Abstract
Epidermal parasitic skin diseases (EPSD) are common in the tropics and sub-tropics. They are caused by mites, lice and other blood-sucking insects. In resource-poor countries they are associated with considerable morbidity. Hitherto, EPSD are treated with insecticides with a neurotoxic mode of action. The efficacy of this treatment is variable, and the development and spread of resistant mites and lice is alarming.
A new concept for treating EPSD is presented which is based on the topical application of dimeticones, silicone oils of low viscosity which rapidly kill insects and mites by a physical mode of action. They creep into the respiratory system and block oxygen supply. The physical mode of action makes the development of resistant parasite strains very unlikely. Due to their safety and efficacy, dimeticones are promising candidates for population-based intervention programmes targeted against EPSD in resource-poor settings.
Keywords: Epidermal parasitic skin diseases, tungiasis, scabies, pediculosis, epidemiology, treatment, control
Introduction
Epidermal parasitic skin disease (EPSD) are a family of parasitoses frequent in the tropics which predominantly affect marginalized populations [1]. In resource-poor settings cutaneous larva migrans, scabies, pediculosis capitis and tungiasis are the most common EPSD. Whereas cutaneous larva migrans, scabies and pediculosis capitis occur on all continents, tungiasis is limited to South America, the Caribbean and sub-Saharan Africa.
In population groups in which these diseases prevail, polyparasitism is frequent and particularly children are simultaneously infested with two, three or even four different ectoparasites. The reason is that the different EPSD share similar risk factors such as lack of sanitation, crowding, precarious housing conditions, low level of education, etc. [1] (Fig. 1). In children polyparasitism is usually an indicator of neglect [2].
Fig. 1.

Resource-poor community in Northeast Brazil with a typical environment favouring the transmission of skin parasites.
Currently available treatments have several constraints: For instance, the insecticides commonly used are potentially toxic, resistance is spreading and repeated applications are necessary; ivermectin is contra-indicated in children < 5 years and pregnant women and, hence, cannot be used for mass treatment. Obviously, a new treatment concept is needed. The optimal drug for treating EPSD should have the following characteristics:
-
–
the drug should work in all (or the majority of) EPSD
-
–
the compound must be non-toxic
-
–
a single application must be effective
-
–
no risk for development of resistance
-
–
applicable with minimal input from the health sector
Before the new treatment concept is described, the clinical and epidemiological characteristics of the three most important EPSD, namely scabies, pediculosis capitis and tungiasis, are outlined.
Scabies
As a rule, scabies prevails were poverty exists. In resource-poor settings the prevalence ranges between 4 and 80% (Table 1). Humans are the only host of Sarcoptes scabiei. Transmission is predominantly from person-to-person and only rarely through fomites. Children and women are the most vulnerable population groups, since in endemic areas frequent body contacts occur between and within these groups (Fig. 2).
Table 1.
Frequency of scabies in developing countries
| Village Egypt | 4% |
| Village/Slum Brazil | 4%/9% |
| Village/Slum India | 10%/15% |
| Aboriginees Australia | 50% |
| Village Papua Neuguinea | 80% |
| Homeless children South America | 90% |
| Madrasah Bangladesh | 98% |
Fig. 2.

Close body contact favours the transmission of sarcoptes mites and head lice.
In a minority of the patients, the intensity of infestation is extremely high and parasites are spread all over the body (disseminated or Norwegian scabies). This condition is associated with an impaired immuno-competence and, hitherto, requires a special and complicated treatment.
Bacterial superinfection of lesions is common and, if caused by group A streptcococci, may cause post-streptococcal glomerulonephritis or rheumatic heart disease (Fig. 3).
Fig. 3.
