to the cytoplasm. Labeling with QDs in xenopus and zebrafish embryos have been approved by microinjection techniques.121 It has successfully been used for multispectral fluorescence imaging.122 Fig. 9 represents five-color spectrally unmixed QDs for detection of lymphatic system anatomy.
Fig. 9 .
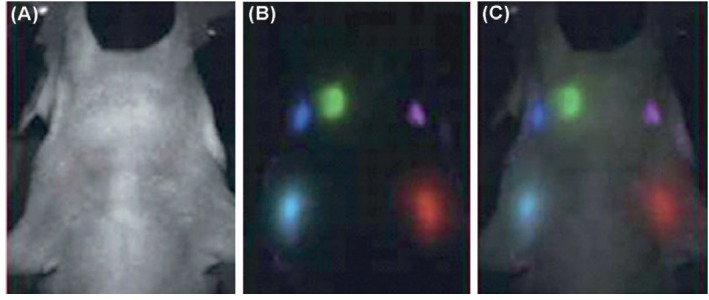
Detection of lymphatic system using five-color spectrally unmixed QDs. A) Autofluorescence image of mouse. B) Fluorescence image of draining lymph nodes after spectral unmixing. C) Superimposed image of autofluorescence and fluorescence images. Data were adapted with permission from a study published by Zhou and El-Deiry.122
It has been shown that QDs can be taken up through endocytic/ non-endocytic pathways. For example, it has been reported that CdSe/ZnS carboxylic-coated QDs (COOH-QDs) are able to enter fibroblast cells through lipid raft/caveolin-mediated endocytosis accumulating in the multivesicular bodies, but not in the lysosomes. In the later phase, the lipid raft/caveolin-dependent endocytosis is inhibited resulting in prevention of intracellular uptake of new COOH-QDs, while the platelet-derived growth factor (PDGF) conjugated QDs could enter fibroblasts through the clathrin-mediated endocytosis accumulating in lysosomes. This clearly means that the intracellular trafficking and the final biological fate and activities of QDs are largely dependent on the surface coating of QDs with the biologically active entities.123 For example, it has also been reported that Tat peptide-conjugated QDs (Tat-QDs) can be internalized through macropinocytosis as a fluid-phase endocytosis process, which are then appear to be trapped in cytoplasmic organelles and actively transported by molecular machineries (e.g., dyneins) along microtubule tracks towards the microtubule organizing center.124
Monodisperse hybrid nanoparticles (38 nm in diameter) of QDs were engineered through mixing with nanogels of cholesterol-bearing pullulan (CHP) modified with amino groups (CHPNH2), which were able to efficiently internalize into various human cells.125 Labelling individual isolated biotinylated F-actin fibres by streptavidin-coated QDs has been processed, in which smaller percentage of labelled filaments were motile in comparison with an organic fluorophore such as Alexa488.126 QDs have also been used in tumor biology for labeling of P-glycoprotein (P-gp) molecules, by which three-dimensional imaging revealed localization of P-gp with QDs allowing consecutive z-sections.127 In other study, concurrent labelling with QDs of nuclear structures/mitochondria and actin filaments have been conducted and successfully produced red labelling of the nucleus and green labelling for the mitochondria.87 Further, QDs played a role in tyramide signal amplification (TSA) in order to expeditor Ab binding. Labeling of multiple targets such as cAMP response element binding protein (CREB) is achievable by merging of QDs with electron microscopy techniques. By means of this methodology, seeking the dynamics of cell surface receptors which has participated in cellular signaling will be possible.128, 129 QDs are competent to visualize the movements of many receptors such as TrkA, GABAc and Glycine.130
QDs are potent imaging agents to visualize/diagnosis several pathogens (Table 3). In addition, in developmental studies, QDs could also be used for the monitoring of microorganisms populations to explore the starvation effects on Dictyostelium discoideum, and they could be tracked with no visible fluorescence destruction.131
Table 3 . Summary of QDs used for diagnosis of infectious pathogens .
