Abstract
Quakingviable (qkv) is a recessive neurological mouse mutation with severe dysmyelination of the CNS and spermiogenesis failure. The molecular lesion in the qkv mutant is a deletion of ≈1 Mb on mouse chromosome 17 that alters the expression of the qk gene in oligodendrocytes. Complementation analysis between the qkv mutation and qk mutant alleles generated through chemical mutagenesis showed that the male sterility is a distinctive feature of the qkv allele. This observation suggested that the sperm differentiation defect in qkv is due to the deletion of a gene(s) distinct from qk. Here, we demonstrate that the deletion of Pacrg is the cause of male sterility in the qkv mutant. Pacrg is the mouse homologue of the human PARKIN-coregulated gene (PACRG), which encodes for a protein whose biochemical function remains unclear. We show that Pacrg is highly expressed in the testes in both mice and humans. In addition, the expression pattern of Pacrg during spermiogenesis suggests that it plays a role in sperm differentiation. In support of this hypothesis, we show that transgenic expression of Pacrg in testes restores spermiogenesis and fertility in qkv males. This finding provides the first in vivo evidence, to our knowledge, for the function of Pacrg in a model organism. Immunolocalization experiments on isolated spermatozoa show that the Pacrg protein is present in mature sperm. Remarkably, the mammalian Pacrg protein shares significant sequence similarities with gene products from flagellated protozoans, suggesting that Pacrg may be necessary for proper flagellar formation in many organisms.
The mouse mutant quakingviable (qkv) has been investigated for nearly four decades for its neurological phenotype. Affected animals display severe tremor of voluntary movements, with onset ≈10 days of age (1). The main neuropathological finding in the CNS of qkv mice is a severe lack of myelin (1). In addition to the CNS abnormalities, qkv homozygous males are sterile due to a severe oligospermia (2). Very few morphologically abnormal immotile spermatozoa are present in the semen of qkv males, because spermatids fail to complete differentiation. A recent report (3) showed that sperm from qkv semen and testes are unable to fertilize mouse oocytes in vitro. However, the intracytoplasmic injection of qkv spermatozoa resulted in normal live offspring. These findings indicate that although the qkv mutation affects functions necessary for proper sperm differentiation and fertilization, it does not affect other epigenetic factors necessary for syngamy and normal embryonic development (3).
The molecular defect in qkv is a large deletion spanning ≈1Mb of mouse chromosome 17 (4, 5). The qk gene, which encodes for RNA binding proteins involved in posttranscriptional mRNA regulation (6, 7), is in close proximity to the proximal deletion breakpoint of qkv (8). The qkv deficiency affects the region upstream of the qk gene to reduce the expression of qk mRNAs in oligodendrocytes, resulting in the CNS myelination defect (9, 10). In addition to the spontaneous qkv deletion, several N-ethyl-N-nitrosourea (ENU)-induced alleles of quaking have been isolated (11-13). Each of these ENU-induced mutations (qke) selectively complements the male sterility associated with the qkv allele. This observation suggests that the spermiogenesis defect in qkv is the result of the loss of function of a locus (or loci) distinct from qk, mapping within the qkv deletion boundaries.
To identify loci involved in the qkv spermiogenesis defect, we determined the gene content of the deleted interval. Two large genes map to the qkv deletion (14). One of these genes is Parkin, the mouse homologue of the human PARKIN gene. Loss-of-function mutations in PARKIN cause an autosomal recessive form of early-onset Parkinson's disease (15). Although Parkin protein expression is lost in qkv homozygous mice (14, 16), qkv mutants do not recapitulate neuropathological features of early-onset Parkinson's disease (14). The second gene mapping to the qkv interval is the mouse homologue of a human gene termed PARKIN coregulated gene (PACRG) and therefore it will be referred to hereafter as Pacrg (14, 16).
In this study, we show that the Pacrg transcript is highly expressed in mouse testes during spermatogenesis and its gene product is present in mature spermatozoa. Transgenic expression of the mouse Pacrg cDNA in testes rescues the sperm differentiation defect and restores normal fertility in qkv males. These results demonstrate that deletion of Pacrg is the cause of male sterility in qkv and validate the hypothesis that the reproductive phenotype in this mutant is due to the loss of function of a gene distinct from qk. Furthermore, the involvement of Pacrg in sperm differentiation provides evidence of its function in vivo in model organisms.