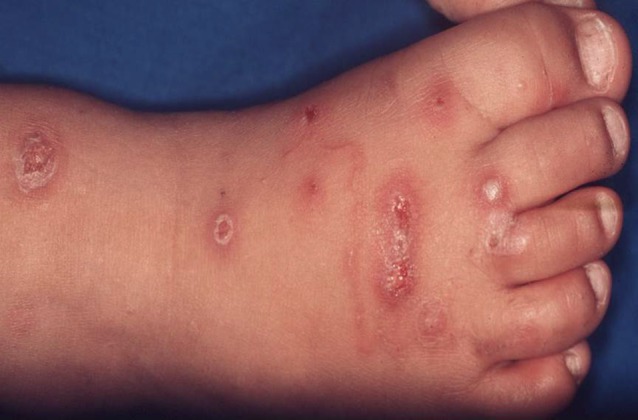
Multiple scabies lesions with bacterial superinfection in a young child.
The predominant symptom is itching. In a village in Northeast Brazil, 94% of the patients reported pruritus and 72% itching-related sleep disturbances [3] (Table 2). Itching-related sleep disturbances may result in violence: when the whole family sleeps in a single room and a child with scabies wakes up in the night and starts to cry, other family members will wake up, too. Mother or father may then slap the diseased child to prevent a riot.
Table 2.
Complaints of 196 patients with scabies in a rural community in Brazil
| Itching | 94% |
| - light | 31% |
| - moderate | 30% |
| - severe | 39% |
| Sleep disturbance | 72% |
| - sleep initiation | 16% |
| - sleep maintainence | 71% |
It was shown that in patients living in resource-poor settings—where quality of life is low anyway—scabies significantly impairs the quality of life [4]. However, effective treatment leads to a rapid restoration of the quality of life [4].
Pediculosis Capitis
Head lice are very specific parasites. They can only propagate on human scalp. In the children population, pediculosis capitis is heterogeneously distributed with spatiotemporal clusters. Where and when cluster arise is not predictable.
Head lice infestation is usually considered to be a nuisance, not a disease. However, in resource-poor settings pediculosis capitis may be an individual and a public health threat.
Similar to scabies, head lice lesions are very itching and cause the patient to scratch its scalp. Repeated scratching leads to excoriations which facilitate bacterial superinfection (Fig. 4). Pathogens, such as Staphylococcus aureus and streptococci are passively transported by head lice when they move from an infected lesion to other parts of the scalp. Head lice can also transmit important pathogens actively (Table 3). Probably, all infectious agents known to be transmitted by body lice are also transmitted by head lice. Since head lice take a blood meal every 2–3 hours and change their host frequently, the transmission of a pathogen becomes likely, when the infectious agent circulates in a substantial part of the population.
Fig. 4.
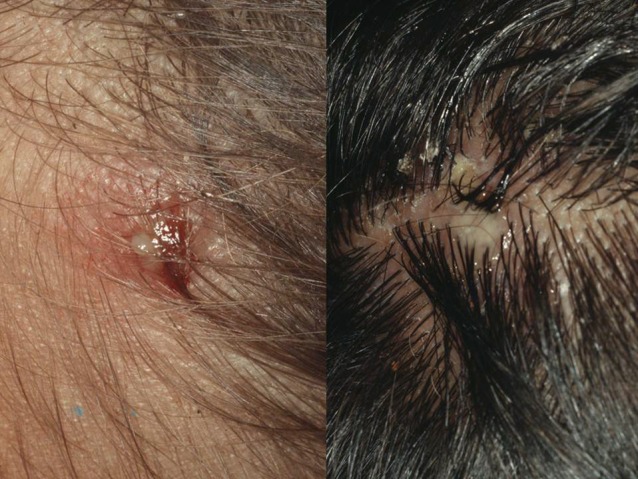
Bacterial superinfection as a consequence of sratching.
Table 3.
Pediculus humanus capitis as a vector of pathogenic bacteria
| Pathogen | Disease | Vector function |
|
|---|---|---|---|
| proved | suspected | ||
| Yersinia pestis | Plague | + | |
| Rickettsia prowazekii | Epidemic typhus | + | |
| Bartonella quintana | Trench fever | + | |
| Francisella tularensis | Tularaemia | + | |
| Borellia recurrentis | Louse-borne relapsing fever | + | |
Eggs are attached to the hair at certain predilection sites (Fig. 5). If there are hundreds of eggs, they cannot be hidden. This may lead to stigmatization and social exclusion.