| Pathogen | Recognition | Methodology of detection | Detection limit | References |
| E. coli O157:H7 | Biotinylated antibody | Fluorescence microscopy | 2 orders more sensitive than other dyes | 132 |
| E. coli O157:H7 | Fim-H mannose specific lectin | Fluorometry | 104 bacteria/ml | 133 |
| E. coli, Salmonella | Antibody | Fluorescence spectroscopy | 104 CFU/ml | 134 |
| Cholera toxin | Antibody | Fluroimmunoassay | In ng/ml quantity | 134 |
| RSV | Antibody | Color change | Single step/short time | 135 |
| HBV, HCV, HIV | Antibody | Fluorescence | 100 μl sample/50 times more sensitive | 136 |
QDs-based detection technology for in vivo imaging of cancer
QDs conjugated with primary/secondary Abs for over-expressed receptors which are perfect targets for imaging cancers, were extensively used. On the basis of such method, Gao et al. labeled human prostate cancer cells or C4-2, utilizing a conjugate of QDs and an Ab intended for PSMA.5 Prostate cancer cells positive for PSMA were labeled efficiently. Well-known cancer markers that over-expressed in many breast cancers such as Her2 receptor has widely been investigated in detection and therapy. Trastuzumab has been used for conjugation to QDs and anti-Her2 Ab was used for selectively labeling.56 A unique QD nanoprobe has been manufactured for bio-sensing of glioma cells on the basis of the tenascin-C (extracellular matrix glycoprotein) over-expression which is involved in tissue remodeling mechanism and engaged in assaulting of glioblastoma into the surrounding tissue.137, 138
Furthe, Tenascin-C was targeted by the CdSe/ZnS QDs by means of the single-stranded DNA aptamer which was formerly selected by systematic evolution of ligands by exponential enrichment (SELEX) and fluorescence microscopy exposed that the QDs has labeled glioma cells.139 Other study revealed that transferrin were conjugated to phospholipid micelle-encapsulated silicon QDs which attach to pancreatic cancer cells.23 There was 95% cell viability after 24 h and subsequently, concentration of silicon QDs approved to the cells was not toxic, nevertheless higher concentrations caused cell death.23
QDs have been used to sense the delivery of chemotherapeutic drugs (e.g., DOX) to different cancerous cell. In a study, DOX interposed the PSMA positive cells through a targeted system using QD-RNA aptamer, and then fluorescent signal was shaped while QDs went through prostate cancer cells and released DOX.140 In other study, Wu et al. has labeled human breast cancer cells (SK-BR-3) with QD-IgG conjugates using HerceptinTM as homing device. They utilized a humanized anti-Her2 Ab intended for targeting the cells via the QD-streptavidin conjugate for labeling the SK-BR-3.56 Using visible and NIR QDs, MCF-7 and BT-474 breast cancer cells have been labeled with QDs armed with Abs specific to some antigens such as ER, EGFR and m-TOR.105 Furthermore, EGFR single-molecules in human ovarian epidermoid carcinoma cells have been targeted by CdSe/ZnS QDs conjugated with EGF Ab.141
Recently, non-Cd-based QDs has been exploited, as highly efficient and nontoxic bioimaging agent, for targeting and live visualizing of pancreatic cancer cells. In this approach, InP/ZnS QDs were functionalized with mercaptosuccinic acid and further conjugated with pancreatic cancer specific mAbs, and then were successfully used for in vitro and in vivo targeted bioimaging.142
Encapsulation of CdSe/ZnS QDs in Fluorine-18 labeled phospholipids micelle resulted in bimodal imaging probes in combined in vivo fibered confocal fluorescence microscopy and positron emission tomography (PET).143 Overall, biomedical imaging in cancer biology or pathology is supposed to receive a new dimension and impulsion by means of manufacturing appropriate and efficient probes based on QDs.
Concluding remarks
To date, fluorescence bioimaging has significantly changed the face of molecular diagnosis in vitro and in vivo. Because of non-invasiveness with high temporal resolution and lower cost, it is an interesting alternative to the currently used molecular detection methods. Of fluorescence bioimaging methods, QD-based nanoprobes have been considered as stable optical imaging and/or sensing probes that are competent to improve biological monitoring and have been created a prominent achievement in multimodal nanomedicines. There exist compelling evidence that such multifunctional NSs have capability to revolutionize molecular diagnosis of diseases in particular malignancies. They appear to offer simultaneous imaging and therapy with minimized undesired consequences. For detection of cancer biomarkers, different QDs-based assays have been designed successfully, in which QDs provide stable optical characteristics beneficial for multicolor bioassays. In addition, they can be used for development of nanobiosensors which can open several prospects towards identification of a large number of diseases’ molecular markers as well as chemical/biochemical entities. However, despite providing great fluorescence potential, the group II-VI QDs contain toxic heavy metals that often limit their in vivo applications. Therefore, various surface modification approaches have been conducted to reduce their undesired toxicity. Further, some safer QDs have been developed. For example, silicon (Si), shows desirable optic properties with great biocompatibility, upon which Si QDs have been exploited for safe real time monitoring, imaging and targeting of tumors as a multicolor NIR bioimaging tool in vivo. Si QDs provides unique surface functionalization and bioconjugation, producing stable luminescence nanospheres with long (>40 h) tumor accumulation time in vivo.144 We envision that QD-based multicolor arrays will be used for advancing the optical bioimaging techniques with greater stability and less photo-bleaching. And, in near future, we should largely capitalize on implementation of QDs for development of next-generation high throughput sensing and imaging techniques which would benefit early detection and on-demand monitoring and therapy of malignancies.
Acknowledgements
This work has financially been supported by the Deputy of Research Affairs at Tabriz University of Medical Sciences (grant RCPN002: development of QD-based nanobiosensors).
Ethical issues
The authors declare no ethical issues.
Competing interests
The authors announce no conflict of interests.