Materials and Methods
Cloning, Expression, and Sequence Analysis of Pacrg. The assembly of the bacterial artificial chromosome contig spanning the qkv deletion and the sequence analysis were described elsewhere (14). Several cDNA clones corresponding to ESTs matching the AK005771 sequence deposited in GenBank were obtained through the IMAGE consortium, and their inserts were fully sequenced. The IMAGE clone 516103, from a mouse cDNA testis library (dbEST accession no. AA089000), was found to contain the entire mouse Pacrg coding region and most of its 5′ and 3′ UTRs. Multiple sequence alignments of metazoan and protozoan Pacrg proteins were generated by using the clustalw algorithm (version 1.8) supported by the Baylor College of Medicine Search Launcher server, which can be accessed at www.hgsc.bcm.tmc.edu/SearchLauncher. Conserved amino acids functional motifs were identified by using the subprograms of the psortii algorithm, which can be accessed at http://psort.nibb.ac.jp.
The insert from cDNA clone 516103 was used as a probe for both Northern blot and for in situ hybridization experiments on mouse testis. For Northern blot analysis, total RNA was isolated from mouse tissues samples by using the RNA STAT-60 reagent (Tel-Test, Friendswood, TX) according to the manufacturer's protocol. Ten micrograms of total RNA per lane was electrophoresed on a 1% 4-morpholinepropanesulfonic acid-formamide agarose gel and was transferred onto a nylon membrane. Hybridization was carried out overnight at 65°C, by using the cDNA inserts radiolabeled with a random primer kit (PrimeIt, Stratagene). In situ hybridization was carried out by using the Pacrg cDNA insert as described by Lu and Bishop (17). For the expression analysis of PACRG in human tissues, a FirstChoice Northern human blot 2 membrane (Ambion, Austin, TX) was hybridized at 55°C by using the ULTRAhyb solution (Ambion) according to the manufacturer's protocol. A human PACRG testis cDNA insert derived from the IMAGE clone 1292856 (dbEST accession no. AA776722) was used as a probe.
Assembly of the PGK2-Pacrg Minigene and Generation of Transgenic Mice. A 1.4-Kb SalI/HindIII fragment containing the upstream regulatory region of the human phosphoglycerate kinase 2 gene (PGK2), derived from the pSVOCAT construct (ref. 18; a kind gift of R. Erickson, University of Arizona, Tucson), was subcloned in the pBluescript-II-KS vector (Stratagene) and was then ligated to a PCR-amplified EcoRV/NotI fragment containing the Pacrg ORF. Subsequently, a NotI/StuI fragment from the plasmid vector pIRES-EGFP (Clontech) containing simian virus 40 polyadenylation signal sites was ligated to the 3′ end of the Pacrg ORF. For microinjection, the 2.5-kb PGK-Pacrg transgene was cut free from the vector backbone with a KpnI/AflI digestion and was purified by using the QIAEX-II Gel extraction kit (Qiagen, Valencia, CA). The pronuclei of C57BL/6J one-cell embryos were injected with the PGK2-Pacrg transgene. Six transgenic founder animals were identified by Southern blot analysis on EcoRV-digested genomic DNA by using the 1.4-kb PGK2 promoter region as a probe. Five of the founder animals were crossed to BTBR mice carrying the qkv deletion, and four transmitted the PGK2-Pacrg transgene to their offspring. The qkv/qkv animals carrying the transgene were generated through a two-generation breeding scheme and were analyzed for sperm count and fertility.
Testis Histology, Sperm Analysis, and Fertility Tests. Testes were treated for histological analyses essentially as described by Clark et al. (19). Sperm was collected from the epididymis and vas deferens of transgenic and control animals in 2 ml of PBS solution. The sperm suspension was incubated at 37°C for 15 min and the concentration was obtained by counting sperm in a Neubauer hemocytometer (Hausser Scientific, Horsham, PA).
To assess fertility of transgenic and wild-type control animals, sexually mature males were placed in a cage with two wild-type BTBR females for 8 weeks.
Antibodies, Western Blotting, and Immunofluorescence. Polyclonal antibodies were raised against the following two peptides derived from the conceptual translation of the AK005771 cDNA nucleotide sequence: RPAKPTTFRKCYERGD (corresponding to amino acid residues 48-64) and YSQQKRENIGDLI (amino acid residues 197-209). The peptides were conjugated to keyhole limpet hemocyanin as carrier and were combined for injection in two rabbits. Peptide synthesis, conjugation, and immunization were carried out by Bethyl Laboratories (Montgomery, TX). Affinity purification of the immune serum was carried out by using the Sulfolink kit (Pierce) following the manufacturer's protocol. Western blot analysis was carried out as described (14). Protein extracts from semen were prepared by collecting sperm by centrifugation (3,000 × g for 5 min at 4°C). Pellets were washed twice in PBS, resuspended in 100 μl of lysis buffer (50 mM Hepes, pH 7.5/150 mM NaCl/10 mM EDTA/1% IGEPAL CA-630 (Sigma), containing Complete proteinase inhibitors mixture (Roche, Mannheim, Germany) and incubated on ice for 30 min. Total anti-Pacrg serum or affinity-purified anti-Pacrg antibodies were used for Western blot analysis on testes and semen protein extracts at a 1:2,000 and 1:500 dilutions, respectively.