Fig. 5.
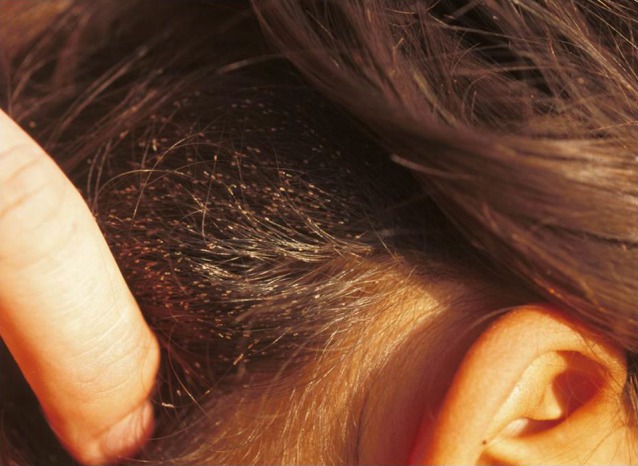
Multiple eggs attached to the hair.
Resistance against pediculocides with a neurotoxic mode of action such as permethrin and malathion occurs worldwide and is spreading. Cross-resistance and double-resistance are common. Hence, it is impossible to predict the efficacy of a classical pediculocide in a defined setting.
Tungiasis
Tungiasis (sand flea disease) is caused by the penetration of female sand fleas (Tunga penetrans) into the skin. Sand flea disease is a zoonosis affecting a broad spectrum of domestic and sylvatic animals with dogs, cats, pigs, cows and rats as typical reservoirs. Tungiasis is a disease of the poorest of the poor. It thrives in the underdeveloped rural hinterland, in isolated communities along the sea shore, in the periphery of rural towns and in the slums of big cities in South America, The Caribbean and sub-Saharan Africa. The prevalence of tungiasis in people living in resource-poor rural and urban communities may be up to 60% [5]. Reports in electronic media indicate that in East Africa tungiasis has re-emerged in recent years with several hundred thousand cases in Kenya alone.
Frequently, lesions occur in clusters at certain predilection sites with up to 30 embedded sand fleas aggregated in a small area accompanied by inflammation and necrosis of the surrounding tissues (Fig. 6).
Fig. 6.
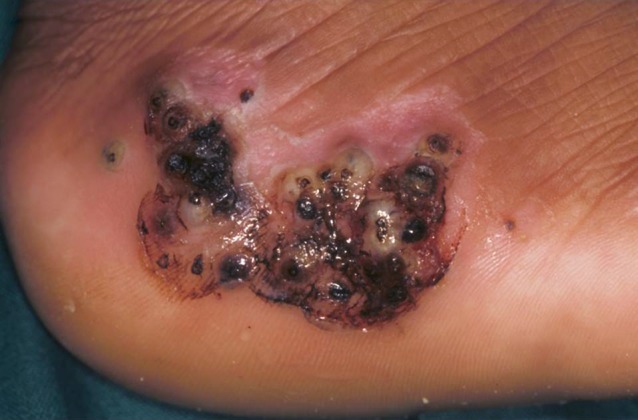
Cluster of embedded sand fleas at the lateral rim of the heel.
Repeated infections and the unavailability of an appropriate treatment cause a persistent inflammation of the feet (Fig. 7). If the lesions are located at the sole, deep fissures and ulcers develop (Fig. 8). Chronic sand flea disease is debilitating and disabling, and eventually causes mutilation of the feet [6]. This results in difficulty in walking and restricted mobility. In the endemic areas walking difficulties are so common that they have been recognized as an indicator of a high prevalence of tungiasis.
Fig. 7.