References
- 1.Rasooly A, Jacobson J. Development of biosensors for cancer clinical testing. Biosens Bioelectron. 2006;21:1851–8. doi: 10.1016/j.bios.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:513–20. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–29. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 4.Barar J, Omidi Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts. 2014;4:3–14. doi: 10.5681/bi.2014.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Hong H, Cai W. Photoacoustic imaging. Cold Spring Harb Protoc. 2011:2011. doi: 10.1101/pdb.top065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. J Am Chem Soc. 2005;127:3870–8. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]
- 8.Jin T, Fujii F, Yamada E, Nodasaka Y, Kinjo M. Control of the optical properties of quantum dots by surface coating with calix[n]arene carboxylic acids. J Am Chem Soc. 2006;128:9288–9. doi: 10.1021/ja062021k. [DOI] [PubMed] [Google Scholar]
- 9.Efros AL, Rosen M, Kuno M, Nirmal M, Norris DJ, Bawendi M. Band-edge exciton in quantum dots of semiconductors with a degenerate valence band: Dark and bright exciton states. Phys Rev B Condens Matter Mater Phys. 1996;54:4843–56. doi: 10.1103/physrevb.54.4843. [DOI] [PubMed] [Google Scholar]
- 10.Tan A, Yildirimer L, Rajadas J, De La Pena H, Pastorin G, Seifalian A. Quantum dots and carbon nanotubes in oncology: a review on emerging theranostic applications in nanomedicine. Nanomedicine (Lond) 2011;6:1101–14. doi: 10.2217/nnm.11.64. [DOI] [PubMed] [Google Scholar]
- 11.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J Am Chem Soc. 1993;115:8706–15. doi: 10.1021/ja00072a025. [DOI] [Google Scholar]
- 13.Manzoor K, Johny S, Thomas D, Setua S, Menon D, Nair S. Bio-conjugated luminescent quantum dots of doped ZnS: A cyto-friendly system for targeted cancer imaging. Nanotechnology. 2009:20. doi: 10.1088/0957-4484/20/6/065102. [DOI] [PubMed] [Google Scholar]
- 14.Yaghini E, Seifalian AM, MacRobert AJ. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine. 2009;4:353–63. doi: 10.2217/nnm.09.9. [DOI] [PubMed] [Google Scholar]
- 15.Papagiannaros A, Levchenko T, Hartner W, Mongayt D, Torchilin V. Quantum dots encapsulated in phospholipid micelles for imaging and quantification of tumors in the near-infrared region. Nanomedicine: Nanotechnology, Biology, and Medicine. 2009;5:216–24. doi: 10.1016/j.nano.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Xiong HM, Xu Y, Ren QG, Xia YY. Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J Am Chem Soc. 2008;130:7522–3. doi: 10.1021/ja800999u. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Fisher B, Eisler HJ, Bawendi M. Type-II quantum dots: CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures. J Am Chem Soc. 2003;125:11466–7. doi: 10.1021/ja0361749. [DOI] [PubMed] [Google Scholar]
- 18.Kirchner C, Liedl T, Kudera S, Pellegrino T, Munoz Javier A, Gaub HE. et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–8. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Chen JY, Idowu M, Nyokong T. Generation of singlet oxygen via the composites of water-soluble thiol-capped CdTe quantum dots-sulfonated aluminum phthalocyanines. J Phys Chem B. 2008;112:4465–9. doi: 10.1021/jp711537j. [DOI] [PubMed] [Google Scholar]
- 20.Pinaud F, Michalet X, Bentolila LA, Tsay JM, Doose S, Li JJ. et al. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials. 2006;27:1679–87. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nirmal M, Brus L. Luminescence Photophysics in Semiconductor Nanocrystals. Acc Chem Res. 1998;32:407–14. doi: 10.1021/ar9700320. [DOI] [Google Scholar]
- 22.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW. et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300:1434–6. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 23.Erogbogbo F, Yong KT, Roy I, Xu G, Prasad PN, Swihart MT. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano. 2008;2:873–8. doi: 10.1021/nn700319z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang LJ, Shen XC, Liang H, Guo S, Liang ZH. Hot-injection synthesis of highly luminescent and monodisperse CdS nanocrystals using thioacetamide and cadmium source with proper reactivity. J Colloid Interface Sci. 2010;342:236–42. doi: 10.1016/j.jcis.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Kucur E, Boldt FM, Cavaliere-Jaricot S, Ziegler J, Nann T. Quantitative analysis of cadmium selenide nanocrystal concentration by comparative techniques. Anal Chem. 2007;79:8987–93. doi: 10.1021/ac0715064. [DOI] [PubMed] [Google Scholar]
- 26.Xie R, Peng X. Synthesis of Cu-doped InP nanocrystals (d-dots) with ZnSe diffusion barrier as efficient and color-tunable NIR emitters. J Am Chem Soc. 2009;131:10645–51. doi: 10.1021/ja903558r. [DOI] [PubMed] [Google Scholar]
- 27.Moreels I, Fritzinger B, Martins JC, Hens Z. Surface chemistry of colloidal PbSe nanocrystals. J Am Chem Soc. 2008;130:15081–6. doi: 10.1021/ja803994m. [DOI] [PubMed] [Google Scholar]
