Abstract
Summary
Background and objectives
Demand for hemodialysis among elderly patients is increasing worldwide. Although clinical care of this high-risk group is complex and challenging, no guidelines exist to inform hemodialysis practices. The Dialysis Outcomes and Practice Patterns Study (DOPPS) provides a unique opportunity to assess dialysis practices and associated outcomes among elderly versus younger patients on chronic in-center hemodialysis in 12 countries.
Design, setting, participants, & measurements
Clinical characteristics, dialysis practices, and outcomes of elderly versus younger patients were compared among participants in four DOPPS regions in 2005 through 2007.
Results
Although participant mean age increased over time in all DOPPS countries, the percentage of elderly varied widely. Overall, comorbidities and malnutrition were more common in the elderly. Fistulae were used less frequently among elderly versus younger patients in Europe and North America but not in Australia, New Zealand, and Japan. No difference in treatment time was observed between elderly and younger patients after normalizing for body weight. In all regions, ultrafiltration rates were lower among elderly patients. Elderly patients reported poorer quality of life with respect to the physical but not mental component scores. Mortality risk was three- to sixfold higher in the elderly group, whereas causes of death overall were similar for elderly and younger patients.
Conclusions
Elderly patients represent a different proportion of DOPPS participants across countries, possibly reflecting differences in policies and clinical practices. In general, hemodialysis practices in the elderly reflected each region's clinical patterns, with some variation by age group depending upon the practice.
Introduction
The incidence of chronic kidney disease is increasing worldwide because of an aging population and higher prevalence of diabetes, hypertension, and cardiovascular diseases (1). As a result, in most Western countries the demand for dialysis is increasing more rapidly in elderly patients than in younger age groups (2–4). Data from the United States Renal Data System indicate that incidence of ESRD is increasing among persons aged >65 years and especially among those aged >80 years (4). Similarly in Europe the proportion of incident ESRD patients aged >65 years increased from 22% in 1980 to 55% in 2005 (3,5). As expected, mortality among elderly ESRD patients is high (4,6–8). However, the aging phenomenon in the dialysis population is amplified by a more liberal acceptance of older patients on dialysis, better survival of dialysis patients, and reduced access to transplantation for elderly patients.
Treatment of ESRD in the elderly is complex and views on how to best manage these patients divide the nephrology community. Once hemodialysis is started, it is not clear whether dialysis prescription for the elderly should be different than that of younger patients. In fact, no established guidelines exist to inform the practice of hemodialysis in the elderly population.
To our knowledge, no study thus far has compared dialysis prescription and clinical practices among the elderly to those of younger patients. In this study, we compared hemodialysis practices and outcomes of elderly (aged ≥75 years) versus younger patients in the international cohort of the Dialysis Outcomes and Practice Patterns Study (DOPPS).
Materials and Methods
Data Source
The DOPPS design and sampling strategy have been described previously (9,10). The DOPPS received local and national institutional review board approval as required. The current study analyzed data collected from 2005 through 2007 in 8161 chronic in-center hemodialysis patients participating in the third phase of the DOPPS (DOPPS III). Patients meeting DOPPS inclusion criteria (age >18 years; on chronic in-center hemodialysis) were selected in 295 dialysis facilities randomly chosen to represent the hemodialysis population in 12 countries (Belgium, France, Germany, Italy, Spain, Sweden, United Kingdom, Australia, New Zealand, Japan, Canada, and the United States). Data are based upon a cross-section of chronic hemodialysis patients treated in each dialysis unit at the time of starting participation in DOPPS III. Participants were followed until the earliest of death, or up to 7 days after departure from the facility for kidney transplantation, change of treatment modality, withdrawal from dialysis, return of renal function, or transfer to another facility. Follow-up time was censored at study end for patients who did not depart from the facility. The trend in mean age at study entry was analyzed among cross-sections of participants in DOPPS I (1996 through 2001) (n = 8626), II (2002 through 2004) (n = 9117), and III (2005 through 2007) (n = 8161).
Data on patient demographic characteristics, medical history, laboratory data, and hemodialysis treatment were obtained by medical record abstraction, and reflected the most recent data for study patients at the time of study entry. Typically within 2 months of study entry, DOPPS participants completed self-administered questionnaires that included the Kidney Disease Quality of Life Short Form (KDQoL-SF) (11) and the Center for Epidemiologic Studies Depression Rating Scale. Mental and physical component summary scores were derived from the KDQoL-SF. Quality of life data presented are those collected at the time point closest to study entry (mean time: 1.8 months [95% confidence interval (CI): 1.7 to 2.0]). Intradialytic hypotension was defined as change of systolic BP during dialysis ≥30 mm Hg and postdialysis systolic BP <100 mm Hg.
Statistical Analyses
Participants were categorized into three age groups (<45 years, 45 to 74 years, and ≥75 years, the latter being the elderly group) based on age at the time of entry into the DOPPS or at initiation of dialysis, depending on the analysis (see below). Descriptive analyses of patient characteristics were performed by age group at study entry and by geographic region (Europe, European countries; ANZ, Australia and New Zealand; Japan; North America, United States and Canada) accounting for patient sampling weights. Patient sampling weights are designed to account for differences in the fraction of patients randomly sampled in each dialysis unit. Separate unadjusted linear regression or logistic regression models accounting for facility clustering effects and patient weights were used to compare each patient characteristic in the <45 years or 45 to 74 years group versus those in the ≥75 years (using age at DOPPS entry) group within each region.
The odds of achieving selected clinical outcomes (single pool Kt/V >1.2, serum albumin >4 g/dl, and Center for Epidemiologic Studies Depression Rating Scale score ≥10) by age group at study entry were estimated by logistic regression accounting for facility clustering effects within each region. Generalized estimating equations were used to account for clustering at the facility level, assuming a compound symmetry covariance structure (12,13). The probability of patient survival from the start of dialysis, by age group at the initiation of dialysis, was estimated within each region using a generalization of Kaplan-Meier estimates to accommodate left truncation at the time of DOPPS enrollment (14). With this method, only time when the patients were under observation was included in the calculation.
The association between age at study entry and mortality was assessed within each region using Cox regression models (14) adjusted for sex, race, body mass index (BMI), years with ESRD, and 14 summary comorbid conditions as assessed at study entry, accounting for facility clustering effects and stratified by country. Sensitivity analyses were conducted using left-truncated models, with time from start of dialysis to death or censoring as the time axis, time at risk beginning at DOPPS enrollment, and age at start of dialysis as the predictor. The proportionality assumption in all of the Cox proportional hazard models was confirmed by Kolmogorov-Smirnov–type supremum tests using 1000 simulated patterns. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). Statistical significance is reported for P values < 0.05.
Results
The study sample was comprised of 8161 in-center hemodialysis patients participating in DOPPS III; the median follow-up was 18 months. During the study period, 1337 participants died (crude mortality rate for all study participants: 12.2/100 patient years, with mortality rates of 3.7, 10.4, and 21.4 deaths per 100 patient years for patients <45, 45 to 74, and ≥75 years old, respectively). Other causes of censoring were switch to peritoneal dialysis (n = 89), kidney transplant (n = 583), and transfer out of a DOPPS facility (n = 944).