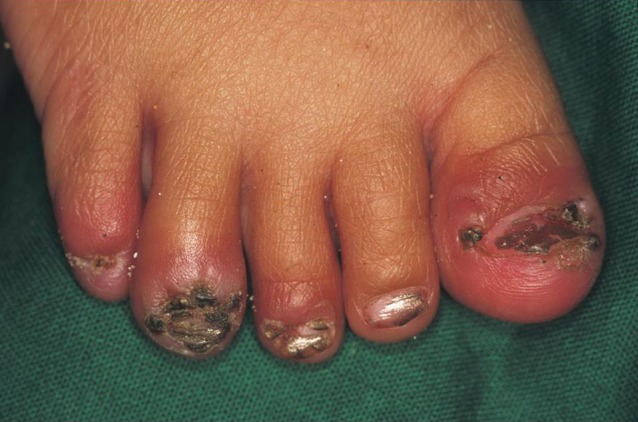
Intense inflammation as a consequence of repeated infection with sand fleas.
Fig. 8.
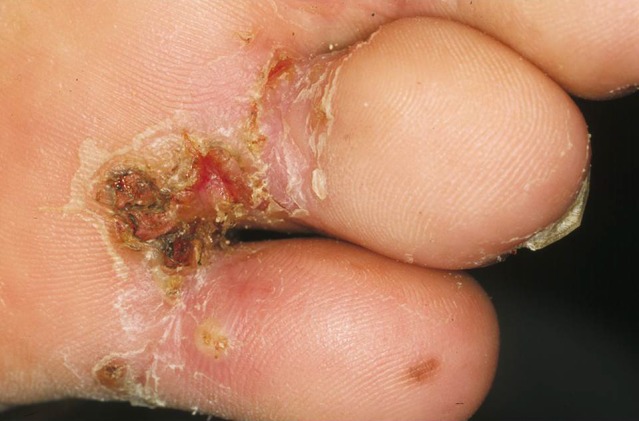
Tungiasis-associated ulcers and fissures at the sole of the foot.
In an act of desperation, affected individuals try to get rid of the parasites by using sharp instruments such as safety pins, needles, scissors, a knife, a thorn or a sharply pointed piece of wood. The instruments are not disinfected and are subsequently used in several household members. They are also shared between neighbors. Since a burrowed sand flea cannot be extracted without causing a haemorrhage, the traditional treatment is supposed to transmit blood-borne pathogens such as hepatitis B and C virus from one person to another [7].
Bacterial superinfection is an almost constant finding and may consist of an array of aerobe, micro-aerophilic and anaerobe bacteria including clostridia [8] (Fig. 9). In non-vaccinated individuals tungiasis is a risk factor for tetanus.
Fig. 9.
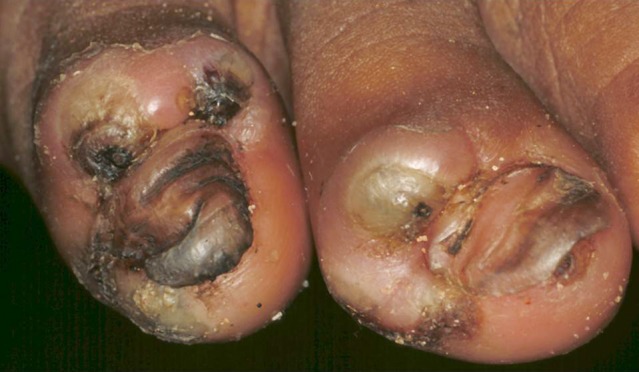
Multiple abscesses in patients with sand flea disease.
New Treatment Concept
A paradigm shift is needed for the treatment of EPSD. Rather than killing ectoparasites through a potentially toxic chemical compound and only active against a single species, a drug is needed which targets an Achilles heel present in all ectoparasites. The respiratory tract seems to be such a target. For instance, in lice 14 narrow openings let oxygen enter and guarantee oxygen supply of vital organs (Fig. 10). If these openings are blocked, lice will rapidly die from suffocation. However, the openings have a diameter of ≤ 10 μm, preventing liquids with a normal surface tension to enter. With 2 μm diameter the respiratory openings in the lid of lice eggs are even smaller. Scabies mites breathe through very small pores distributed over the body. Sand fleas are almost totally embedded in the epidermis, but remain in contact with the environment through an opening in the skin of about 200 μm. The intestinal, the genital and the respiratory tract jointly enter in the so-called abdominal cone through which eggs are expelled, faeces is excreted and oxygen enters trachea-like structures (Fig. 11).