- 28.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–40. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A. et al. Thiol-capping of CDTe nanocrystals: An alternative to organometallic synthetic routes. J Phys Chem B. 2002;106:7177–85. doi: 10.1021/jp025541k. [DOI] [Google Scholar]
- 30.Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WC. In vivo quantum-dot toxicity assessment. Small. 2010;6:138–44. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 31.Aleksejenko G, Pačebutas V, Krotkus A. Optical nonlinearities in PbSe nanocrystals. Acta Physica Polonica A. 2005;107:294–7. [Google Scholar]
- 32.Matsumoto Y, Kanemoto R, Itoh T, Nakanishi S, Ishikawa M, Biju V. Photoluminescence quenching and intensity fluctuations of CdSe-ZnS quantum dots on an Ag nanoparticle film. J Phys Chem C. 2008;112:1345–50. doi: 10.1021/jp076659+. [DOI] [Google Scholar]
- 33.Yong KT, Law WC, Roy I, Jing Z, Huang H, Swihart MT. et al. Aqueous phase synthesis of CdTe quantum dots for biophotonics. J Biophotonics. 2011;4:9–20. doi: 10.1002/jbio.201000080. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Xu L, Chen J, Gao H, Wang S, Fang J. et al. Hydrothermal synthesis of GSH-TGA co-capped CdTe quantum dots and their application in labeling colorectal cancer cells. Colloids Surf B Biointerfaces. 2012;95:247–53. doi: 10.1016/j.colsurfb.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Rogach AL, Ogris M. Near-infrared-emitting semiconductor quantum dots for tumor imaging and targeting. Curr Opin Mol Ther. 2010;12:331–9. [PubMed] [Google Scholar]
- 36.Kirby PJ, Shines CJ, Taylor GJ, Bousquet RW, Price HC, Everitt JI. et al. Pleural effects of indium phosphide in B6C3F1 mice: nonfibrous particulate induced pleural fibrosis. Exp Lung Res. 2009;35:858–82. doi: 10.3109/01902140902980961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougherty TJ, Grindey GB, Fiel R, Weishaupt KR, Boyle DG. Photoradiation therapy II Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 1975;55:115–21. doi: 10.1093/jnci/55.1.115. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Tao H, Yang T, Kong L, Qin D, Chen J. A surfactant-free recipe for shape-controlled synthesis of CdSe nanocrystals. Nanotechnology. 2011;22:045604. doi: 10.1088/0957-4484/22/4/045604. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Zhong Y, Wang J, Su Y, Peng F, Zhou Y. et al. Aqueous synthesized near-infrared-emitting quantum dots for RGD-based in vivo active tumour targeting. Nanotechnology. 2013;24:135101. doi: 10.1088/0957-4484/24/13/135101. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino A, Manabe N, Fujioka K, Suzuki K, Yasuhara M, Yamamoto K. Use of fluorescent quantum dot bioconjugates for cellular imaging of immune cells, cell organelle labeling, and nanomedicine: surface modification regulates biological function, including cytotoxicity. J Artif Organs. 2007;10:149–57. doi: 10.1007/s10047-007-0379-y. [DOI] [PubMed] [Google Scholar]
- 42.Dayal S, Burda C. Surface effects on quantum dot-based energy transfer. J Am Chem Soc. 2007;129:7977–81. doi: 10.1021/ja071457c. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Zhang Y, Lei J, Xue Y, Cheng L, Ju H. Quantum dots based electrochemiluminescent immunosensor by coupling enzymatic amplification with self-produced coreactant from oxygen reduction. Anal Chem. 2010;82:7351–6. doi: 10.1021/ac1013942. [DOI] [PubMed] [Google Scholar]
- 44.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4:435–46. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Chakrabortty S, Gropeanu RA, Wilhelmi J, Xu Y, Er KS. et al. pH-Responsive quantum dots via an albumin polymer surface coating. J Am Chem Soc. 2010;132:5012–4. doi: 10.1021/ja909570v. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7:385–402. doi: 10.1016/j.nano.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Shi L, Rosenzweig N, Rosenzweig Z. Luminescent quantum dots fluorescence resonance energy transfer-based probes for enzymatic activity and enzyme inhibitors. Anal Chem. 2007;79:208–14. doi: 10.1021/ac0614644. [DOI] [PubMed] [Google Scholar]
- 48.Medintz IL, Pons T, Delehanty JB, Susumu K, Brunel FM, Dawson PE. et al. Intracellular delivery of quantum dot-protein cargos mediated by cell penetrating peptides. Bioconjug Chem. 2008;19:1785–95. doi: 10.1021/bc800089r. [DOI] [PubMed] [Google Scholar]
- 49.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG. et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2010;5:42–7. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhang SH, Won N, Lee TJ, Jin H, Nam J, Park J. et al. Hyaluronic acid-quantum dot conjugates for in vivo lymphatic vessel imaging. ACS Nano. 2009;3:1389–98. doi: 10.1021/nn900138d. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X, Ahmed M, Deng Z, Narain R. Biotinylated glyco-functionalized quantum dots: synthesis, characterization, and cytotoxicity studies. Bioconjug Chem. 2009;20:994–1001. doi: 10.1021/bc800566f. [DOI] [PubMed] [Google Scholar]
