Abstract
The World Health Organization (WHO) has, for some time, encouraged countries endemic for schistosomiasis to control morbidity from this disease through mass drug administration (MDA) of the well-tolerated drug, praziquantel (PZQ). With the London Declaration in January 2012 and the promise by Merck Serono to eventually donate 250 million PZQ tablets per year, most endemic countries in sub-Saharan Africa have now developed national plans to do MDA for schistosomiasis morbidity control. More recently, based on two World Health Assembly (WHA) resolutions (WHA 54.19 & WHA 65.21) on schistosomiasis, countries are further encouraged to eliminate schistosomiasis, where feasible. The fight against schistosomiasis is therefore in a critical period of tremendous opportunities and equal challenges. How do we do the most effective job of MDA? What tools do we need to do this job better? How will we know when to move from morbidity control to elimination? What combinations of interventions, beyond MDA, are needed to eliminate transmission? The Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) has its Secretariat at the University of Georgia and with programs in more than 26 institutions in 19 countries it is trying to answer these very practical questions through multiple large field-based studies and the evaluation or development of better diagnostics for schistosomiasis. This presentation will summarize the current status of morbidity control and elimination programs and the operational research by SCORE that we hope will provide much-needed answers for national program managers so they can most effectively pursue these critical public health programs.
Keywords: Schistosomiasis, Control, Elimination, Operational Research, Diagnostics, Mass Drug Administration
Greeting
Thank you very much for this opportunity. I greatly appreciate coming back to Nagasaki to see many friends and participate in this meeting of the Japanese Society of Tropical Medicine. Ten years ago, I was privileged to participate in the centenary symposium for the discovery of Schistosoma japonicum here in Japan. I spoke at that time about morbidity control and schistosomiasis in a very theoretical way. But today I am going to talk about mass drug administration (MDA) in a much more practical, public health manner. But, when I think back to the symposium on the discovery of S. japonicum, it makes me realize that I am talking to the wrong audience about control and elimination of schistosomiasis. Why is that?
It is because Japan is the first and best example of where schistosomiasis has already been eliminated. You, the members of this venerable Society already know how to do this. However, because it has been a long time since this major accomplishment, I will remind you of some aspects of control and elimination, and discuss the situation in Africa and some of the challenges we have now.
Schistosomiasis Background: Transmission and Control
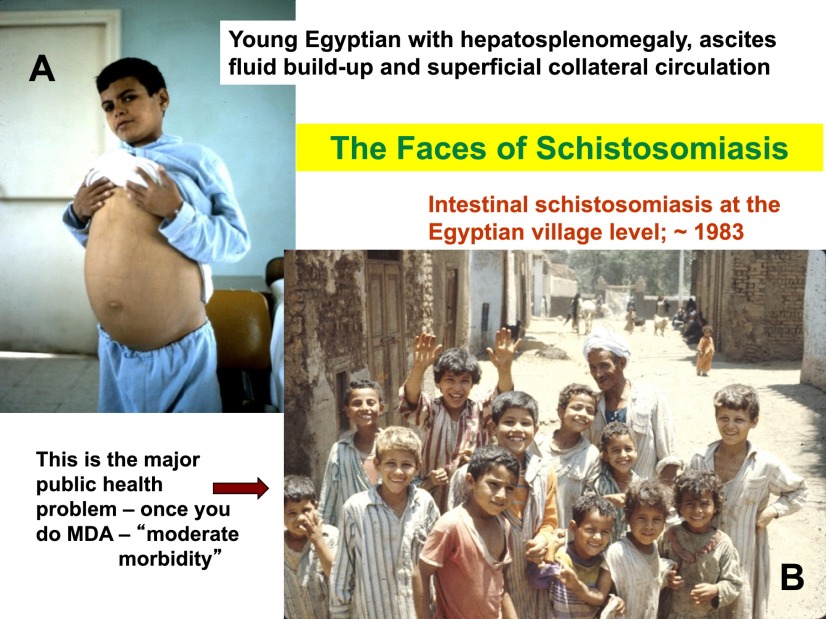
This Egyptian adolescent is the textbook face of schistosomiasis (Fig. 1A), presenting with periportal fibrosis and hepatosplenomegaly. However, with the advent of MDA with praziquantel we really need to think about the broader public health problem of schistosomiasis at the village level. When I took this picture (Fig. 1B) in Qalyub province outside of Cairo, in this age group (excluding the interloper in the photograph), ~80% of these boys were infected with schistosomes. Only one of them—we still do not know how to tell which one—would be likely to progress to the hepatosplenic form. But the public health problem is really this whole group, who suffer from what is called “subtle morbidity” and is seen as developmental deficiencies, anemia, hematuria and other symptoms, and results in life-long challenges [1–3].