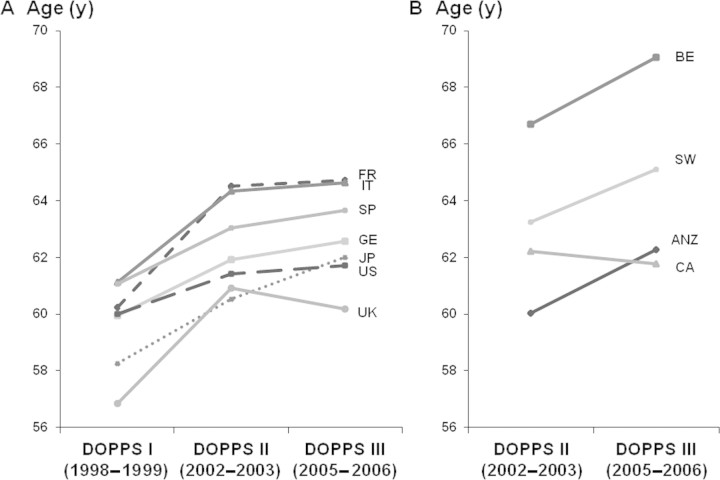
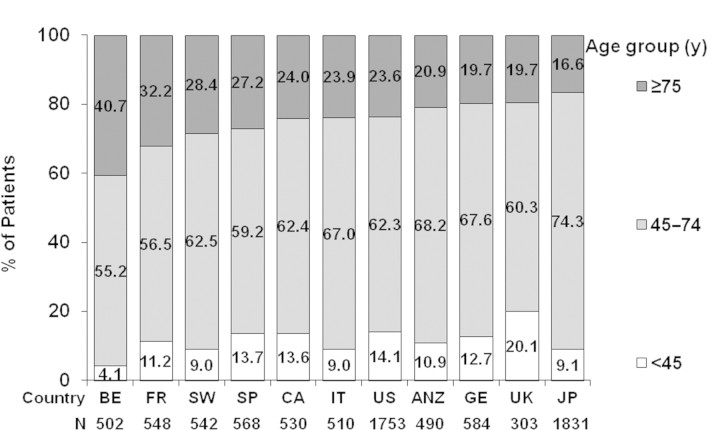
Overall, the mean age of DOPPS participants at study entry increased over time (Figure 1). Mean age increased significantly from DOPPS I to DOPPS II in all countries participating in the first three DOPPS phases except for the United States, which showed a similar trend but failed to reach statistical significance (P = 0.08). Between DOPPS II and DOPPS III a significant age increase was observed in ANZ, Belgium, Japan, and Sweden. However, the proportion of patients who were elderly (≥75 years old) varied widely across countries (Figure 2) and differed in the four DOPPS regions (27.4% in Europe, 20.9% in ANZ, 16.6% in Japan, and 23.7% in North America).
Figure 1.
Mean age of Dialysis Outcomes and Practice Patterns Study (DOPPS) participants at study entry across DOPPS phases. On the basis of cross sections of participants in DOPPS I (1998 through 1999, with the exception of the United States (US), where most DOPPS I participants were enrolled in 1997; n = 8542), DOPPS II (2002 through 2003; n = 9117), and DOPPS III (2005 through 2006; n = 8161). Panel A depicts countries that participated in DOPPS I, II, and III; panel B depicts countries that participated only in DOPPS II and DOPPS III. P value for comparison between DOPPS I and DOPPS II: <0.05 in all countries except the United States; P value for comparison between DOPPS II and DOPPS III: <0.05 in Belgium (BE), Japan (JP), Sweden (SW), and Australia and New Zealand (ANZ). FR, France; IT, Italy; SP, Spain; GE, Germany; UK, United Kingdom; CA, Canada.
Figure 2.
Distribution of patients in age categories across DOPPS countries. Cross section of participants in DOPPS III (2005 through 2007; n = 8161).
Patient Characteristics
The distribution of patient characteristics by age group across DOPPS regions is shown in Table 1. Elderly patients had shorter duration of ESRD in all regions. The majority of elderly participants in Europe (43.6%), ANZ (23.6%), and North America (42.8%) had ESRD for <2 years. By contrast, in Japan, elderly participants had much longer duration of ESRD, with 15.1% of elderly participants having been on dialysis for >10 years. BMI and estimated dry weight were lower for elderly patients in each region except Europe. Furthermore, the fraction of patients who were able to walk without assistance substantially declined with older age in each region.
Table 1.
Patient characteristics by age categories across DOPPS regions
| Patient characteristics | Europe |
Australia and New Zealand |
Japan |
North America |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
|||||||||||||
| mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | |
| Male (%) | 60.1 | (54.6–65.5) | 58.8 | (56.6–61.1) | 54.2 | (50.8–57.6) | 43.8 | (30.3–57.4) | 54.3 | (48.8–59.9) | 73.1 | (64.5–81.6) | 71.1 | (63.4–78.8) | 61.7 | (58.9–64.6) | 53.1 | (47.2–59) | 59.4 | (53.1–65.7) | 56.5 | (53.5–59.5) | 53.1 | (48.3–57.9) |
| Black race (%) | 4.3 | (1.3–7.4) | 1.9 | (1.2–2.6) | 0.7 | (0.2–1.3) | 2.1 | (0–6.1) | 0.2 | (0–0.7) | 1.7 | (0–4) | 0 | 0 | 0 | 33.6 | (27.9–39.3) | 29.1 | (26.5–31.7) | 13 | (9.9–16) | |||
| Duration of ESRD (year) (mean) | 6.8 | (5.8–7.8) | 5.1 | (4.8–5.4) | 3.7 | (3.4–3.9) | 6.5 | (4.5–8.6) | 4.9 | (4.4–5.4) | 4.5 | (3.9–5.2) | 7.7 | (6.7–8.7) | 9.2 | (8.8–9.7) | 5.7 | (5–6.3) | 5.1 | (4.5–5.8) | 4 | (3.8–4.3) | 2.9 | (2.7–3.2) |
| BMI (mean) | 23.8 | (23.1–24.5) | 25.6 | (25.4–25.8) | 24.3 | (24–24.6) | 27.8 | (25.6–30) | 27.7 | (27–28.5) | 25.5 | (24.5–26.4) | 21.4 | (20.8–22.1) | 21.1 | (20.9–21.3) | 20.3 | (20–20.6) | 27.9 | (26.8–29) | 27.9 | (27.5–28.3) | 25 | (24.4–25.5) |
| Dry weight (kg) (mean) | 68.2 | (65.4–71) | 71.7 | (70.9–72.4) | 65.8 | (64.9–66.7) | 77.7 | (70.4–85.1) | 77 | (74.8–79.3) | 68.3 | (65.4–71.2) | 58 | (55.6–60.4) | 54.4 | (53.8–55.1) | 48.3 | (47.3–49.3) | 79.3 | (76.1–82.6) | 80.4 | (79–81.8) | 67.7 | (66.2–69.3) |
| Unable to walk without assistance (%) | 8.1 | (5.2–11) | 20.7 | (18.8–22.6) | 35 | (31.7–38.3) | 11.5 | (2.8–20.1) | 23.4 | (18.6–28.2) | 39.9 | (30.3–49.5) | 4.5 | (1.3–7.7) | 10.7 | (8.9–12.5) | 32 | (26.6–37.4) | 11.8 | (7.8–15.7) | 30.7 | (27.9–33.6) | 47.1 | (42.2–52) |