- 52.Kang WJ, Chae JR, Cho YL, Lee JD, Kim S. Multiplex imaging of single tumor cells using quantum-dot-conjugated aptamers. Small. 2009;5:2519–22. doi: 10.1002/smll.200900848. [DOI] [PubMed] [Google Scholar]
- 53.Zhelev Z, Ohba H, Bakalova R, Jose R, Fukuoka S, Nagase T. et al. Fabrication of quantum dot-lectin conjugates as novel fluorescent probes for microscopic and flow cytometric identification of leukemia cells from normal lymphocytes. Chem Commun (Camb) 2005:1980–2. doi: 10.1039/b419305a. [DOI] [PubMed] [Google Scholar]
- 54.Orndorff RL, Rosenthal SJ. Neurotoxin quantum dot conjugates detect endogenous targets expressed in live cancer cells. Nano Lett. 2009;9:2589–99. doi: 10.1021/nl900789e. [DOI] [PubMed] [Google Scholar]
- 55.Zhao MX, Xia Q, Feng XD, Zhu XH, Mao ZW, Ji LN. et al. Synthesis, biocompatibility and cell labeling of L-arginine-functional beta-cyclodextrin-modified quantum dot probes. Biomaterials. 2010;31:4401–8. doi: 10.1016/j.biomaterials.2010.01.114. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP. et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 57.Jokerst JV, Raamanathan A, Christodoulides N, Floriano PN, Pollard AA, Simmons GW. et al. Nano-bio-chips for high performance multiplexed protein detection: determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosens Bioelectron. 2009;24:3622–9. doi: 10.1016/j.bios.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolatabadi JEN, Mashinchian O, Ayoubi B, Jamali AA, Mobed A, Losic D. et al. Optical and electrochemical DNA nanobiosensors. TrAC-Trend Anal Chem. 2011;30:459–72. doi: 10.1016/j.trac.2010.11.010. [DOI] [Google Scholar]
- 59.Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153:16–22. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Jia X, Lv XJ, Deng YL, Xie HY. Fluorescent quantum dot-labeled aptamer bioprobes specifically targeting mouse liver cancer cells. Talanta. 2010;81:505–9. doi: 10.1016/j.talanta.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 61.Cheng AK, Su H, Wang YA, Yu HZ. Aptamer-based detection of epithelial tumor marker mucin 1 with quantum dot-based fluorescence readout. Anal Chem. 2009;81:6130–9. doi: 10.1021/ac901223q. [DOI] [PubMed] [Google Scholar]
- 62.Algar WR, Krull UJ. Toward a multiplexed solid-phase nucleic acid hybridization assay using quantum dots as donors in fluorescence resonance energy transfer. Anal Chem. 2009;81:4113–20. doi: 10.1021/ac900421p. [DOI] [PubMed] [Google Scholar]
- 63.Jamali AA, Pourhassan-Moghaddam M, Dolatabadi JEN, Omidi Y. Nanomaterials on the road to miRNA detection with optical and electrochemical nanobiosensors. TrAC-Trend Anal Chem. 2014;55:24–42. doi: 10.1016/j.trac.2013.10.008. [DOI] [Google Scholar]
- 64.Zhang H, Zeng X, Li Q, Gaillard-Kelly M, Wagner CR, Yee D. Fluorescent tumour imaging of type I IGF receptor in vivo: comparison of antibody-conjugated quantum dots and small-molecule fluorophore. Br J cancer. 2009;101:71–9. doi: 10.1038/sj.bjc.6605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaman MB, Baral TN, Jakubek ZJ, Zhang J, Wu X, Lai E. et al. Single-domain antibody bioconjugated near-IR quantum dots for targeted cellular imaging of pancreatic cancer. J Nanosci Nanotechnol. 2011;11:3757–63. doi: 10.1166/jnn.2011.4167. [DOI] [PubMed] [Google Scholar]
- 66.Mittal R, Bruchez MP. Biotin-4-fluorescein based fluorescence quenching assay for determination of biotin binding capacity of streptavidin conjugated quantum dots. Bioconjug Chem. 2011;22:362–8. doi: 10.1021/bc100321c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shao D, Zeng Q, Fan Z, Li J, Zhang M, Zhang Y. et al. Monitoring HSV-TK/ganciclovir cancer suicide gene therapy using CdTe/CdS core/shell quantum dots. Biomaterials. 2012;33:4336–44. doi: 10.1016/j.biomaterials.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 68.Genin E, Carion O, Mahler B, Dubertret B, Arhel N, Chameau P. et al. CrAsH-quantum dot nanohybrids for smart targeting of proteins. J Am Chem Soc. 2008;130:8596–7. doi: 10.1021/ja802987q. [DOI] [PubMed] [Google Scholar]
- 69.Chan P, Yuen T, Ruf F, Gonzalez-Maeso J, Sealfon SC. Method for multiplex cellular detection of mRNAs using quantum dot fluorescent in situ hybridization. Nucleic Acids Research. 2005;33:1–8. doi: 10.1093/nar/gni162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Khullar O, Frangioni JV, Grinstaff M, Colson YL. Image-guided sentinel lymph node mapping and nanotechnology-based nodal treatment in lung cancer using invisible near-infrared fluorescent light. Semin Thorac Cardiovasc Surg. 2009;21:309–15. doi: 10.1053/j.semtcvs.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang K, Zhang FJ, Tang H, Zhao C, Cao YA, Lv XQ. et al. In-vivo imaging of oral squamous cell carcinoma by EGFR monoclonal antibody conjugated near-infrared quantum dots in mice. Int J Nanomedicine. 2011;6:1739–45. doi: 10.2147/IJN.S23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A. et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–34. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubois F, Mahler B, Dubertret B, Doris E, Mioskowski C. A versatile strategy for quantum dot ligand exchange. J Am Chem Soc. 2007;129:482–3. doi: 10.1021/ja067742y. [DOI] [PubMed] [Google Scholar]