Fig. 1.
Photographs of Egyptians with schistosomiasis mansoni. A) An adolescent male with periportal fibrosis of the liver, esophageal varices and hepatosplenic disease. B) A group of (mainly) grade school children in a village with ~80% prevalence of Schistosoma mansoni infection in this age group.
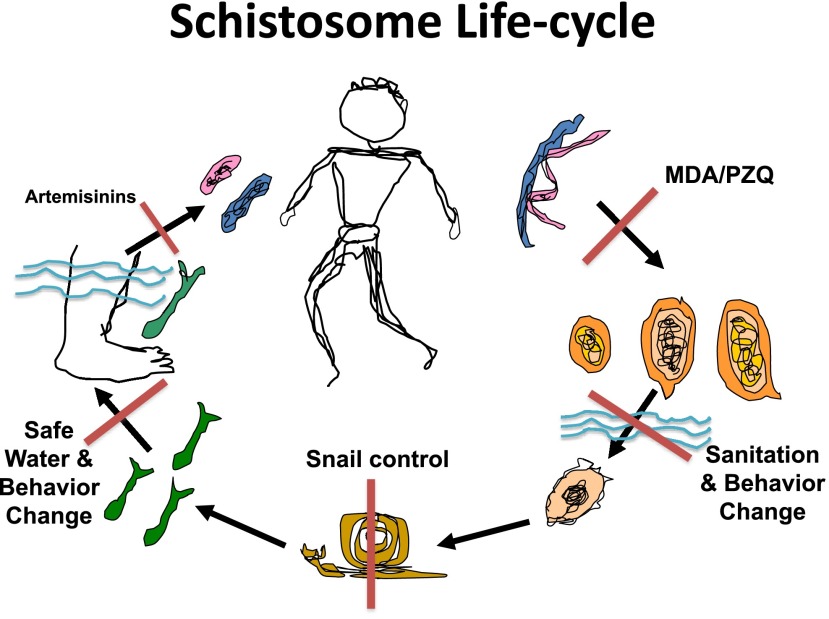
To control and eliminate this major public health threat we need to think about the transmission dynamics of schistosomiasis. If you have fresh water, specific intermediate host/vector snails, human contact with that water and human contamination of that water, you can have transmission. But, if you eliminate any one of those, transmission stops. So, as seen in this diagrammatic life-cycle (Fig. 2), the current possible points of attack for controlling or eliminating schistosomiasis include sanitation, water supply, and education, behavior change, snail control, and chemotherapy. When you think about stopping transmission, these are the interventions you can use. You can kill the adult worms, through MDA with praziquantel. You can keep the eggs from getting in the water through sanitation. You can kill the snails with molluscicides. You can have safe water for people to get into or to drink or to fetch with wells. If you know when infection occurred (which is rare) you can kill juvenile worms with artemisinins. Thus there are several ways to stop transmission of this disease. But remember, the most common public health plan for schistosomiasis, based on World Health Assembly Resolution 54.19, is that all countries with schistosomiasis should be doing morbidity control through MDA, also known as preventive chemotherapy.
Fig. 2.
Cartoon of human schistosome life cycles depicting adult worms, eggs of the three main human schistosomes, a miracidium, snail vector, cercariae, and schistosomula and showing the means by which and the points at which transmission can be controlled (red bars).
Morbidity control by MDA is not the same as elimination by MDA, and this distinction is important in regard to the last question for Dr. Ichimori that followed her presentation. As she stated, your goal with each disease is dependent on the biology of the organism in question and its lifecycle. For example, lymphatic filariasis (LF) may be eliminated by MDA, while schistosomiasis and STH differ from LF and from each other in the life-span of the worms and the ease of transmission.