| Comorbid conditions (%) | ||||||||||||||||||||||||
| coronary artery disease | 12.4 | (9–15.9) | 41 | (38.8–43.3) | 46 | (42.6–49.3) | 15.8 | (5.5–26) | 56.2 | (50.7–61.7) | 55 | (45.3–64.6) | 11.6 | (6.1–17.1) | 27.5 | (24.8–30.1) | 38.5 | (32.8–44.2) | 25.7 | (20.1–31.3) | 50.9 | (47.8–53.9) | 63.3 | (58.6–67.9) |
| cancer other than skin | 6.6 | (4–9.3) | 14.2 | (12.6–15.8) | 18.9 | (16.3–21.4) | 3.7 | (0–8.8) | 13 | (9.3–16.7) | 22.6 | (14.6–30.5) | 0.6 | (0–1.8) | 9 | (7.3–10.6) | 11.4 | (7.6–15.2) | 3.5 | (0.6–6.5) | 12.2 | (10.2–14.2) | 20.8 | (16.8–24.9) |
| other cardiovascular disease | 20.1 | (15.8–24.4) | 40.1 | (37.9–42.3) | 60.2 | (56.9–63.5) | 11.4 | (2.8–20.1) | 35.1 | (29.8–40.4) | 46.6 | (36.9–56.3) | 17.7 | (10.9–24.6) | 31.7 | (28.9–34.5) | 46.1 | (40.2–52) | 16.6 | (11.9–21.3) | 34.2 | (31.3–37.1) | 51.9 | (47.1–56.7) |
| cerebrovascular disease | 7.6 | (4.9–10.4) | 18 | (16.2–19.7) | 25.6 | (22.6–28.6) | 6.8 | (0.3–13.3) | 19.9 | (15.5–24.3) | 29.8 | (21–38.6) | 1.6 | (0–3.3) | 12.8 | (10.8–14.8) | 18.5 | (14.2–22.7) | 4.2 | (1.9–6.5) | 19.4 | (17–21.7) | 22.9 | (18.7–27.1) |
| congestive heart failure | 23.9 | (19.1–28.7) | 41.2 | (38.9–43.4) | 47.3 | (44–50.7) | 31.4 | (18.3–44.4) | 46 | (40.5–51.6) | 49.2 | (39.5–58.9) | 13.2 | (7.3–19.2) | 22.6 | (20.2–25.1) | 36 | (30.4–41.6) | 24.1 | (19–29.1) | 47.8 | (44.7–50.8) | 58 | (53.2–62.7) |
| diabetes | 16.3 | (12.2–20.4) | 33.5 | (31.3–35.7) | 29.6 | (26.5–32.6) | 34.4 | (21.1–47.7) | 46.4 | (40.8–52) | 40 | (30.4–49.6) | 16.7 | (10.5–22.9) | 32.8 | (30–35.6) | 35.2 | (29.5–40.9) | 24 | (18.8–29.1) | 60.1 | (57.1–63.2) | 48.8 | (44–53.6) |
| GI bleeding in prior 12 months | 1.4 | (0.3–2.5) | 4.7 | (3.8–5.5) | 6 | (4.4–7.6) | 5 | (0–10.6) | 3.4 | (1.5–5.4) | 11.8 | (5.6–18) | 3.4 | (0.4–6.4) | 3.9 | (2.8–5) | 4.4 | (2.2–6.7) | 3.4 | (1.4–5.5) | 6.1 | (4.6–7.6) | 8.9 | (5.9–11.8) |
| HIV/AIDS | 1.5 | (0.2–2.8) | 0.4 | (0.1–0.8) | 0.1 | (0–0.2) | 0 | 0.3 | (0–0.9) | 0 | 0.7 | (0–1.8) | 0.4 | (0.1–0.7) | 0.4 | (0–0.9) | 3.1 | (1.1–5.1) | 0.9 | (0.3–1.4) | 0 | |||
| hypertension | 73.5 | (68.4–78.6) | 79.1 | (77.2–81.1) | 79 | (76.1–81.9) | 70.8 | (58.8–82.9) | 85 | (81–88.9) | 85.2 | (78.3–92) | 58.8 | (50.2–67.4) | 70.2 | (67.5–72.9) | 78.2 | (73.5–82.9) | 86.6 | (81.8–91.3) | 87.5 | (85.4–89.6) | 87.3 | (83.9–90.7) |
| lung disease | 3.4 | (1.6–5.3) | 13 | (11.5–14.5) | 16.4 | (14–18.8) | 0 | 19.5 | (15–24.1) | 25.8 | (17–34.6) | 1.1 | (0–2.7) | 2.4 | (1.4–3.4) | 4.3 | (2–6.6) | 5.9 | (3.2–8.6) | 19.1 | (16.8–21.4) | 24.5 | (20.5–28.6) | |
| neurological disease | 9.8 | (6.8–12.9) | 10.3 | (8.9–11.6) | 14 | (11.8–16.3) | 13.1 | (4.5–21.7) | 9 | (5.9–12.2) | 12.2 | (6.1–18.3) | 4 | (1.2–6.8) | 6.3 | (4.9–7.6) | 25 | (19.9–30.1) | 14.8 | (10.7–18.8) | 11.8 | (9.8–13.8) | 15.1 | (11.8–18.5) |
| dementia | 0.5 | (0–1.1) | 2.1 | (1.5–2.8) | 3.6 | (2.4–4.9) | 0 | 1.4 | (0.2–2.7) | 7.4 | (2.4–12.4) | 0 | 2 | (1.2–2.8) | 22 | (17.1–26.8) | 0.6 | (0–1.5) | 2 | (1.2–2.7) | 7.5 | (5–10) | ||
| other psychiatric disorder | 10.4 | (7.2–13.7) | 11.6 | (10.1–13.1) | 9.7 | (7.6–11.8) | 15.4 | (5.4–25.5) | 18.9 | (14.3–23.4) | 12.3 | (5.9–18.7) | 1.9 | (0–3.9) | 3.8 | (2.7–4.9) | 1.9 | (0.4–3.3) | 27.4 | (21.9–33) | 20.2 | (17.8–22.6) | 12.9 | (9.5–16.3) |
| peripheral vascular disease | 9.7 | (6.6–12.9) | 32.2 | (30.1–34.3) | 35.7 | (32.5–39) | 11.7 | (2.7–20.7) | 33.8 | (28.6–39.1) | 49.2 | (39.5–58.9) | 4.7 | (0.4–9.1) | 17.7 | (15.4–20) | 21.6 | (17–26.3) | 11.7 | (7.9–15.5) | 35.9 | (33–38.9) | 35.5 | (30.8–40.2) |
| recurrent cellulitis, gangrene | 6.1 | (2.8–9.4) | 10.9 | (9.5–12.3) | 7 | (5.2–8.8) | 6.3 | (0–13.5) | 14.1 | (10.1–18) | 13.7 | (6.9–20.5) | 1.4 | (0–3.4) | 3.8 | (2.6–5) | 4.9 | (2.4–7.4) | 7.6 | (4.3–11) | 12.9 | (10.7–15) | 7.3 | (4.6–9.9) |
Cross section of hemodialysis patients in DOPPS III (2005 through 2007) (n = 8161). Bold numbers represent P < 0.05 compared to the elderly group (≥75 years) within each region. Europe includes United Kingdom, France, Germany, Italy, Spain, Belgium, and Sweden; North America includes United States and Canada. Abbreviation: 95% CI = 95% confidence interval; DOPPS, Dialysis Outcomes and Practice Patterns Study; BMI, body mass index; GI, gastrointestinal.