Immunolocalization of Pacrg was performed on methanol-fixed isolated spermatozoa essentially as described by Quill et al. (20). Anti-Pacrg serum was diluted 1:200 in PBS and 10% normal goat serum and incubated overnight at 4°C. After washing in 1× PBS, slides were incubated with an anti-rabbit Alexa Fluor 488-conjugated antibody (dilution 1:4,000, Molecular Probes). Slides were overlaid with Vectashield mounting medium (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole before observation under the microscope.
Results
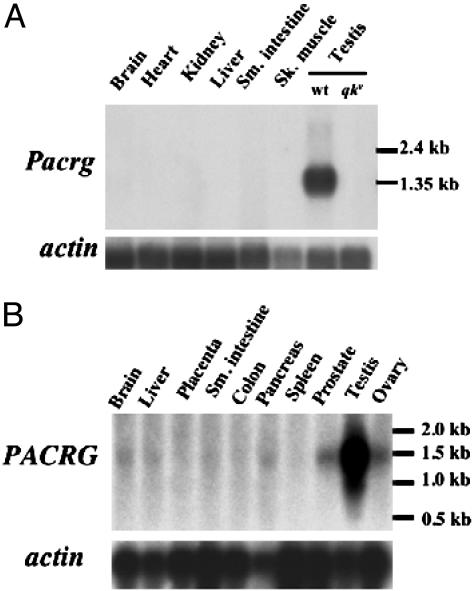
Pacrg Transcript Is Highly Expressed in Mouse Testes. To determine the tissue distribution of Pacrg mRNA in the mouse, we performed Northern blot analysis on total RNA from multiple tissues. The Pacrg mRNA is most abundant in mouse testes, where it could be detected with a short exposure time (≈5 h) of the Northern blot filter autoradiograph (Fig. 1A). In addition, RT-PCR amplification showed expression of the Pacrg transcript in brain, skeletal muscle, and kidney (data not shown). The qkv deletion encompasses the entire Pacrg locus, thus, as expected, the Pacrg transcript is absent from testes as well as from all other tissues tested in qkv homozygous animals (Fig. 1 A, qkv/qkv testes lane, and data not shown). To compare the expression pattern of the mouse Pacrg with its human counterpart, we performed Northern blot analysis on a panel of human tissues by using a human PACRG cDNA probe (Fig. 1B). Similar to the mouse, PACRG mRNA was most abundant in the testes.
Fig. 1.
Pacrg transcript is highly expressed in testis in both mouse and humans. (A) Northern blot on multiple mouse tissues shows that Pacrg mRNA is detected only wild-type testis (testis lane wt). The Pacrg mRNA is completely absent in testes from qkv mutant animals (testis, qkv/qkv lane). (Lower) Hybridization with an actin probe as loading control. (B) Northern blot on human tissues shows that PACRG mRNA is most abundant in testis. Lower levels of expression are also detected in brain, liver, small intestine, pancreas, prostate, and ovaries. (Lower) Hybridization with an actin probe is shown as a loading control.
Pacrg Is Expressed in Germ Cells During Spermiogenesis. Given the high levels of Pacrg mRNA detected in testes, we performed analyses aimed at determining both the developmental expression and cellular distribution of the Pacrg transcript in this tissue. The Pacrg transcript was absent from the testes of Odd sex mutant males, which completely lack germ cells (21), suggesting that Pacrg is predominantly expressed in this cell type (data not shown).
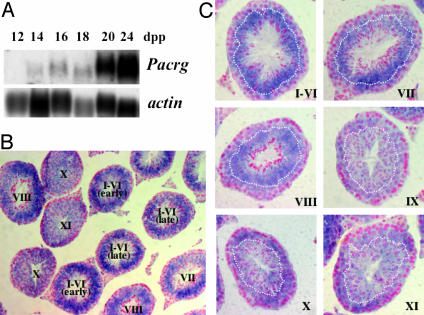
The developmental expression pattern of Pacrg in the postnatal period was determined by Northern blot analysis on testes of newborn mice starting at 2 days postpartum (dpp) up to 28 dpp. Pacrg mRNA was first detected in 14-dpp testes (Fig. 2A). This time point in the mouse developing testis corresponds to the initiation of pachytene phase of meiosis in spermatocytes (22). The level of expression of Pacrg appears to reach its maximum at 20 dpp, when spermatids first appear, and then remains constant at later ages (Fig. 2 A and data not shown). The cellular distribution of the Pacrg mRNA in adult testes was determined by in situ hybridization experiments. Pacrg is expressed only in the spermatogenic cells of the testis and it is not present in interstitial cells or in the epididymis (Fig. 2B and data not shown). The highest Pacrg expression is detected in seminiferous tubules at stages I-VI (Fig. 2B). The Pacrg transcript is most abundant in the round spermatids, but it is also present at lower levels in pachytene spermatocytes (Fig. 2C, stages I-VI). Expression of Pacrg gradually decreases from stage VII onward, as nuclear condensation proceeds in elongating spermatids. Pacrg expression appears to be at its lowest level at stage IX, and gradually increases in pachytene spermatocytes at subsequent stages (Fig. 2C). The tissue distribution as well as the developmental expression pattern of Pacrg is highly consistent with a potential role in the spermiogenesis defect associated with the qkv mutation.