There is evidence that regular treatment with praziquantel prevents severe, hepatosplenic schistosomiasis and subtle morbidity due to schistosomiasis [4,5]. Yes, children will get re-infected, but then you come back with the MDA again and kill the new adult worms. Thus children are ‘worm-free’ for a sufficient period of time that they do not develop severe disease, and if done regularly MDA will decrease subtle morbidity, but in most transmission settings this will not lead to elimination.
The Bill and Melinda Gates Foundation funded the Schistosomiasis Control Initiative (SCI; http://www3. imperial.ac.uk/schisto), in Imperial College in London to show that MDA with praziquantel could actually be rolled-out countrywide. SCI succeeded in doing this over a number of years in several different countries. They showed that with enough money, time and sustained work in countries with their ministries, it could be done by the National Programs.
There are now major ongoing efforts by WHO/NTD, SCI, USAID, DFID, Merck Serono, and many others showing that you can do morbidity control through MDA on a countrywide level. The next question might be what is the best way to do this? We know it works if done regularly, but is one way more effective than another?
Overview of the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE)
To address such questions, the Bill and Melinda Gates Foundation funded a program called the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE). Now, before I get to that, I wanted to introduce another title for my talk. I know that many people in Japan follow baseball. So this is going to be a baseball title, ‘Schistosomiasis—What inning are we in—and what is the score?’ By the end of the talk I hope to tell you what I think the score is in the fight against schistosomiasis.
SCORE is a consortium to do operational research on control and elimination of schistosomiasis caused by Schistosoma mansoni and S. haematobium. The definition of operational research is a challenge, because it means different things to different people. Our definition is: “finding out what current and future program managers need to do the job better—both programmatically and in terms of the tools needed.” It is truly research, because we do not know the answers that we will get. But the questions are such that if we get answers they will directly help control program managers in the field. Our hope is that country program managers will be able to implement the findings of SCORE’s research to do a more effective job of controlling and eventually eliminating schistosomiasis.
I am the Director of this consortium, but I do not do the research. The SCORE Secretariat designs the studies with a lot of input from many people. We have an Advisory Committee made of people who know various aspects of schistosomiasis and representation from the NTD Office of WHO/Geneva. SCORE is a consortium of over 55 investigators doing projects in 19 different countries, 26 different institutions. So, it is a large consortium doing a lot of research. Again, I am not doing the actual research. The consortium members are doing the research. The SCORE website is http://score.uga.edu and you can look up more details about SCORE there.
SCORE is currently asking several different operational research questions (Table 1). SCORE is trying to find a better mapping tool than the Kato-Katz stool examination for S. mansoni. For S. haematobium, you can do hemastix assays for heme in the urine to determine prevalence, but for S. mansoni we still have to do stool exams and that slows things down, is unpleasant and can impede integration of NTD control programs [6]. SCORE is also trying to perfect a highly sensitive and specific diagnostic for when control moves to elimination. In several very large studies SCORE is trying to evaluate the best means of praziquantel distribution to gain and sustain morbidity control by MDA, and then what are the best combinations of interventions to achieve elimination. For example, how can we improve delivery of snail control? Also, while reporting MDA coverage is very important, it would also be good to actually show the impact of your MDA on the health and well-being impact of children, and SCORE is looking for better ways to do that. SCORE is also determining if MDA has an impact on the schistosome genomic population structure. We have only one drug to treat schistosomiasis and we are advocating MDA. Does that bother anyone? It should. We should always be concerned about the possible development of drug resistance. We do not have clinical resistance to praziquantel yet, but maybe the operative word is “yet.” We do not even know the mechanism of action of praziquantel, so we cannot look for resistance, but we can look at the genomic populations of schistosomes under heavy drug pressure, so that is what SCORE is doing at the village level. SCORE is also looking at whether data on vector snails can predict how well MDA will do in a given area. Lastly, Dr. Charlie King of the SCORE Secretariat is pursuing several questions we call Rapid Answer Projects (RAPs). These are questions for which we believe there is already plenty of literature, but it needs to be brought together in either meta-analyses or systematic literature reviews. Some of these are already published [7, 8].