In all regions, the prevalence of cardiovascular diseases, congestive heart failure, and malignancies was higher in the elderly group versus the <45 year old group (P < 0.05).
Dialysis Treatment Characteristics
The type of vascular access used in the elderly group reflected the region practices in younger age groups: arteriovenous fistulae (AVFs) were the most common type of access used for elderly patients in Europe (68.5%), ANZ (80.5%), and Japan (91.4%), but not in North America (44.2%) (Table 2). However, within Europe and North America, elderly patients were less likely (P < 0.05) to have an AVF than patients <45 years old. Permanent catheters were used more frequently among elderly patients in Europe (23.1 versus 12.2% of participants aged <45 years and 19% of those aged 45 to 74 years; P < 0.05) with a similar trend seen in North America.
Table 2.
Hemodialysis treatment characteristics by age categories across DOPPS regions
| Hemodialysis treatment characteristics | Europe |
Australia and New Zealand |
Japan |
North America |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
|||||||||||||
| mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | mean or % | 95% CI | |
| Vascular access (%) | ||||||||||||||||||||||||
| AV fistula | 78.5 | (73.9–83.1) | 71 | (68.8–73.2) | 68.5 | (65.2–71.7) | 75.5 | (63.7–87.2) | 76.8 | (72–81.6) | 80.5 | (72.7–88.3) | 93.1 | (88.8–97.3) | 93.5 | (92–95) | 91.4 | (88–94.7) | 55 | (48.7–61.4) | 46.3 | (43.2–49.4) | 44.2 | (39.4–49.1) |
| graft | 7.8 | (5.2–10.4) | 8.2 | (7–9.5) | 7.1 | (5.4–8.8) | 11.2 | (2.6–19.7) | 12.7 | (9–16.4) | 10.3 | (4.4–16.2) | 5.9 | (1.8–10) | 6 | (4.6–7.4) | 8.4 | (5.1–11.7) | 17.8 | (13.2–22.5) | 24.6 | (22–27.3) | 23.2 | (19.2–27.2) |
| permanent catheter | 12.2 | (8.4–16.1) | 19 | (17.1–21) | 23.1 | (20.2–26.1) | 11.3 | (2.7–20) | 9.5 | (6.1–12.8) | 6.9 | (1.9–11.8) | 0 | 0.3 | (0–0.8) | 0 | 25 | (19.5–30.5) | 27.6 | (24.7–30.5) | 31.8 | (27.1–36.5) | ||
| temporary catheter | 1.5 | (0–3.1) | 1.8 | (1.1–2.4) | 1.3 | (0.6–2) | 2 | (0–5.9) | 1 | (0–2.2) | 2.4 | (0–5.6) | 1 | (0–2.3) | 0.2 | (0–0.3) | 0.2 | (0–0.7) | 2.1 | (0.5–3.7) | 1.5 | (0.7–2.3) | 0.8 | (0–1.8) |
| Dialysis prescriptionsa (mean) | ||||||||||||||||||||||||
| treatment time (min) | 246 | (241–251) | 245 | (244–247) | 232 | (231–234) | 269 | (259–279) | 272 | (268–275) | 253 | (247–260) | 248 | (243–253) | 239 | (238–241) | 225 | (222–228) | 237 | (231–242) | 230 | (227–232) | 213 | (209–217) |
| treatment time (min/kg) | 3.8 | (3.7–4) | 3.6 | (3.5–3.6) | 3.6 | (3.6–3.7) | 3.8 | (3.4–4.1) | 3.7 | (3.6–3.8) | 3.8 | (3.6–4) | 4.4 | (4.3–4.6) | 4.6 | (4.5–4.6) | 4.8 | (4.7–4.9) | 3.2 | (3.1–3.4) | 3 | (3–3.1) | 3.3 | (3.2–3.4) |
| blood flow (ml/min) | 315 | (304–326) | 302 | (298–305) | 295 | (290–300) | 308 | (295–321) | 304 | (299–309) | 309 | (298–320) | 207 | (201–213) | 199 | (197–201) | 184 | (180–188) | 395 | (386–405) | 386 | (381–390) | 375 | (368–383) |
| ultrafiltration rate (ml/h) | 562 | (527–597) | 547 | (533–561) | 482 | (464–499) | 524 | (457–591) | 484 | (457–511) | 404 | (358–449) | 629 | (591–668) | 564 | (552–577) | 468 | (446–489) | 708 | (655–761) | 691 | (670–712) | 569 | (540–598) |
| Hypotension (%) | 5.5 | (1.8–9.2) | 3.7 | (2.8–4.6) | 2.3 | (1.2–3.4) | 2 | (0–6) | 1.9 | (0.4–3.4) | 0 | 3.6 | (0–7.9) | 3.9 | (2.7–5) | 3.4 | (1.1–5.8) | 1.6 | (0–3.5) | 2.3 | (1.2–3.4) | 1.4 | (0.4–2.4) | |
Cross section of hemodialysis patients in DOPPS III (2005 through 2007) (n = 8161). Bold numbers represent P < 0.05 compared to the elderly group (≥75 years) within each region.
Restricted to patients with three dialysis sessions per week (n = 7599). Europe includes United Kingdom, France, Germany, Italy, Spain, Belgium, and Sweden; North America includes United States and Canada. Abbreviation: AV, arteriovenous.
Among patients receiving three hemodialysis sessions per week (n = 7599), the mean prescribed treatment time was shorter for elderly patients. However, once treatment time was normalized for body weight, no differences were observed for elderly patients versus those aged <45 years in all regions except for Japan. Although statistically significant, the difference in prescribed blood flow between the elderly and younger age group within each region was not clinically meaningful. Blood flow varied greatly across regions, with overall higher blood flow in North America compared with other regions. In all regions, the mean ultrafiltration rate was significantly lower in the elderly age group than in the younger age groups. Intradialytic hypotension tended to be less common among elderly patients in all regions.
Biochemical Parameters
The distribution of biochemical parameters by age category across DOPPS regions is shown in Table 3. Overall, elderly participants were at least as or more likely to meet current guidelines for dialysis dose (single pool Kt/V ≥1.2) (15) as younger participants in the same geographic region (Europe: odds ratio [OR] 0.95 [95% CI: 0.66 to 1.39]; ANZ: OR 1.39 [95% CI: 0.54 to 3.53]; Japan: OR 0.94 [95% CI: 0.63 to 1.40]; North America: OR 1.99 [95% CI: 1.19 to 3.35]). Hemoglobin >11 g/dl was achieved in 77.8% to 81.2% of elderly patients in Europe, ANZ, and North America but in only 29.1% of elderly patients in Japan. The erythropoietin resistance index (weekly erythropoietin dose per body weight divided by hemoglobin levels [16]) among elderly versus younger patients was higher in Japan (11.3 versus 8.3 g/dl per week per kilogram; P < 0.001), whereas no significant differences (P > 0.05) were observed in Europe, ANZ, and North America.