Fig. 2.
Pacrg developmental and cellular expression during mouse spermatogenesis. (A) Northern blot analysis of Pacrg in neonatal testes shows the onset of expression of Pacrg at 14 dpp. Higher levels of Pacrg transcript are detected from 20 dpp onward, when sperm differentiation initiates. (Lower) Hybridization with an actin probe as loading control. (B) In situ hybridization analysis by using a Pacrg RNA probe on adult mouse testis shows expression in the seminiferous epithelium. Roman numerals indicate the stage(s) assigned to each seminiferous tubule (×200 magnification). (C) Cellular localization of Pacrg mRNA by in situ hybridization on testis seminiferous tubules at different stages (indicated by Roman numerals in the bottom right corner, ×400 magnification). The dotted curves delineate the approximate boundary between the spermatocyte (toward the basal membrane) and the spermatid cellular layers (toward the lumen) in each of the stages showed.
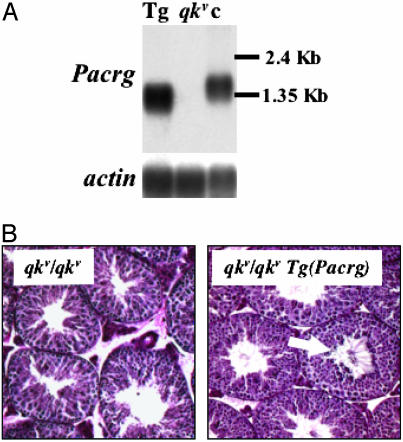
Transgenic Expression of Pacrg Restores Fertility in qkv Mutant Males. To determine whether the deletion of Pacrg is responsible for the spermiogenesis defect in the qkv mutant, we attempted to restore its function in the testes by transgenic expression in sterile qkv males. Given the large genomic interval covered by the Pacrg-coding region (14), expression of the Pacrg transcript cannot be achieved by generating transgenic mouse lines harboring single bacterial artificial chromosome clones. We therefore assembled a transgenic construct containing the Pacrg cDNA under the control of the testis-specific promoter of the human phosphoglycerate kinase 2 (PGK2) gene. This promoter has been previously used to drive expression of transgenes in the male germ line at developmental time points similar to Pacrg (18). Four independent transgenic lines carrying the PGK2-driven Pacrg minigene: Tg(Pacrg)6Jus, Tg(Pacrg)15Jus, Tg(Pacrg)16Jus, and Tg(Pacrg)21Jus, were established by crossing founder animals to mice heterozygous for the qkv deletion. Because each of these lines had similar levels of expression of the transgene and produced similar results, they are hereafter referred to collectively as Tg(Pacrg). The offspring of these matings were then backcrossed to mice carrying the qkv deletion to generate qkv homozygous animals carrying the PGK2-Pacrg transgene [qkv/qkv Tg(Pacrg)]. Northern blot analysis showed that the PGK2-Pacrg minigene is expressed in testes of qkv mutant mice at similar levels in all four transgenic Tg(Pacrg) lines (Fig. 3A and data not shown). The PGK2-Pacrg transgene produces a shorter transcript compared with its endogenous counterpart because the 5′ and 3′ UTRs of the Pacrg cDNA were not included in the minigene construct (Fig. 3A).
Fig. 3.
Transgenic expression of Pacrg restores spermiogenesis in qkv males. (A) Northern blot analysis on testis total RNA from qkv/qkv Tg(Pacrg) transgenic (Tg), qkv/qkv mutant (qkv), and unaffected control mice (c). (Lower) Hybridization with an actin cDNA probe as a loading control. (B) Periodic acid Schiff staining of testes sections from qkv/qkv mutant and from a qkv/qkv Tg(Pacrg) transgenic littermate. The arrow points to sperm tails in the lumen of the seminiferous tubules in the qkv/qkv Tg(Pacrg) testes.