Table 1.
Public health operational research questions SCORE is currently pursuing or will soon ask
| • | A better mapping tool than Kato-Katz stool exam for S. mansoni |
| • | A highly sensitive and specific diagnostic tool for use when approaching elimination of transmission |
| • | Best means of PZQ distribution to gain & sustain morbidity control by MDA |
| • | Best combination of interventions to eliminate S. haematobium and S. mansoni transmission |
| • | How can we improve and best deliver effective snail control |
| • | Better way to show impact of MDA on morbidity |
| • | Does MDA impact schistosome population structures—as clues to the development of drug resistance |
| • | Can snail data predict the effectiveness of MDA for a given village |
| • | Rapid Answer Projects—analyzing existing data for: “double treatment”; “hemastix efficacy post-treatment campaigns”; “reinfection of adults with S. haematobium”; “uses and impact of niclosamide” |
A Better Mapping Tool for Schistosomiasis Mansoni
Now I will tell you a little bit about some of these projects. The first is about a better mapping tool for S. mansoni prevalence. National NTD program MDA strategies for schistosomiasis depend on the starting prevalence in school-age children. Therefore, you first have to create prevalence maps to determine what level of MDA you should do. This is a big barrier to integrating S. mansoni control into other control programs because it takes time to get a stool. It’s not easy. It’s nasty. People don’t like it. The technicians have to be good. The microscopes have to be good. So SCORE’s goal was to evaluate a commercially available cassette, point-of-contact mapping tool for S. mansoni prevalence that is done on urine. We focused on this point of contact circulating cathodic antigen (POC-CCA) assay, because it was commercially available in South Africa (Rapid Medical Diagnostics, Pretoria, RSA; info@rapid-diagnostics.com), and easy to use and it had performed well in high prevalence areas [9–11]. SCORE wanted to know how well it would perform in moderate and low prevalence areas compared to the Kato-Katz fecal exam.
SCORE funded groups in five countries; Cote d’Ivoire, Cameroon, Uganda, Kenya, and Ethiopia to do parallel Kato-Katz assays and this POC-CCA assay, to see if it would be “just as good as Kato-Katz.” If it is just as good, then we think it is better, because it is done on-site, using urine instead of feces. This 5-country study was done in 63 schools on a total of 4305 children. Figure 3 shows this being done in a school in western Kenya and the cassettes. The positive assay points to two lines (orange arrow). Remember, this is a urine assay to detect S. mansoni infection. The results of the 5-country study have been published and show that a single POC-CCA assay was much more sensitive than a single Kato-Katz stool examination with 2 slides [12]. Based on this study in 5 African countries, we believe that the POC-CCA assay is “just as good as a Kato-Katz” and is actually more sensitive than a Kato-Katz at intensities of infection below ~60 eggs/gram of stool. At intensities below 10 eggs/gram the sensitivity of the POC-CCA begins to drop. SCORE is currently pursuing other evaluations of this assay, but we believe that it is a better mapping tool than the Kato-Katz assay and it is now being used for this in a number of country control programs. Because it is more sensitive, its widespread use would necessitate consideration of a change in the prevalence guidelines for control programs, which is of course challenging.
Fig. 3.
Photographs of the Point-of-contact Circulating Cathodic Antigen (POC-CCA) assay being done in western Kenya and of the POC-CCA cassettes with one cassette (orange arrow) showing a positive “test” line, in addition to the internal control line seen in all the cassettes.
Because there is no gold standard for schistosomiasis diagnosis, you have to use biostatistical programs like latent class analysis (LCA) to compare diagnostic tests. Subjecting these data to LCA we see that the Kato-Katz is 100% specific (because when you see an egg it is real), but it is only 62% sensitive, whereas the POC-CCA is 86% sensitive. However, the POC-CCA does not look as good in terms of specificity, which is only 72% by this analysis. However, we think this could be misleading because when the POC-CCA was tested in Ethiopia, in an area that was not endemic for S. mansoni but had a high prevalence of soil-transmitted helminths, the POC-CCA gave only 1% false-positivity. Nevertheless, SCORE is continuing to evaluate the POC-CCA in different settings and with more rigorous training prior to its use. Our recommendation is that it be further tried and used for mapping of S. mansoni.