Table 3.
Distribution of biochemical parameters by age categories across DOPPS regions
| Biochemical parameters | Europe |
Australia and New Zealand |
Japan |
North America |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
<45 years |
45 to 74 years |
≥75 years |
||||||||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Single pool Kt/V | <1.20 | 17.1 | (11.7–22.5) | 18.6 | (16.5–20.6) | 16 | (13.2–18.9) | 19.4 | (6.5–32.3) | 11.3 | (7.1–15.5) | 11.8 | (4–19.6) | 31.2 | (22.7–39.7) | 26.3 | (23.5–29) | 29.9 | (23.9–35.8) | 15.5 | (10.4–20.6) | 13.3 | (10.8–15.7) | 9.6 | (6.3–12.8) |
| 1.20 to 1.39 | 22.3 | (16.9–27.7) | 23.8 | (21.5–26.1) | 20.2 | (17–23.3) | 18.9 | (5.9–32) | 20.2 | (14.9–25.5) | 15.4 | (6.5–24.2) | 33.6 | (24.7–42.4) | 30 | (27.1–32.9) | 27 | (21–33.1) | 17.2 | (12.2–22.1) | 18.2 | (15.6–20.8) | 14.1 | (10.5–17.6) | |
| 1.40 to 1.59 | 25 | (19.2–30.7) | 25.3 | (22.9–27.6) | 29.8 | (26.2–33.5) | 20.2 | (7.2–33.1) | 26.5 | (20.6–32.4) | 29.5 | (18.3–40.8) | 25.8 | (17.4–34.2) | 25.8 | (23–28.6) | 25.6 | (19.9–31.3) | 26.5 | (20.5–32.4) | 32.4 | (29.3–35.6) | 26.9 | (22.2–31.6) | |
| 1.60 to 1.79 | 17.8 | (12.9–22.6) | 18.7 | (16.6–20.8) | 18.2 | (15.2–21.2) | 23.2 | (10.2–36.1) | 18.3 | (13.2–23.4) | 25.5 | (14.8–36.1) | 6.3 | (2.3–10.3) | 12.3 | (10.3–14.3) | 11.8 | (7.7–16) | 20.9 | (15.4–26.5) | 19 | (16.4–21.6) | 23.5 | (19.2–27.8) | |
| ≥1.80 | 18 | (12.8–23.1) | 13.7 | (11.8–15.6) | 15.7 | (12.8–18.6) | 18.4 | (6.5–30.2) | 23.7 | (17.9–29.5) | 17.8 | (9.1–26.5) | 3.1 | (0.4–5.9) | 5.7 | (4.3–7) | 5.7 | (2.6–8.8) | 20 | (14.2–25.7) | 17.1 | (14.5–19.7) | 26 | (21.2–30.8) | |
| Hemoglobin (g/dl) | <8.0 | 2.2 | (0.6–3.8) | 1.1 | (0.6–1.6) | 0.3 | (0–0.7) | 4.6 | (0–10.9) | 0.6 | (0–1.3) | 1.3 | (0–3.7) | 2.5 | (0–5.2) | 3.1 | (2.1–4.1) | 3.6 | (1.5–5.7) | 0.8 | (0–1.8) | 0.3 | (0–0.6) | 0.1 | (0–0.4) |
| 8.0 to 9.9 | 10.5 | (7.4–13.6) | 9.5 | (8.1–10.8) | 6.7 | (5.1–8.3) | 11.1 | (2.6–19.7) | 13 | (9.1–16.9) | 6.2 | (1.3–11.1) | 23.8 | (17–30.6) | 31.2 | (28.4–33.9) | 34.1 | (28.5–39.7) | 7.7 | (4.4–11) | 7.6 | (5.9–9.3) | 4.6 | (2.8–6.4) | |
| 10.0 to 10.9 | 15.3 | (10.5–20.1) | 16.3 | (14.6–18.1) | 14.9 | (12.3–17.5) | 12.9 | (3.7–22.1) | 14.3 | (10.3–18.2) | 14.8 | (8–21.6) | 28.1 | (20.4–35.8) | 32.5 | (29.7–35.3) | 33.2 | (27.6–38.7) | 12 | (7.7–16.3) | 13.6 | (11.4–15.8) | 14 | (10.6–17.5) | |
| 11.0 to 11.9 | 22 | (17.5–26.6) | 28.7 | (26.6–30.9) | 30.9 | (27.7–34.1) | 20.9 | (9.5–32.3) | 26.6 | (21.5–31.6) | 24.6 | (16.1–33) | 29.9 | (21.7–38.2) | 23.8 | (21.3–26.4) | 22.5 | (17.3–27.6) | 23 | (17.6–28.4) | 30.7 | (27.8–33.6) | 30.1 | (25.6–34.6) | |
| ≥12.0 | 50 | (44.2–55.8) | 44.4 | (42.1–46.7) | 47.2 | (43.8–50.6) | 50.4 | (36.5–64.3) | 45.6 | (40–51.2) | 53.2 | (43.5–62.9) | 15.7 | (8.8–22.6) | 9.4 | (7.6–11.1) | 6.6 | (3.8–9.5) | 56.5 | (50.1–62.9) | 47.8 | (44.7–50.9) | 51.1 | (46.2–56) | |
| Serum albumin (g/dl) | <3.2 | 5.6 | (2.8–8.4) | 10.7 | (9.2–12.3) | 14.1 | (11.5–16.7) | 0 | 9.3 | (6–12.6) | 13.7 | (6.7–20.7) | 1.5 | (0–3) | 5.7 | (4.4–7.1) | 10.7 | (7.4–14) | 8.4 | (4.6–12.2) | 9.9 | (8.2–11.7) | 13.2 | (9.9–16.5) | |
| 3.2 to 3.4 | 6.8 | (4.1–9.6) | 14.3 | (12.5–16.1) | 17.1 | (14.5–19.7) | 10.7 | (2.5–18.9) | 10 | (6.7–13.4) | 19.6 | (11.6–27.7) | 6.5 | (1.7–11.4) | 8.8 | (7.1–10.5) | 14.5 | (10.2–18.8) | 10.4 | (6.6–14.3) | 13 | (10.9–15.1) | 17.6 | (13.8–21.5) | |
| 3.5 to 3.7 | 20.6 | (15.7–25.4) | 22.2 | (20.1–24.2) | 25.9 | (22.8–29) | 22.1 | (10.4–33.7) | 24.3 | (19.5–29.2) | 26.5 | (17.5–35.5) | 10.6 | (4.8–16.4) | 21.2 | (18.7–23.7) | 29.6 | (24–35.1) | 16.3 | (11.2–21.4) | 26.5 | (23.7–29.4) | 26.4 | (22.1–30.8) | |
| 3.8 to 3.9 | 14.2 | (10–18.3) | 17.7 | (15.8–19.7) | 20.3 | (17.2–23.4) | 13 | (3.7–22.3) | 18.1 | (13.7–22.5) | 22.5 | (14.2–30.7) | 21.9 | (14.5–29.4) | 24.5 | (21.9–27.1) | 21.5 | (16.8–26.2) | 20.1 | (14.8–25.3) | 17.9 | (15.7–20.2) | 20.1 | (16.2–24) | |
| ≥4 | 52.9 | (46.8–59) | 35.1 | (32.6–37.5) | 22.6 | (19.6–25.6) | 54.2 | (40.4–68) | 38.2 | (32.8–43.6) | 17.8 | (10.6–24.9) | 59.5 | (50.6–68.4) | 39.7 | (36.8–42.7) | 23.7 | (18.4–29) | 44.9 | (38.5–51.2) | 32.5 | (29.7–35.4) | 22.6 | (18.7–26.6) | |