Semen collected from the genital tracts of qkv/qkv Tg(Pacrg) male mice contained numerous motile spermatozoa, whereas only rare abnormal sperm were seen in the semen of nontransgenic qkv/qkv males (data not shown). Accordingly, histological analysis on testes from qkv/qkv Tg(Pacrg) males showed several seminiferous tubules with flagella in their lumen, indicating that transgenic expression of Pacrg restores spermiogenesis in qkv/qkv mutants (Fig. 3B). Sperm titers were compared between qkv/qkv Tg(Pacrg) and qkv heterozygous or wild-type littermates. Sperm counts from qkv/qkv Tg(Pacrg) males showed an ≈30% reduction compared with control littermates (qkv/+ or wild-type: 3.07 ± 0.25 × 106 sperm per ml, n = 13; qkv/qkv Tg(Pacrg): 2.14 ± 0.16 × 106 sperm per ml, n = 19, P < 0.005, two-tailed t test). This moderate reduction in sperm count in the transgenic animals may be the result of the expression of Pacrg under the control of an exogenous human promoter.
To determine whether the PGK2-Pacrg transgene could rescue male sterility in qkv/qkv mutants, we performed fertility tests on qkv/qkv Tg(Pacrg) transgenic males, qkv/qkv males and control nontransgenic littermates. All of the qkv/qkv Tg(Pacrg) males tested were fertile and sired a number of litters similar to the control males (Table 1). In addition, the average litter size did not differ significantly between qkv/qkv Tg(Pacrg) and control fertile males (Table 1). As expected, no offspring were produced from the matings of qkv/qkv males.
Table 1. Fertility test on qkv/qkv Tg (Pacrg) transgenic males.
| Genotype | Fertile males (tested) | No. of litters | Pups per litter (mean ± SEM) |
|---|---|---|---|
| qkv/qkv Tg (Pacrg)* | 10 (n = 10) | 30 | 10.85 ± 0.37† |
| qkv/qkv | 0 (n = 7) | 0 | 0 |
| qkv/+ or wt | 11 (n = 11) | 31 | 10.57 ± 0.5† |
At least two animals for each of the four Pacrg transgenic lines were tested
P > 0.05, t test for independent samples (t = 0.4628, df = 17)
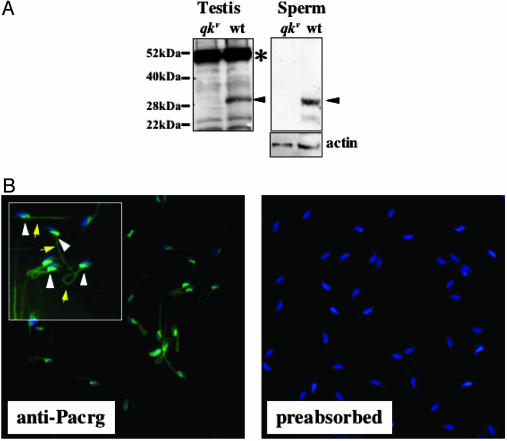
Pacrg Is Present in Mature Spermatozoa. To gain insight into the cellular function that Pacrg plays in spermiogenesis, we raised polyclonal antibody against peptides derived from the mouse Pacrg-predicted amino acid sequence. Western blot analysis using an anti-Pacrg serum on protein extracts of wild-type testis detected a band of ≈28 kDa, corresponding to the predicted molecular weight of Pacrg (Fig. 4A, testis). This 28-kDa species was absent in testis protein extracts from qkv/qkv mutant animals, indicating that this band corresponds to Pacrg (Fig. 4A, testis). A 50-kDa cross-reacting band was also detected in both wild-type and mutant testes (Fig. 4A, testis). The presence of this unidentified cross-reacting species hampered attempts to unequivocally determine the localization of the Pacrg protein by immunohistochemistry in testes sections (data not shown). However, when Western blot analysis was performed on sperm extracts from wild-type animals by using anti-Pacrg antiserum, a single 28-kDa band corresponding to Pacrg was detected (Fig. 4A, sperm representation). To determine to which sperm structure the Pacrg protein localizes, we performed immunofluorescence on isolated spermatozoa by using anti-Pacrg serum. A signal was detected in the postacrosomal region of the sperm head, directly underlying the nucleus (Fig. 4B, anti-Pacrg representation, arrowheads in Inset). Also, the anti-Pacrg antibodies stained the midpiece of the spermatozoa flagellum (Fig. 4B, anti-Pacrg, arrows in Inset). No fluorescent signal in these structures was detected when the anti-Pacrg serum was preabsorbed with the immunizing peptides (Fig. 4B, preabsorbed).
Fig. 4.