- 75.Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J Am Chem Soc. 2007;129:13987–96. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe M, Fujioka K, Akiyama N, Takeyama H, Manabe N, Yamamoto K. et al. Conjugation of quantum dots and JT95 IgM monoclonal antibody for thyroid carcinoma without abolishing the specificity and activity of the antibody. IEEE Trans Nanobioscience. 2011;10:30–5. doi: 10.1109/TNB.2011.2125800. [DOI] [PubMed] [Google Scholar]
- 77.Charalambous A, Andreou M, Antoniades I, Christodoulou N, Skourides PA. In vivo, site-specific, covalent conjugation of quantum dots to proteins via split-intein splicing. Methods Mol Biol. 2012;906:157–69. doi: 10.1007/978-1-61779-953-2_11. [DOI] [PubMed] [Google Scholar]
- 78.Ruan J, Song H, Qian Q, Li C, Wang K, Bao C. et al. HER2 monoclonal antibody conjugated RNase-A-associated CdTe quantum dots for targeted imaging and therapy of gastric cancer. Biomaterials. 2012;33:7093–102. doi: 10.1016/j.biomaterials.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 79.Albrecht MA, Evans CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chemistry. 2006;8:417–32. doi: 10.1039/B517131H. [DOI] [Google Scholar]
- 80.Su Y, He Y, Lu H, Sai L, Li Q, Li W. et al. The cytotoxicity of cadmium based, aqueous phase - synthesized, quantum dots and its modulation by surface coating. Biomaterials. 2009;30:19–25. doi: 10.1016/j.biomaterials.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 81.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–72. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma-Hock L, Brill S, Wohlleben W, Farias PM, Chaves CR, Tenorio DP. et al. Short term inhalation toxicity of a liquid aerosol of CdS/Cd(OH)(2) core shell quantum dots in male Wistar rats. Toxicol Lett. 2012;208:115–24. doi: 10.1016/j.toxlet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 83.Su Y, Peng F, Jiang Z, Zhong Y, Lu Y, Jiang X. et al. In vivo distribution, pharmacokinetics, and toxicity of aqueous synthesized cadmium-containing quantum dots. Biomaterials. 2011;32:5855–62. doi: 10.1016/j.biomaterials.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 84.Galeone A, Vecchio G, Malvindi MA, Brunetti V, Cingolani R, Pompa PP. In vivo assessment of CdSe-ZnS quantum dots: coating dependent bioaccumulation and genotoxicity. Nanoscale. 2012;4:6401–7. doi: 10.1039/c2nr31826a. [DOI] [PubMed] [Google Scholar]
- 85.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–8. doi: 10.1021/Nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009;238:280–8. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan WH, Shiao NH, Lu PZ. CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol Lett. 2006;167:191–200. doi: 10.1016/j.toxlet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 88.Pons T, Pic E, Lequeux N, Cassette E, Bezdetnaya L, Guillemin F. et al. Cadmium-free CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano. 2010;4:2531–8. doi: 10.1021/nn901421v. [DOI] [PubMed] [Google Scholar]
- 89.Subramaniam P, Lee SJ, Shah S, Patel S, Starovoytov V, Lee KB. Generation of a library of non-toxic quantum dots for cellular imaging and siRNA delivery. Adv Mater. 2012;24:4014–9. doi: 10.1002/adma.201201019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmadian S, Barar J, Saei AA, Fakhree MA, Omidi Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J Vis Exp. 2009 doi: 10.3791/1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barar J, Omidi Y. Intrinsic bio-signature of gene delivery nanocarriers may impair gene therapy goals. Bioimpacts. 2013;3:105–9. doi: 10.5681/bi.2013.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target. 2007;15:83–8. doi: 10.1080/10611860601151860. [DOI] [PubMed] [Google Scholar]
- 93.Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. Bioimpacts. 2011;1:23–30. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakhlband A, Barar J, Bidmeshkipour A, Heidari HR, Omidi Y. Bioimpacts of anti epidermal growth factor receptor antisense complexed with polyamidoamine dendrimers in human lung epithelial adenocarcinoma cells. J Biomed Nanotechnol. 2010;6:360–9. doi: 10.1166/jbn.2010.1131. [DOI] [PubMed] [Google Scholar]
- 95.Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol. 2009;28:113–22. doi: 10.1177/1091581809335177. [DOI] [PubMed] [Google Scholar]
- 96.Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv. 2005;2:429–41. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- 97.Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods. 2008;18:369–78. doi: 10.1080/15376510801891286. [DOI] [PubMed] [Google Scholar]
- 98.Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target. 2003;11:311–23. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- 99.Omidi Y, Hollins AJ, Drayton RM, Akhtar S. Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target. 2005;13:431–43. doi: 10.1080/10611860500418881. [DOI] [PubMed] [Google Scholar]