| Serum calciumalb (mg/dl) | <8.4 | 15.2 | (10.9–19.5) | 9.8 | (8.3–11.2) | 7 | (5.2–8.8) | 10.9 | (2.4–19.3) | 6.8 | (4–9.7) | 2.3 | (0–5.4) | 25.1 | (17–33.2) | 14.6 | (12.4–16.8) | 21.9 | (16.5–27.2) | 14.6 | (10.2–19) | 12.1 | (10–14.2) | 8.2 | (5.4–10.9) |
| 8.4 to 9.4 | 44.9 | (38.7–51.1) | 45.3 | (42.8–47.9) | 47.4 | (43.7–51.1) | 37.9 | (24.2–51.6) | 42.8 | (37.2–48.4) | 38.9 | (29.1–48.6) | 42.7 | (33.8–51.6) | 47.8 | (44.8–50.9) | 52.9 | (46.9–59) | 47.7 | (41.1–54.2) | 49.3 | (46.1–52.4) | 53.7 | (48.8–58.7) | |
| 9.5 to 10.1 | 26.1 | (20.4–31.8) | 29.7 | (27.4–32) | 27.1 | (23.8–30.4) | 28.5 | (16–41.1) | 29.9 | (24.8–35) | 37.6 | (27.7–47.4) | 21.8 | (14.1–29.6) | 21.7 | (19.2–24.1) | 17.3 | (13–21.7) | 26.4 | (20.7–32.2) | 27.7 | (24.9–30.6) | 27.2 | (22.8–31.5) | |
| ≥10.2 | 13.8 | (9.1–18.6) | 15.2 | (13.3–17.1) | 18.5 | (15.6–21.4) | 22.7 | (11.1–34.2) | 20.5 | (16–24.9) | 21.3 | (13.1–29.5) | 10.4 | (4.8–15.9) | 15.9 | (13.6–18.1) | 7.9 | (4.8–10.9) | 11.3 | (7.2–15.5) | 10.9 | (9–12.8) | 10.9 | (7.5–14.4) | |
| Serum phosphorus (mg/dl) | <3.5 | 7.1 | (4.3–9.8) | 12.3 | (10.8–13.8) | 18.7 | (16–21.3) | 12 | (2.9–21.2) | 12.2 | (8.5–16) | 16 | (8.9–23.1) | 1.8 | (0–3.8) | 3.8 | (2.8–4.9) | 11.6 | (7.7–15.5) | 7 | (2.9–11) | 9.6 | (7.7–11.4) | 16.9 | (13.1–20.8) |
| 3.5 to 5.4 | 38.4 | (32.8–44) | 47.1 | (44.8–49.5) | 54.7 | (51.3–58.2) | 44.6 | (30.8–58.4) | 43 | (37.4–48.5) | 45.6 | (35.8–55.3) | 33.9 | (25.6–42.2) | 43 | (40.1–45.9) | 55.1 | (49.2–61) | 31.3 | (25.4–37.3) | 46.7 | (43.6–49.8) | 58.4 | (53.6–63.2) | |
| ≥5.5 | 54.6 | (48.8–60.3) | 40.6 | (38.3–42.9) | 26.6 | (23.6–29.7) | 43.4 | (29.7–57) | 44.8 | (39.2–50.4) | 38.4 | (28.9–47.9) | 64.3 | (55.9–72.7) | 53.2 | (50.2–56.1) | 33.3 | (27.7–38.9) | 61.7 | (55.3–68) | 43.7 | (40.6–46.8) | 24.7 | (20.6–28.7) | |
| Serum PTH (pg/ml) | <150 | 35.1 | (29.1–41) | 39.8 | (37.4–42.2) | 43.8 | (40.1–47.4) | 36.6 | (22.7–50.5) | 40.2 | (34.3–46.1) | 43 | (32.2–53.9) | 46.5 | (36.8–56.3) | 45.1 | (41.8–48.4) | 55.1 | (48.3–61.8) | 30.1 | (22.9–37.4) | 30.1 | (26.7–33.5) | 39.4 | (33.3–45.4) |
| 150 to 299 | 26 | (20.9–31.1) | 27.2 | (25–29.5) | 30.5 | (27.1–34) | 11 | (2.4–19.5) | 17.8 | (13.1–22.4) | 26.4 | (16.9–35.9) | 28.7 | (20.3–37.1) | 32.5 | (29.3–35.7) | 32.9 | (26.4–39.3) | 20.6 | (14.8–26.4) | 28.6 | (25.4–31.9) | 34 | (28.3–39.6) | |
| 300 to 599 | 18.4 | (14–22.8) | 22.3 | (20.2–24.3) | 17.9 | (15.1–20.7) | 17.4 | (6.7–28.1) | 21 | (16.2–25.9) | 19.6 | (10.9–28.3) | 17.6 | (9.7–25.5) | 18.3 | (15.6–21.1) | 11.4 | (7.2–15.5) | 26.3 | (19.7–33) | 25.3 | (22.1–28.5) | 21 | (16.3–25.7) | |
| ≥600 | 20.5 | (15.4–25.7) | 10.7 | (9.1–12.3) | 7.8 | (5.7–9.9) | 35 | (21.1–49) | 21 | (16.2–25.7) | 11 | (4.4–17.5) | 7.2 | (2.3–12.1) | 4 | (2.7–5.4) | 0.7 | (0–2.1) | 22.9 | (16.8–29.1) | 16 | (13.3–18.7) | 5.6 | (2.9–8.4) | |
Cross section of hemodialysis patients in DOPPS III (2005 through 2007) (n = 8161). Bold numbers represent P < 0.05 compared with the elderly group (≥75 years) within each region. Europe includes United Kingdom, France, Germany, Italy, Spain, Belgium, and Sweden; North America includes United States and Canada. Abbreviation: PTH, parathryroid hormone.
Hypoalbuminemia was common in the elderly group. In all regions patients aged ≥75 years were less likely to have a normal serum albumin (>4.0 g/dl) (17) compared with patients aged <45 years (Europe: OR 0.32 [95% CI: 0.25 to 0.42]; ANZ: OR 0.41 [95% CI: 0.17 to 1.00]; Japan OR 0.19 [95% CI: 0.12 to 0.30]; North America OR 0.35 [95% CI: 0.26 to 0.47]). Similarly, elderly patients were less likely to have a serum albumin >3.5 g/dl (Europe: OR 0.33 [95% CI: 0.25 to 0.44]; ANZ: OR 0.34 [95% CI: 0.19 to 0.63]; Japan OR 0.21 [95% CI: 0.12 to 0.36]; North America OR 0.53 [95% CI: 0.38 to 0.73]). In addition, the percentage of patients with serum phosphorus <3.5 mg/dl was higher among elderly participants, whereas the percentage with serum phosphorus >5.5 mg/dl was significantly lower among elderly patients in all regions with a similar pattern seen in ANZ.