The Pacrg protein is present in mature spermatozoa. (A) Western blot analysis on protein extracts from testis (Left) and isolated sperm (Right) from mutant (qkv) or control wild-type (wt) animals. The arrowheads identify the 28-kDa band corresponding to Pacrg in both testes and sperm; the asterisk indicates the cross-reacting band present in the testis extract. (B) Immunofluorescence on methanol-fixed epididymal spermatozoa with anti-Pacrg antiserum (Left, ×200 magnification; Inset, ×400 magnification). White arrowheads and yellow arrows identify the Pacrg staining (green fluorescence) in the postacrosomal region and in the midpiece of the sperm flagellum, respectively. No specific fluorescence is detected in sperm incubated with anti-Pacrg sera preabsorbed with 5 μg/ml of the antigen peptides (Right). Sperm were counterstained for nucleic acids with 4′,6-diamidino-2-phenylindole (blue fluorescence).
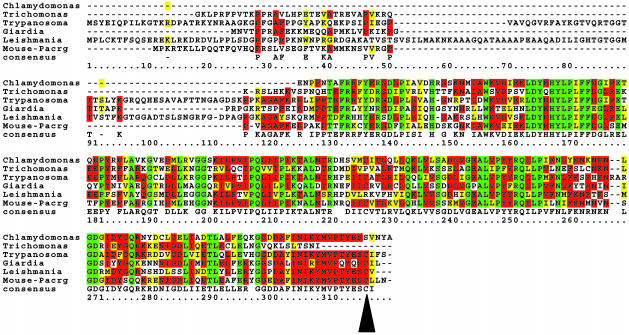
Pacrg Is Evolutionarily Conserved in Flagellated Protozoa. The conceptual translation of the mouse Pacrg cDNA (GenBank accession no. AK005771) yields a 241-aa protein that does not display any sequence similarities with proteins of known function. Searches of the publicly available databases identified several gene products with significant sequence similarity to Pacrg in metazoan species including Caenorhabditis elegans, Drosophila, Zebrafish, and Xenopus (see Fig. 6, which is published as supporting information on the PNAS web site). Sequence alignment of Pacrg-like proteins showed that a putative C-terminal prenylation site previously described in mammalian Pacrg,¶ is completely conserved in all metazoan species (see Fig. 6). Remarkably, the mammalian Pacrg also showed sequence similarity with gene products from flagellated protozoa (Fig. 5). All of the protozoan Pacrg-like proteins display at least 50% identity at the amino acid level with the mammalian Pacrg. The C-terminal prenylation motif is conserved in most but not all of the protozoan Pacrg proteins (Fig. 5), presumably as the result of incomplete or inaccurate EST sequences from some of these species. In addition to the prenylation site, other highly conserved domains were detected in Pacrg throughout all species, which include amino acid motifs found in proteins that are targeted to cellular organelles (see Fig. 6).
Fig. 5.
Pacrg is evolutionarily conserved in protozoan species. Protein sequence alignment of mouse Pacrg protein with gene products from protozoa. Residues conserved in the majority of sequences are highlighted in red, conservative amino acid substitutions are highlighted in yellow, and residues identical in all sequences are highlighted in green. The consensus sequence is shown in the lower line of the alignment. The arrowhead indicates the partially conserved cysteine residue within the putative prenylation motif. The protein sequences were obtained by conceptual translation of DNA GenBank and dbEST database entries from each species, corresponding to the following accession nos.: Mouse, AK005771; Chlamydomonas, BG856901; Trichomonas, CD664326; Trypanosoma, AC091330; Giardia, BAB56145; Leishmania, AAF69760.1. Alignment was generated by using clustalw version 1.8 (see Materials and Methods).
Discussion
In this study, we show that the deletion of the mouse Pacrg locus is the cause of male sterility in the qkv mutant. This result confirms the hypothesis that the mutation of a gene distinct from qk is responsible for the spermiogenesis defect associated with the qkv deletion (11). The transgenic expression of Pacrg in testis is necessary and sufficient to rescue the severe oligospermia associated with the qkv mutation and restores normal fertility in mutant males. Therefore, our work also implies that the deletion of the Parkin gene does not play a role in the infertility of qkv males. In further support of this conclusion, mice lacking Parkin have been reported to be fertile (23, 24). In mice as in humans, Pacrg and Parkin are closely linked and share a bidirectional minimal promoter (25). It has been therefore proposed that PACRG and PARKIN may be functionally related and may both have role in the pathogenesis of Parkinson's disease (25). However, qkv mutants do not display any Parkinson's disease-like neuropathology (14), suggesting that like Parkin, Pacrg is not necessary for the survival of dopaminergic neurons in the mouse brain (23, 24).