- 100.Saei AA, Omidi Y. A glance at DNA microarray technology and applications. Bioimpacts. 2011;1:75–86. doi: 10.5681/bi.2011.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biju V, Mundayoor S, Omkumar RV, Anas A, Ishikawa M. Bioconjugated quantum dots for cancer research: present status, prospects and remaining issues. Biotechnol Adv. 2010;28:199–213. doi: 10.1016/j.biotechadv.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Hu R, Law WC, Lin G, Ye L, Liu J, Reynolds JL. et al. PEGylated Phospholipid Micelle-Encapsulated Near-Infrared PbS Quantum Dots for in vitro and in vivo Bioimaging. Theranostics. 2012;2:723–33. doi: 10.7150/thno.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han S, Mu Y, Zhu Q, Gao Y, Li Z, Jin Q. et al. Au:CdHgTe quantum dots for in vivo tumor-targeted multispectral fluorescence imaging. Anal Bioanal Chem. 2012;403:1343–52. doi: 10.1007/s00216-012-5921-y. [DOI] [PubMed] [Google Scholar]
- 104.Yong KT. Biophotonics and biotechnology in pancreatic cancer: cyclic RGD-peptide-conjugated type II quantum dots for in vivo imaging. Pancreatology. 2010;10:553–64. doi: 10.1159/000283577. [DOI] [PubMed] [Google Scholar]
- 105.Yezhelyev MV, Al-Hajj A, Morris C, Marcus AI, Liu T, Lewis M. et al. In Situ Molecular Profiling of Breast Cancer Biomarkers with Multicolor Quantum Dots. Adv Mater. 2007;19:3146–51. doi: 10.1002/adma.200701983. [DOI] [Google Scholar]
- 106.Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Chung LW. et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2007;2:1152–65. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 107.Kawashima N, Nakayama K, Itoh K, Itoh T, Ishikawa M, Biju V. Reversible dimerization of EGFR revealed by single-molecule fluorescence imaging using quantum dots. Chemistry. 2010;16:1186–92. doi: 10.1002/chem.200902963. [DOI] [PubMed] [Google Scholar]
- 108.Ren D, Xia Y, You Z. Multiplexed living cells stained with quantum dot bioprobes for multiplexed detection of single-cell array. J Biomed Opt. 2013;18:096005. doi: 10.1117/1.JBO.18.9.096005. [DOI] [PubMed] [Google Scholar]
- 109.Weng KC, Noble CO, Papahadjopoulos-Sternberg B, Chen FF, Drummond DC, Kirpotin DB. et al. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett. 2008;8:2851–7. doi: 10.1021/nl801488u. [DOI] [PubMed] [Google Scholar]
- 110.Nair LV, Nagaoka Y, Maekawa T, Sakthikumar D, Jayasree RS. Quantum dot tailored to single wall carbon nanotubes: a multifunctional hybrid nanoconstruct for cellular imaging and targeted photothermal therapy. Small. 2014;10:2771–5, 40. doi: 10.1002/smll.201400418. [DOI] [PubMed] [Google Scholar]
- 111.Yong KT. Mn-doped near-infrared quantum dots as multimodal targeted probes for pancreatic cancer imaging. Nanotechnology. 2009;20:015102. doi: 10.1088/0957-4484/20/1/015102. [DOI] [PubMed] [Google Scholar]
- 112.Kaul Z, Yaguchi T, Harada JI, Ikeda Y, Hirano T, Chiura HX. et al. An antibody-conjugated internalizing quantum dot suitable for long-term live imaging of cells. Biochem Cell Biol. 2007;85:133–40. doi: 10.1139/o06-205. [DOI] [PubMed] [Google Scholar]
- 113.Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat Mater. 2003;2:630–8. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 114.Hohng S, Ha T. Single-molecule quantum-dot fluorescence resonance energy transfer. Chemphyschem. 2005;6:956–60. doi: 10.1002/cphc.200400557. [DOI] [PubMed] [Google Scholar]
- 115.Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL. et al. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat Mater. 2006;5:581–9. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 116.Shi L, De Paoli V, Rosenzweig N, Rosenzweig Z. Synthesis and application of quantum dots FRET-based protease sensors. J Am Chem Soc. 2006;128:10378–9. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 117.Clapp AR, Medintz IL, Fisher BR, Anderson GP, Mattoussi H. Can luminescent quantum dots be efficient energy acceptors with organic dye donors? J Am Chem Soc. 2005;127:1242–50. doi: 10.1021/ja045676z. [DOI] [PubMed] [Google Scholar]
- 118.Ho CL, Kurman RJ, Dehari R, Wang TL, Shih Ie M. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004;64:6915–8. doi: 10.1158/0008-5472.CAN-04-2067. [DOI] [PubMed] [Google Scholar]
- 119.Bailey VJ, Easwaran H, Zhang Y, Griffiths E, Belinsky SA, Herman JG. et al. MS-qFRET: a quantum dot-based method for analysis of DNA methylation. Genome Res. 2009;19:1455–61. doi: 10.1101/gr.088831.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang E, Miller JS, Sun J, Yu WW, Colvin VL, Drezek R. et al. Protease-activated quantum dot probes. Biochem Biophys Res Commun. 2005;334:1317–21. doi: 10.1016/j.bbrc.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 121.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 122.Zhou L, El-Deiry WS. Multispectral fluorescence imaging. J Nucl Med. 2009;50:1563–6. doi: 10.2967/jnumed.109.063925. [DOI] [PubMed] [Google Scholar]