A Highly Sensitive and Specific Laboratory Assay for Us As We Approach Elimination
In addition to the POC-CCA for mapping, SCORE is also funding studies on a very sensitive and very specific diagnostic tool called the UCP-CAA assay [13]. This assay detects the circulating anodic antigen (CAA) and the people at Leiden University’s medical school, Govert van Dam and Paul Corstjens, are doing this work. SCORE is funding them to make this very good assay even better, and to evaluate it in very low prevalence areas. They now have the UCP-CAA assay to a point where it can detect one worm in an infected baboon—when the baboons are perfused to determine the actual number of worms, which of course cannot be done on humans. I am not going to go through this system in detail, but it can be done on either serum or urine and is highly sensitive, highly specific and detects active infection by any of the major human schistosomes.
We think this is a very good assay. SCORE is now taking this assay to the field to see how well it does in very low prevalence areas, areas with very low intensities of infection, such as Zanzibar and other locations selected in conjunction with WHO. We hope this test will soon be moving down a pathway to commercialization.
Programmatic Questions Being Asked by SCORE
SCORE’s questions on MDA are how often and how long do you need to do it, is it better to do School-Based Treatment (SBT) or Community-Wide Treatment (CWT) and does it matter what the starting prevalence is, if you want to gain or sustain control? As I indicated earlier and Dr. Ichimori said, for LF the number of MDAs until elimination is based on the expected life-spans of the adult worms. But for schistosomiasis control we simply do not know how long MDA should go on, or what it costs to lower the prevalence and intensity to an “acceptable” level. SCORE is trying to ask these questions in 5 different African countries. We call these our Gaining and Sustaining control activities.
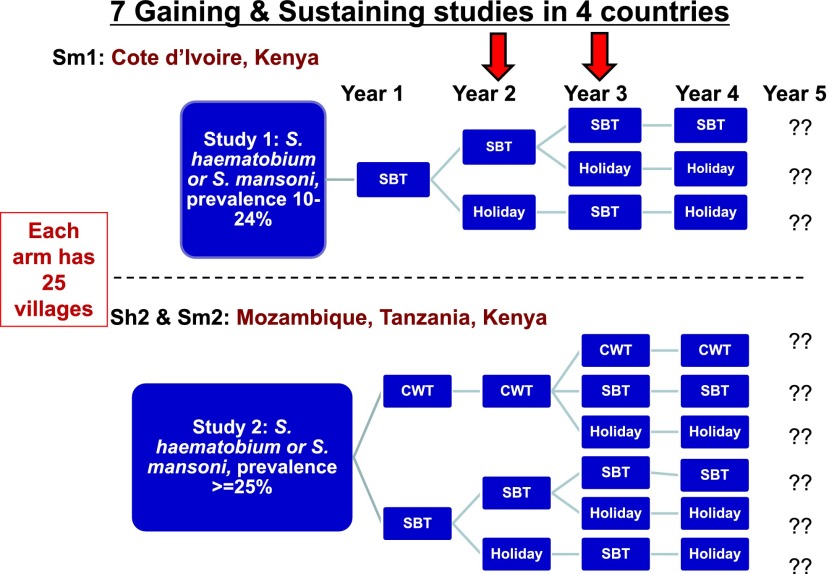
Sustaining control means we start with a prevalence of between 10% and 24%. Gaining control means we start at 25% or higher prevalence and compare the results of different regimens of MDA over 4 years (Fig. 4). Each of these arms has 25 villages. These very large studies are being done in Cote d’Ivoire, Mozambique, Tanzania and Kenya. The Gaining control studies compare SBT with CWT and different arms are treated every year or every other year, or two years in a row and then left untreated (drug holidays).
Fig. 4.