The Pacrg mRNA is highly expressed in the male mouse germ cells and, consistent with its role in sperm differentiation, Pacrg is abundant during the postmeiotic phase of spermatogenesis, in the round and elongating spermatids. Pacrg is also expressed in several mouse tissues other than testes, although at much lower levels (data not shown). Confirming previous reports (25, 26), our Northern blot analysis detected human PACRG mRNA in several tissues, including the brain. However, our study shows that the PACRG transcript is most abundant in testes, indicating that this gene may play a role in human spermiogenesis as well.
We show that the mouse Pacrg protein is a component of mature sperm and that it localizes to the postacrosomal region of the sperm head and to the flagellum midpiece. In qkv mutants, morphological abnormalities in both of these structures have been described (2). Abnormally shaped sperm heads are evident in qkv animals in late spermiogenesis at the onset of chromatin condensation. In mutant spermatids condensation proceeds normally, but the membrane structures surrounding the nucleus fail to conform to the changing shape of the chromatin. Also contributing to the abnormal head morphology in qkv spermatids are frequent finger-shaped intrusions of Sertoli cell cytoplasm. Pacrg has been reported to bind to tubulins (26), suggesting that it may be associated with microtubular cytoskeletal elements. All metazoan homologues of Pacrg contain a potential binding site for a lipid moiety as well as other evolutionary conserved amino acid motifs, indicating that Pacrg may associate with cellular organelles (see also Fig. 6). In agreement with both of these observations, Pacrg appears to localize to axonal processes and cytoplasmic vesicles in neuronal cells (26). It is possible that in the testis Pacrg may be necessary for the proper association between the membrane structures (i.e., plasma membrane and nuclear envelope) and the microtubular elements of the spermatid (i.e., manchette and nuclear ring), which are thought to be involved in head shaping during spermiogenesis. Interestingly, mutation of the Hook1 mouse gene, which encodes for a protein known to interact with both microtubules and cellular organelles, results in sperm head defects that resemble those described in qkv (27).
In addition to the sperm head malformations, qkv mutants display a marked flagellar defect. In qkv spermatids the flagellum begins to develop normally but the microtubular filaments fail to maintain their regular 9 plus 2 arrangement at late stages of spermiogenesis. This finding suggests that Pacrg may be also essential for the assembly or the maintenance of the axonemal microtubule array in the developing flagellum. In support of this hypothesis, we report that the Pacrg proteins display significant sequence similarities with gene products in several flagellated protozoan species (Fig. 5). Therefore, the mammalian Pacrg may have evolved from an ancestral protein necessary for flagellar development in monocellular organisms. Structural and functional conservation of flagellar components between protozoa and mammals has been described. For instance, mice lacking Sperm antigen 6, the mammalian homologue of the Chlamydomonas axonemal protein PF16, are sterile due to sperm flagellar abnormalities (28). Ultrastructural localization of Pacrg in the developing sperm as well as additional biochemical analyses will be needed to confirm its function(s) in spermiogenesis.
In conclusion, we have shown that Pacrg is necessary for spermiogenesis and male fertility in mice. This is the first report, to our knowledge, to describe the function of Pacrg in vivo in any model organism. Because it is likely that the role of Pacrg in spermiogenesis may be conserved in other mammalian species, including humans, the understanding of its cellular function may also help elucidate the molecular basis for some infertility cases in men. Furthermore, its conservation in monocellular organisms may reveal an essential role for Pacrg in flagellar development throughout evolution.
Acknowledgments
We thank Cavatina Truong for technical help with the in situ experiments and Dr. Marvin Meistrich for assistance with the staging of testes sections and for ongoing discussions. This work was supported by a grant from the Kleberg Foundation and by Public Health Service Grant U01 HD39372 (to M.J.J.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: dpp, days postpartum.
Footnotes
Imai, Y., Soda, M., Kataoka, A. & Takahashi, R., 33rd Annual Meeting Society for Neuroscience, Nov. 8-12, 2003, New Orleans, abstr. 78.3.