Low parathyroid hormone (<150 pg/ml) levels were more common, whereas high parathyroid hormone (>600 pg/ml) was less common in the elderly group in all regions.
Quality of Life
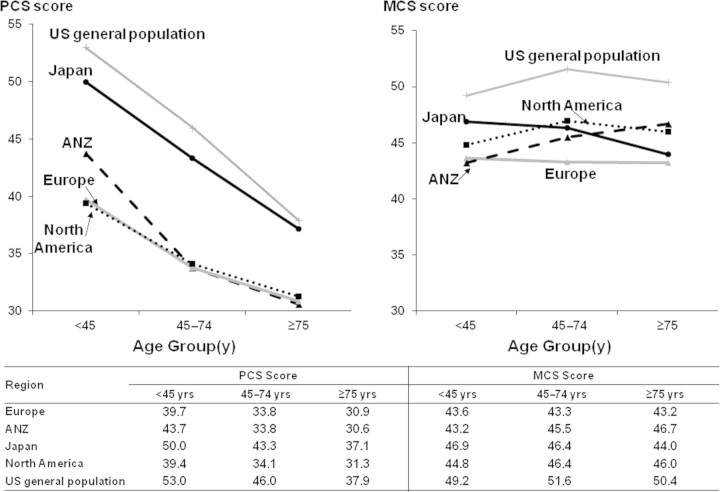
KDQoL mental (MCS) and physical component summary (PCS) scores for the three age groups among DOPPS participants and in the U.S. general population are shown in Figure 3. As expected, in all regions hemodialysis patients reported worse quality of life compared with an age-matched normal population. Japanese patients had higher PCS scores in all age groups (P < 0.0001 for each age group). However, PCS scores decreased with age in all regions (P < 0.0001). MCS scores were not affected by age. In Europe and Japan, but not in North America and ANZ, elderly patients were more likely to have a Center of Epidemiologic Studies Depression Rating Scale score ≥10, which is considered to be indicative of possible clinical depression (Europe: OR of having a Center of Epidemiologic Studies Depression score ≥10 was 1.56 [95% CI: 1.15 to 2.11] versus patients <45 years old; Japan: OR 1.62 [95% CI: 1.00 to 2.63]; North America: OR 0.91 [95% CI: 0.65 to 1.26]; and ANZ: OR 0.84 [95% CI: 0.43 to 1.65]).
Figure 3.
Kidney Disease Quality of Life (KDQoL) physical component summary (PCS) and mental component summary (MCS) scores by age categories across DOPPS regions versus U.S. population norm. Cross section of participants in DOPPS III (2005 through 2007; n = 8161). Europe includes United Kingdom, France, Germany, Italy, Spain, Belgium, and Sweden; North America includes United States and Canada; ANZ represents Australia and New Zealand.
Mortality
Patient survival by age categories within each region is shown in Figure 4. Median survival since the start of dialysis was shorter in the elderly group (Europe: 3.3 years; ANZ: 1.6 years; Japan: 5.4 years; North America: 2.5 years) compared with participants aged 45 to 74 years (Europe: 5.5 years; ANZ: 5.7 years; Japan: 11.9; North America: 4.5 years) and <45 years (Europe: 13.9 years; ANZ: 11.1 years; Japan: 22.5 years; North America: 7.9 years).
Figure 4.
Survival by age at start of dialysis across DOPPS regions. Probability of survival from start of dialysis by region and age group at dialysis start (calculated using unadjusted Cox regression within each region with delayed entry at time of study enrollment, stratified by age group, and accounting for facility clustering effects). Total number of patients = 8161, and the number of deaths = 1337. P value for 75 years versus 45 years: 0.05 in each region. Europe includes United Kingdom, France, Germany, Italy, Spain, Belgium, and Sweden; North America includes United States and Canada; ANZ represents Australia and New Zealand.
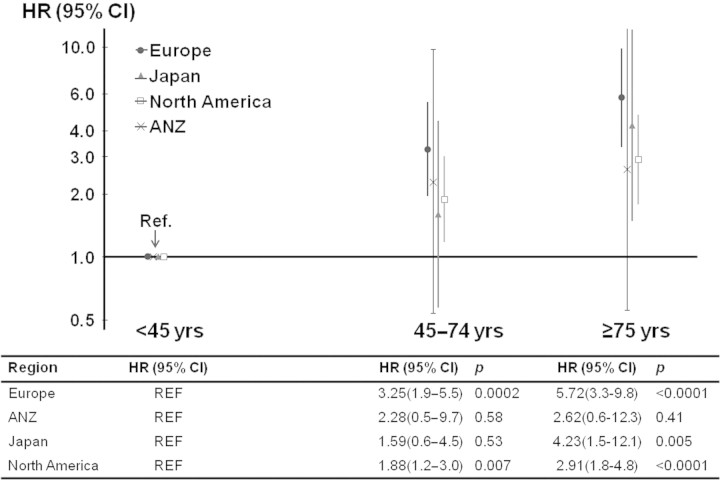
In Cox models adjusted for demographics and a wide range of comorbidities, elderly patients had a three- to sixfold higher mortality risk compared with participants <45 years of age. Overall, the higher mortality risk in the elderly group tended to be greater compared with that of participants <45 years of age in Europe and Japan than in ANZ and North America (Figure 5). The association of age with mortality was similar in analyses using continuous age at the time of study entry, adjusted for time receiving hemodialysis (hazard ratio [HR] per 20 years older: 1.94 [95% CI: 1.74 to 2.16]) and in left-truncated models using age at the start of dialysis (HR 1.95 [95% CI: 1.76 to 2.17]) as the predictor.
Figure 5.
Mortality risk by age at study entry across DOPPS regions. Cross section of participants in DOPPS III (2005 through 2007; n = 8161); number of deaths n = 1337. Reference (Ref) group: patients <45 years of age. Model adjusted for sex, race, body mass index, duration of ESRD, 14 summary comorbid conditions listed in Table 1 (dementia was included in the neurologic diseases), stratified by country, and accounted for facility clustering effect. Mean age in category: <45 years = 36, 45 to 75 years = 62, ≥75 years = 80. Europe includes United Kingdom, France, Germany, Italy, Spain, Belgium, and Sweden; North America includes United States and Canada; ANZ represents Australia and New Zealand; HR, Hazard Ration; CI, confidence interval.