Diagram of the large SCORE field studies of Gaining control (starting prevalence of >/= 25%) and Sustaining control (starting prevalence of 10%–24%). Each arm is comprised of 25 villages or schools and the red arrows indicate that some of the studies are finishing their Year 2 and others are finishing their Year 3 of four years of MDA and 5 years of data collection. CWT = Community-wide Treatment; SBT = School-based Treatment. These studies are being done in four African countries: Cote d’Ivoire, Kenya, Tanzania and Mozambique. For more detail please see the SCORE website: http://score.uga.edu
The primary outcomes will be to find what the prevalence and intensity of infection is in these randomized villages in Year 5, after 4 years of these different MDA schedules. The differences in the starting prevalence levels may show us that what works at one level does not work as well at the higher level. The comparison of SBT and CWT will also be interesting. CWT every year is probably as much as most ministries would like to do, but maybe you could do CWT for two years and then drop back to doing SBT for two years, and get as good a result. Some of the studies have just completed Year 2 and others are finishing Year 3. These are very large studies requiring hundreds of thousands of stool samples and many people. They are complicated to do and they will generate a lot of data. But for now we will just have to wait and see what they will tell us.
Layered on top of the large studies with the six arms, SCORE is also looking in Arm 1 (the most drug pressure) compared to Arm 6 (the least drug pressure) at the genomic structure of the schistosomes. I mentioned this earlier. Also in these two Arms SCORE is also doing morbidity measurements and snail studies in some villages to see whether we can see any differences based on the two different levels of MDA. In Niger, they went off target by not properly randomizing the villages in their studies (both a 10%–24% study and a >24% study on S. haematobium) and we had to change their protocol in the third year. Now the Niger project is evaluating twice a year MDA versus once a year MDA.
Onward to Elimination and How Best to Eliminate Transmission
In Zanzibar, SCORE is evaluating what it takes to eliminate S. haematobium transmission, because this is very, very different than morbidity control [14]. SCORE’s operational research on elimination is part of a larger effort by an umbrella consortium called Zanzibar Elimination of Schistosome Transmission (ZEST) to actually accomplish elimination on Zanzibar [15]. After making the decision to move from morbidity control to elimination you can no longer just depend on MDA. Some of the reasons for this are listed in Table 2. Now you have to go to combinations of interventions. So in Zanzibar, SCORE is trying to do operational research on elimination by comparing: 1) MDA twice a year; 2) MDA twice a year plus snail control; and 3) MDA twice a year plus behavior change. For such a major undertaking you need to have a countrywide commitment from the backing of the President of the country down to the village volunteer. Because SCORE recently received a supplement from the Bill and Melinda Gates Foundation, we will also soon be doing operational research on elimination of S. mansoni transmission in Rwanda and Burundi. These are small countries, but they are very heavily populated. This will be challenging.
Table 2.
Why is Mass Drug Administration (or Preventive Chemotherapy) not sufficient to eliminate schistosomiasis transmission by itself?
| • | 100% MDA Coverage is very hard to achieve—and will only get more difficult as prevalence and intensities approach zero |
| • | Praziquantel does not kill juvenile worms—which then grow up to be adults |
| • | Production of enough praziquantel is a challenge—if we really treat everyone who is infected |
| • | There may be a prevalence at which it is not longer cost-effective to keep doing MDA |
| • | There may be a prevalence at which it is no longer acceptable to the population to undergo MDA |
| • | Reinfection of treated people can continue to occur if infected snails remain in the environment |
| • | The multiplicative phase of the life cycle is in the snail host (vector) |
| • | If poor sanitation, vector snails and a few infected people remain—transmission will continue unless a “break point” in one of these parameters is achieved—but we do not know what that “break point” is. |
Elimination is very different than morbidity control and we know that it will take a lot more combinations of things; snail control, water, sanitation, behavior change, all of these things will have to be put together with MDA. What we do not know is which combinations are most effective for the least money or the least time. If you do all of them, yes, we know Japan has done all of them and eliminated schistosomiasis transmission. SCORE’s challenge is to find the right combinations to get there quickest for the least money, and in Africa.
The Broader Perspective
There are many good things now going on in the world of NTDs, but in the interest of time I will not be able to list them. However, please know that SCORE is not operating in a vacuum and every year things change—and that is really good. This means that every year we have to adjust to that change and adjust our studies and change things as we move along because there are a lot of new things going on in MDA and at all levels.