Fig. 10.
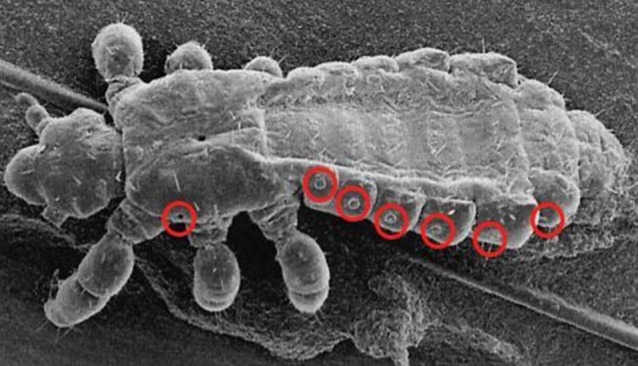
Scanning electron micrograph of Pediculosis humanus capitis (please print name in Italic). Red circles indicate entrance to tracheae.
Fig. 11.
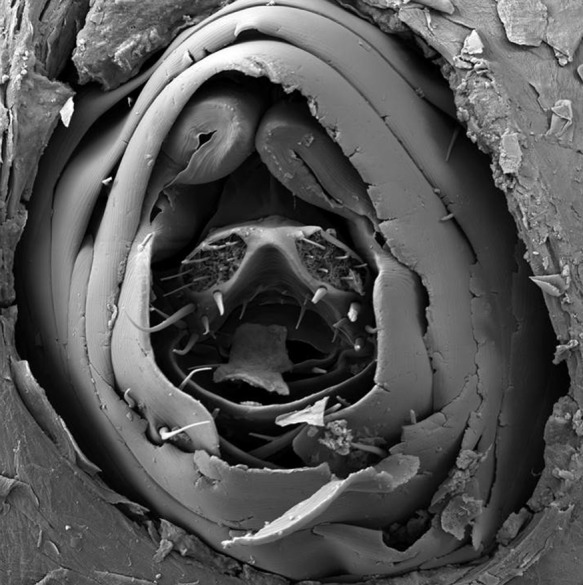
Scanning electron migrograph of the last abdominal segments of a female embedded sand flea protruding through the skin.
Obviously, only compounds with a very low viscosity can creep into such small openings, fill the respiratory tract and, thereby, prevent diffusion of oxygen. Polydimethylsiloxanes (dimeticones) are such compounds (Fig. 12). Dimeticones are chemically inert, non-toxic and have a pure physical mode of action. Hence, it is highly improbable that parasites develop resistance.
Fig. 12.
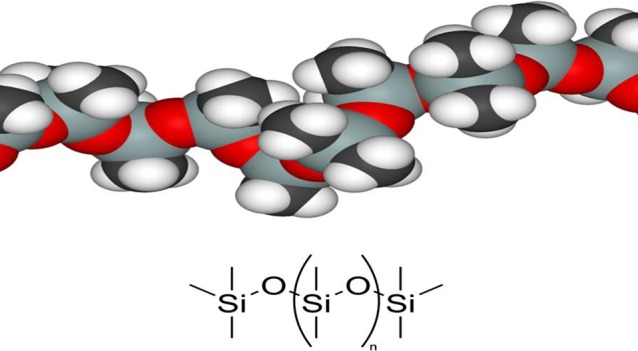
Polydimethylsiloxane (dimeticone)
Validation of the concept
It has been shown that a mixture of two dimeticones (one with an extremely low and the other with a moderate viscosity) kill head lice in vitro within a couple of minutes [9] (Fig. 13). The efficacy of this approach was demonstrated in a randomized clinical trial in Brazilian children with a high intensity of infestation [10]. Recently, it was shown that an appropriate mixture of dimeticone also penetrates into lice eggs and rapidly kills the embryos [11].