- 123.Karabanovas V, Zitkus Z, Kuciauskas D, Rotomskis R, Valius M. Surface properties of quantum dots define their cellular endocytic routes, mitogenic stimulation and suppression of cell migration. J Biomed Nanotechnol. 2014;10:775–86. doi: 10.1166/jbn.2014.1770. [DOI] [PubMed] [Google Scholar]
- 124.Ruan G, Agrawal A, Marcus AI, Nie S. Imaging and tracking of tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J Am Chem Soc. 2007;129:14759–66. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 125.Hasegawa U, Nomura SM, Kaul SC, Hirano T, Akiyoshi K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun. 2005;331:917–21. doi: 10.1016/j.bbrc.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 126.Mansson A, Sundberg M, Balaz M, Bunk R, Nicholls IA, Omling P. et al. In vitro sliding of actin filaments labelled with single quantum dots. Biochem Biophys Res Commun. 2004;314:529–34. doi: 10.1016/j.bbrc.2003.12.133. [DOI] [PubMed] [Google Scholar]
- 127.Sukhanova A, Devy J, Venteo L, Kaplan H, Artemyev M, Oleinikov V. et al. Biocompatible fluorescent nanocrystals for immunolabeling of membrane proteins and cells. Anal Biochem. 2004;324:60–7. doi: 10.1016/j.ab.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 128.Nisman R, Dellaire G, Ren Y, Li R, Bazett-Jones DP. Application of quantum dots as probes for correlative fluorescence, conventional, and energy-filtered transmission electron microscopy. J Histochem Cytochem. 2004;52:13–8. doi: 10.1177/002215540405200102. [DOI] [PubMed] [Google Scholar]
- 129.Rosenthal SJ, Tomlinson I, Adkins EM, Schroeter S, Adams S, Swafford L. et al. Targeting cell surface receptors with ligand-conjugated nanocrystals. J Am Chem Soc. 2002;124:4586–94. doi: 10.1021/ja003486s. [DOI] [PubMed] [Google Scholar]
- 130.Giepmans BN, Deerinck TJ, Smarr BL, Jones YZ, Ellisman MH. Correlated light and electron microscopic imaging of multiple endogenous proteins using Quantum dots. Nat Methods. 2005;2:743–9. doi: 10.1038/nmeth791. [DOI] [PubMed] [Google Scholar]
- 131.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 132.Hahn MA, Tabb JS, Krauss TD. Detection of single bacterial pathogens with semiconductor quantum dots. Anal Chem. 2005;77:4861–9. doi: 10.1021/ac050641i. [DOI] [PubMed] [Google Scholar]
- 133.Mukhopadhyay B, Martins MB, Karamanska R, Russell DA, Field RA. Bacterial detection using carbohydrate-functionalised CdS quantum dots: a model study exploiting E. coli recognition of mannosides Tetrahedron Letters. 2009;50:886–9. doi: 10.1016/j.tetlet.2008.12.029. [DOI] [Google Scholar]
- 134.Yang L, Li Y. Simultaneous detection of Escherichia coli O157:H7 and Salmonella Typhimurium using quantum dots as fluorescence labels. Analyst. 2006;131:394–401. doi: 10.1039/b510888h. [DOI] [PubMed] [Google Scholar]
- 135.Tripp RA, Alvarez R, Anderson B, Jones L, Weeks C, Chen W. Bioconjugated nanoparticle detection of respiratory syncytial virus infection. Int J Nanomedicine. 2007;2:117–24. doi: 10.2147/nano.2007.2.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klostranec JM, Xiang Q, Farcas GA, Lee JA, Rhee A, Lafferty EI. et al. Convergence of quantum dot barcodes with microfluidics and signal processing for multiplexed high-throughput infectious disease diagnostics. Nano Lett. 2007;7:2812–8. doi: 10.1021/nl071415m. [DOI] [PubMed] [Google Scholar]
- 137.Chen XC, Deng YL, Lin Y, Pang DW, Qing H, Qu F. et al. Quantum dot-labeled aptamer nanoprobes specifically targeting glioma cells. Nanotechnology. 2008;19:235105. doi: 10.1088/0957-4484/19/23/235105. [DOI] [PubMed] [Google Scholar]
- 138.Hirata E, Arakawa Y, Shirahata M, Yamaguchi M, Kishi Y, Okada T. et al. Endogenous tenascin-C enhances glioblastoma invasion with reactive change of surrounding brain tissue. Cancer Sci. 2009;100:1451–9. doi: 10.1111/j.1349-7006.2009.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci U S A. 2003;100:15416–21. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R. et al. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7:3065–70. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 141.Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007;67:1138–44. doi: 10.1158/0008-5472.CAN-06-1185. [DOI] [PubMed] [Google Scholar]
- 142.Yong KT, Ding H, Roy I, Law WC, Bergey EJ, Maitra A. et al. Imaging pancreatic cancer using bioconjugated inp quantum dots. ACS Nano. 2009;3:502–10. doi: 10.1021/nn8008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duconge F, Pons T, Pestourie C, Herin L, Theze B, Gombert K. et al. Fluorine-18-labeled phospholipid quantum dot micelles for in vivo multimodal imaging from whole body to cellular scales. Bioconjug Chem. 2008;19:1921–6. doi: 10.1021/bc800179j. [DOI] [PubMed] [Google Scholar]
- 144.Erogbogbo F, Yong KT, Roy I, Hu R, Law WC, Zhao W. et al. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS Nano. 2011;5:413–23. doi: 10.1021/nn1018945. [DOI] [PubMed] [Google Scholar]