I thank you for your time. Now it is time for me to tell you where I think we are in the baseball game called controlling and eliminating schistosomiasis.
What Inning Are We In, and What Is the SCORE?
I think we have just finished the bottom of the fourth inning. I think this is the box score so far (Table 3). In the first inning, the visitors (of course the visitors are the bad guys—the schistosomes) had two runs and we did not have any. They got two more and we still did not have any. Now, in the third inning, we are starting to get somewhere and in this inning, we are winning, but we are still behind. The score at this point is 3 to 6 in favor of the “long-term visitors”—the schistosomes. But there are a lot of innings left. We do not know how we will do, but we hope to hit some homeruns, play smart and win.
Table 3.
In the baseball game against schistosomiasis—What inning are we in, and what is the SCORE?
| Home Team* | Visitors# | |
|---|---|---|
| 1st Inning | 0+ | 2 |
| 2nd Inning | 0 | 2 |
| 3rd Inning | 1 | 2 |
| 4th Inning | 2 | 0 |
* The Home Team is us—the scientists & public health workers fighting schistosomiasis
# The Visitors are the long term visitors—the schistosomes
+ Numbers indicate the number of runs per inning
Thank you very much.
Acknowledgements
This presentation was based on studies made possible by support from the University of Georgia Research Foundation, Inc, which is generously funded by the Bill and Melinda Gates Foundation for the SCORE project.
Questions and Discussion
Shinjiro Hamano Thank you very much. Any comment or question from the audience? Please Osamu [ph] at first.
Male Participant Thank you very much for a nice talk. I was wondering about new methods, maybe someone developed about detecting schistosomiasis. I found the sensitivity of Cote d’Ivoire case is bit similar to Kato-Katz method. Is it depends on the diversity or something else?
Daniel Colley We do not know the answer to that. We think it may depend on reader variability and our Kenyan colleagues have some studies going on in Kisumu starting in about 2 weeks to look at inter-reader variability. We have already tried to use a standard and that really did not work very well. So, we have to try to figure out why Cote d’Ivoire was different. We think that they were reading them differently.
Male Participant Okay, so you don’t think this is diversity.
Daniel Colley We don’t think so. This test, this CCA antigen has been looked at for 35 or 40 years across many, many, many places and it seems to be the same. The monoclonal picks it up everywhere. Thank you.
Male Participant Okay. Thank you.
Shinjiro Hamano Another comment, please.
Hiroshi Ohmae Thank you very much. I am Hiroshi Ohmae from National Institute of Infectious Diseases. I participated in some control programs of schistosomiasis in Asia, but I have never participated in schistosomiasis control programs in Africa. So, I have no experience in S. mansoni, S. haematobium control program. I have some comments and questions. One is for MDA. The impact of MDA on control of morbidity, I have agreed and it’s very strong impact on morbidity control due to each kind of schistosomiasis. I agree. But, for impact on transmission is very different in each kind of schistosomes and the number of reservoir host is a core factor, causes this difference. So to compare the Schistosoma mekongi and Schistosoma japonicum the impact on transmission control is strong in S. mekongi control program and the coverage rate is very important to transmission control. To compare the Cambodia and Lao cases, the high coverage rate continues in Cambodia, more than 90%. The transmission rate is strongly decreasing. But, very changeable in Lao, the prevalence is continuing at high level. So, I think S. mansoni reservoir host is limited, so mass drug administration with high coverage rate more than 90%, I think the prevalence with transmission rate may decrease with high coverage rate of mass drug administration for long time. This is one point.
Another one is side effect or adverse effect of praziquantel is an obstacle for expanding program of MDA. For example, in Lao, last year the three suspected cases of adverse effect of praziquantel were reported and unfortunately three people died, but I have never believed.
Shinjiro Hamano Anyhow in short please.
Hiroshi Oyama Very, very sorry. What is the reporting system and the situation of adverse side effect suspected cases in Africa? I think I have more than many, many cases of praziquantel treatment in Africa. So I need some explanation.