Fig. 13.

Penetration of dimeticone into tracheae of headlice
The new treatment concept was subsequently tested in tungiasis. In a field study in rural Kenya, the feet of 47 children were randomized to either receive the treatment recommended by the Ministry of Health (bathing the whole foot in a 0.05% solution of KMnO4) or a topical application of dimeticone. In the dimeticone group 78% of sand fleas lost all viability signs within seven days and 92% of the parasites developed in an abnormal way. In the KMnO4 group only 39% of the parasites lost viability signs [12].
Conclusion
Dimeticones are a family of compounds with a physical mode of action, targeting an Achilles heel of ectoparasites. Development of resistance is very unlikely. Dimeticones can be used for the treatment of individual patients or in mass treatment campaigns with minimal input from the health sector.
References
- 1.Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ 2009; 87(2): 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldmeier H, Krantz I. A way of measuring poverty that could further a change for the better. Bull World Health Organ 2008; 86: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson A, Heukelbach J, da Silva Filho AF, Barros Campelo Junior E, Feldmeier H. Clinical features and associated morbidity of scabies in a rural community in Alagoas, Brazil. Trop Med Int Health 2007; 12: 493–502. [DOI] [PubMed] [Google Scholar]
- 4.Worth C, Heukelbach J, Fengler G, Walter B, Liesenfeld O, Feldmeier H. Impaired quality of life in adults and children with scabies from an impoverished community in Brazil. Int J Dermatol 2012; 51: 275–282. [DOI] [PubMed] [Google Scholar]
- 5.Ugbomoiko US, Ofoezie IE, Heukelbach J. Tungiasis: high prevalence, parasite load, and morbidity in a rural community in Lagos State, Nigeria. Int J Dermatol 2007; 46: 475–481. [DOI] [PubMed] [Google Scholar]
- 6.Feldmeier H, Eisele M, Saboia Moura RC, Heukelbach J. Severe Tungiasis in underprivileged communities: case series from Brazil. Emerg Infect Dis 2003; 9: 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmeier H, Sentongo E, Krantz I. Tungiasis (sand flea disease): a parasitic disease with intriguing challenges for a transformed public health. Eur J Clin Microbiol Infect Dis 2012; 32: 19–26. [DOI] [PubMed] [Google Scholar]
- 8.Feldmeier H, Heukelbach J, Eisele M, Sousa AQ, Barbosa LM, Carvalho CBM. Bacterial superinfection in human tungiasis. Trop Med Int Health 2002; 7: 559–564. [DOI] [PubMed] [Google Scholar]
- 9.Richling I, Böckeler W. Lethal effects of treatment with a special dimeticone formula on head lice and house crickets (Orthoptera, Ensifera: Acheta domestica and Anoplura, Phthiraptera: Pediculus humanus). Arzneim -Forsch/Drug Res 2008; 58: 248–254. [DOI] [PubMed] [Google Scholar]
- 10.Heukelbach J, Pilger D, Oliveira F, Khakban A, Ariza L, Feldmeier H. A highly efficacious pediculocide based on dimeticone: Randomized observer blinded comparative trial. BMC Infect Dis 2008; 8: 115. doi:10.1186/1471-2334-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heukelbach J, Sonnberg S, Becher H, Melo I, Speare R, Oliveira FA. Ovicidal efficacy of high concentration dimeticone: A new era of head lice treatment. J Am Acad Dermatol 2011; 64(4): e61–e62. [DOI] [PubMed] [Google Scholar]
- 12.Thielecke M, Nordin P, Ngomi N, Feldmeier H. Treatment of Tungiasis with Dimeticone: A proof-of-principle study in rural Kenya. PLOS Negl Trop Dis 2014. (in press) [DOI] [PMC free article] [PubMed]