References
- 1.Sidman, R. L., Dickie, M. M. & Appel, S. H. (1964) Science 144, 309-311. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, W. I., Gall, A. M., Southard, J. L. & Sidman, R. L. (1971) Biol. Reprod. 5, 30-58. [DOI] [PubMed] [Google Scholar]
- 3.Yanagimachi, R., Wakayama, T., Kishikawa, H., Fimia, G. M., Monaco, L. & Sassone-Corsi, P. (2004) Proc. Natl. Acad. Sci. USA 101, 1691-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebersole, T., Rho, O. & Artzt, K. (1992) Genetics 131, 183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, R. D., Shedlovsky, A., Hamvas, R., Goldsworthy, M., Whittington, J., Connelly, C. S., Dove, W. F. & Lehrach, H. (1994) Genomics 21, 77-84. [DOI] [PubMed] [Google Scholar]
- 6.Wu, J. I., Reed, R. B., Grabowski, P. J. & Artzt, K. (2002) Proc. Natl. Acad. Sci. USA 99, 4233-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakiza, O., Villavicencio, E., Walterhouse, D., Goodwin, E. B. & Innacone, P. (2003) Dev. Biol. 259, 522. [DOI] [PubMed] [Google Scholar]
- 8.Ebersole, T. A., Chen, Q., Justice, M. J. & Artzt, K. (1996) Nat. Genet. 12, 260-265. [DOI] [PubMed] [Google Scholar]
- 9.Hardy, R. J., Loushin, C. L., Friedrich, V. L., Jr., Chen, Q., Ebersole, T. A., Lazzarini, R. A. & Artzt, K. (1996) J. Neurosci. 16, 7941-7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu, Z., Zhang, Y., Ku, L., Wang, H., Ahmadian, A. & Feng, Y. (2003) Nucleic Acids Res. 31, 4616-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justice, M. J. & Bode, V. C. (1988) Genet. Res. 51, 95-102. [DOI] [PubMed] [Google Scholar]
- 12.Shedlovsky, A., King, T. R. & Dove, W. F. (1988) Proc. Natl. Acad. Sci. USA 85, 180-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox, R. D., Hugill, A., Shedlovsky, A., Noveroske, J. K., Best, S., Justice, M. J., Lehrach, H. & Dove, W. F. (1999) Genomics 57, 333-341. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzetti, D., Antalffy, B., Vogel, H., Noveroske, J. K., Armstrong, D. & Justice, M. J. (2004) Mamm. Genome 15, 210-217. [DOI] [PubMed] [Google Scholar]
- 15.Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y. & Shimizu, N. (1998) Nature 392, 605-608. [DOI] [PubMed] [Google Scholar]
- 16.Lockhart, P., O'Farrell, C. & Farrer, M. (2004) Movement Disorders 19, 101-104. [DOI] [PubMed] [Google Scholar]
- 17.Lu, B. & Bishop, C. E. (2003) J. Biol. Chem. 278, 16289-16296. [DOI] [PubMed] [Google Scholar]
- 18.Robinson, M. O., McCarrey, J. R. & Simon, M. I. (1989) Proc. Natl. Acad. Sci. USA 86, 8437-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark, A. T., Firozi, K. & Justice, M. J. (2004) Biol. Reprod. 70, 1317-1324. [DOI] [PubMed] [Google Scholar]
- 20.Quill, T. A., Ren, D., Clapham, D. E. & Garbers, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 12527-12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop, C. E., Whitworth, D. J., Qin, Y., Agoulnik, A. I., Agoulnik, I. U., Harrison, W. R., Behringer, R. R. & Overbeek, P. A. (2000) Nat. Genet. 26, 490-494. [DOI] [PubMed] [Google Scholar]
- 22.McCarrey, J. R. (1993) in Cell and Molecular Biology of the Testis, eds. Desjardins, C. & Ewing, L. L. (Oxford Univ. Press, New York), pp. 58-89.
- 23.Goldberg, M. S., Fleming, S. M., Palacino, J. J., Cepeda, C., Lam, H. A., Bhatnagar, A., Meloni, E. G., Wu, N., Ackerson, L. C., Klapstein, G. J., et al. (2003) J. Biol. Chem. 278, 42628-42635. [DOI] [PubMed] [Google Scholar]
- 24.Itier, J. M., Ibanez, P., Mena, M. A., Abbas, N., Cohen-Salmon, C., Bohme, G. A., Laville, M., Pratt, J., Corti, O., Pradier, L., et al. (2003) Hum. Mol. Genet. 12, 2277-2291. [DOI] [PubMed] [Google Scholar]
- 25.West, A. B., Lockhart, P. J., O'Farell, C. & Farrer, M. J. (2003) J. Mol. Biol. 326, 11-19. [DOI] [PubMed] [Google Scholar]
- 26.Imai, Y., Soda, M., Murakami, T., Shoji, M., Abe, K. & Takahashi, R. (2003) J. Biol. Chem. 278, 51901-51910. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza-Lujambio, I., Burfeind, P., Dixkens, C., Meinhardt, A., Hoyer-Fender, S., Engel, W. & Neesen, J. (2002) Hum. Mol. Genet. 11, 1647-1658. [DOI] [PubMed] [Google Scholar]
- 28.Sapiro, R., Kostetskii, I., Olds-Clarke, P., Gerton, G. L., Radice, G. L. & Strauss, I. J. (2002) Mol. Cell. Biol. 22, 6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]