Daniel Colley There are lots of questions there. For one thing I think that the experience in Africa even though it is with S. mansoni and S. haematobium, indicates that mass drug administration will not lead to elimination, that is it will not cut down on the transmission sufficiently. This is, in part, because the coverage is never 100% and it only takes a few people contaminating to start the whole cycle again. As far as I know, in Africa, there has never been anywhere that MDA has actually stopped transmission. That’s why we think we are going to have to do combined interventions to get to elimination.
As far as the severe adverse effects, certainly the minor things of dizziness or occasional vomiting is always a problem and you have to prepare the community or the school to know that this will happen. It is more likely to happen if the patients are heavily infected, because you are killing a lot of worms and you end up with immune complex reactions. You release a lot of antigens and you already have a lot of antibodies. But, those pass in a short time. I have not seen true severe adverse effects so far. This is in the SCORE programs where we were treating thousands of children, where we have pretty good reporting systems. In national programs they may not have as extensive reporting systems as in research projects. It will differ by country. But, I have not heard of any deaths directly due to praziquantel treatment. You always have to be careful of the ancillary things. We had a severe adverse effect in our Kenya study in SCORE. It was reported, and the report went up through the ministry. The ministry team came and investigated. It turned out that the young girl who died had malaria. She died of malaria. She happened to get treated with praziquantel 3 days before she died of malaria. But, the autopsy was clear. So, when you are treating a lot of people, there are always potentials for something to happen—aside from your particular treatment. So far praziquantel has been very safe.
Shinjiro Hamano Thank you very much. It’s time so please ask him outside of the auditorium. Thank you very much.
References
- 1.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helminthic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005; 365(9470): 1561–1569. [DOI] [PubMed] [Google Scholar]
- 2.Ezeamam AE, McGarvey ST, Hogan J, Lapane KL, Bellinger DC, Acosta LP, Leenstra T, Oveda RM, Kurtis JD, Friedman JF. Treatment for Schistosoma japonicum, reduction of intestinal parasite load, and cognitive test score improvements in school-aged children. PLoS Negl Trop Dis 2012; 6(5): e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol 2005; 21(8): 386–392. [DOI] [PubMed] [Google Scholar]
- 4.Savioli L, Hatz C, Dixon H, Kisumku UM, Mott KE. Control of morbidity due to Schistosoma haematobium on Pemba Island: egg excretion and hematuria as indicators of infection. Am J Trop Med Hyg 1990; 43(3): 289–295. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Antihelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006.
- 6.Richards FO Jr, Eigege A, Miri ES, Jinadu MY, Hopkins DR. Integration of mass drug administration programmes in Nigeria: The challenge of schistosomiasis. Bull World Health Organ 2006; 84(8): 673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis 2011; 5(9): e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King CH, Bertsch D. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low-prevalence and previously-treated populations. PLoS Negl Trop Dis 2013; 7(9): e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legesse M, Erko B. Field-based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Trans R Soc Trop Med Hyg 2007; 101(7): 668–673. [DOI] [PubMed] [Google Scholar]
- 10.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PN, Butler SE, Karanja DM, Secor WE. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in western Kenya. PLoS Negl Trop Dis 2011; 5: e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchuem Tchuenté L-A, Kuete Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, Gipwe NF, Nana ED, Stothard JR, Rollinson D. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis 2012; 6: e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuenté LA, N’Goran EK, Erko B, Karanja DM, Kabatereine NB, van Lieshout L, Rathbun S. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg 2013; 88(3): 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam GJ, de Dood CJ, Lewis M, Deelder AM, van Lieshout L, Tanke HJ, van Rooyen LH, Corstjens PL. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 2013; 135(2): 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J. Time to set the agenda for schistosomiasis elimination. Acta Trop 2013; 128(2): 423–440. [DOI] [PubMed] [Google Scholar]
- 15.Knopp S, Mohammed KA, Ali SM, Khamis IS, Ame SM, Albonico M, Gouvras A, Fenwick A, Savioli L, Colley DG, Utzinger J, Person B, Rollinson D. Study and implementation of urogenital schistosomiasis elimination in Zanzibar (Unguja and Pemba islands) using an integrated multidisciplinary approach. BMC Public Health 2012; 12: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]