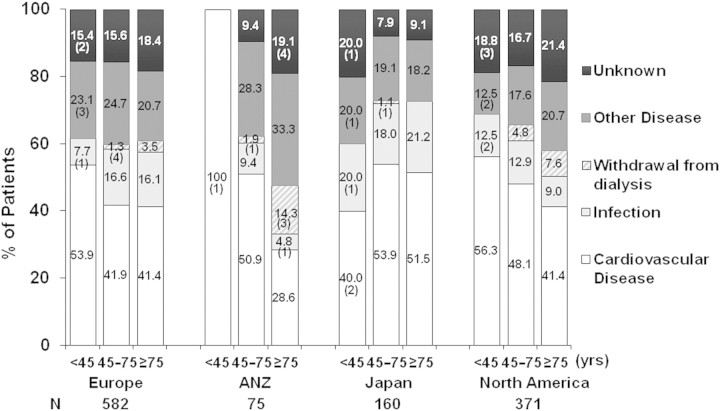
Reported causes of death by age group and region are shown in Figure 6. Although cardiovascular events were the main cause of death in the younger age group, they contributed to a smaller proportion in elderly patients in all regions except Japan. Among elderly participants, withdrawal from dialysis accounted for 3.5% of deaths in Europe, 14.3% in ANZ, and 7.6% in North America; no deaths due to withdrawal were reported in Japan.
Figure 6.
Distribution of cause of death by age at study entry across DOPPS regions. Analysis was limited to patients for whom data on cause of death was available (patient n = 8010; number of deaths n = 1188). Number of deaths shown in parentheses for n <5.
The association between hemoglobin, diabetes, and mortality differed among patients ≥75 versus patients <75 years old in Japan and North America but not in Europe and ANZ. Each 1 g/dl higher hemoglobin concentration was strongly associated with lower mortality among participants aged ≥75 years (Japan: HR 0.66 [95% CI: 0.51 to 0.85]; North America: HR 0.75 [95% CI: 0.64 to 0.88]) but with a weaker association in those <75 years (Japan: HR 0.94 [95% CI: 0.79 to 1.12]; North America: HR 0.91 [95% CI: 0.83 to 1.00]). In Europe and ANZ, no significant interaction between hemoglobin and age category on all-cause mortality was found (P = 0.69 in Europe; P = 0.61 in ANZ).
The relationship of diabetes with mortality was also different among patients ≥75 years versus those aged <75 years in Japan (P = 0.01) and North America (P < 0.0001) but not in Europe and ANZ (P = 0.15 in Europe; P = 0.18 in ANZ). The mortality hazard ratio for persons with diabetes (versus those without diabetes) was not significantly higher for patients aged ≥75 years (Japan: HR 1.12 [95% CI: 0.68 to 1.86, P = 0.66]; North America: HR 0.87 [95% CI: 0.64 to 1.18, P = 0.37]) but was twofold higher for participants aged <75 years (Japan: HR 2.50 [95% CI: 1.41 to 4.41], P < 0.01; North America: HR 1.85 [95% CI: 1.42 to 2.42], P < 0.0001).
Discussion
Elderly patients account for an increasing fraction of patients on renal replacement therapy worldwide, reaching 25% to 30% in most ESRD registries (5,18). This phenomenon translates into higher health care costs and additional clinical challenges for health professionals to meet the often broader and more demanding health care needs of the geriatric population (19,20). To assess hemodialysis practices and outcomes among elderly versus younger patients in 12 countries, we evaluated recent data from the DOPPS, a large international hemodialysis population.
To our knowledge, this is the first study of size to describe hemodialysis practices among elderly patients from a broad international perspective and to report outcome data compared with younger counterparts treated in the same dialysis environments. The international nature of the DOPPS has allowed us to compare practices and outcomes across four geographic regions, representing the vast majority of the hemodialysis population worldwide. Our results are based upon cross-sections of patients representative of the typical in-center hemodialysis patient population seen on a given day in a dialysis unit.
The age distribution of patients enrolled in the DOPPS closely follows the current aging trend observed in the general population, with the mean age of DOPPS participants increasing significantly in most countries over the past decade. However, the proportion of elderly among in-center hemodialysis patients varied widely across countries, indicating possible differences in clinical practices and policies toward starting chronic hemodialysis in this population, and use of transplantation and peritoneal dialysis. The low proportion of elderly in Japan may be due in part to the low rates of kidney transplantation and peritoneal dialysis in Japan compared with other countries, coupled with Japan having one of the highest rates of ESRD incidence and longest hemodialysis patient survival. According to the Japanese Society for Dialysis Therapy Registry in 2005, 29.7% of patients starting dialysis and 21.5% of chronic dialysis patients were aged ≥75 years (21). In other countries having much higher transplantation rates, a substantial fraction of the younger healthier patients received a kidney transplant, thereby removing them from the pool of hemodialysis patients.
Comorbid conditions were common in elderly dialysis patients. Prevalence of coronary artery disease increased with age in all DOPPS countries, and was almost 3 times higher in patients aged ≥75 years versus those aged <45 years. Similarly, malignancies were 3 to 18 times more frequent in the elderly group. These findings are in agreement with epidemiologic studies showing that prevalence of cardiovascular events, and malignancies, increases almost linearly with aging (22,23). Interestingly, neurologic diseases and dementia were much higher among Japanese elderly participants. Because comorbidities are abstracted from the patient medical record, these differences may be in part explained by cultural differences in the diagnosis of these conditions.
Overall, hemodialysis prescription and clinical practices in elderly patients mainly reflected country-specific practices. Permanent central venous catheters were used more frequently in the elderly versus younger patients in Europe, ANZ, and North America but were very rarely used in Japan. Despite the differences in vascular access, mean prescribed blood flow rate was virtually identical in all age groups, between 295 and 395 ml/min, except for Japan, which displayed a mean blood flow rate between 184 and 207 ml/min. In all regions, the duration of dialysis session was about 15 minutes shorter among the elderly than in younger age groups. However, once the prescribed treatment time was normalized for body weight, no significant differences were observed across age groups. No differences were observed in delivered dialysis dose, as measured by single pool Kt/V. Maintenance of a satisfactory dialysis dose can be partially explained by the lower dry weight, lower lean body mass, and low serum albumin, which were common in the elderly group.
The mean ultrafiltration rate was lower in the elderly than in the younger age groups. This is likely explained by the lower dry weight as well as the high prevalence of cardiovascular disease and congestive heart failure observed among elderly patients, as reported in Table 1.
In Europe, ANZ, and North America, a higher percentage of elderly patients achieved the hemoglobin target value. This fact confirms that anemia correction is particularly a case-sensitive concern in the elderly dialysis population because benefits extend from enhancement of cardiac function and physical capacity to improvement of brain and cognitive functioning (24–26). Interestingly, in North America, Europe, and ANZ—but not in Japan—the erythropoietin resistance index was lower in elderly patients than in younger participants. Differences in clinical practices (e.g., targeted hemoglobin values) as well as in patient case-mix may contribute to differences in erythropoietin stimulating agent prescription observed across regions.
Malnutrition was consistently observed among elderly patients across the four regions as indicated by low serum albumin and low serum phosphorus. These findings suggest that two major mortality risk factors, low dietary protein intake and hypoalbuminemia, are common among elderly dialysis patients and require specific attention.
Quality of life for dialysis patients is reduced by almost 50% compared with that of the population worldwide (27). The deterioration of quality of life affects all dimensions, including the physical component, mental component, and burden of kidney disease with regional variations. Interestingly, among DOPPS elderly participants the physical component was significantly more affected than the mental component. These observations suggest that elderly patients are more restricted in their physical function than in their mental function. These results also correlate well with the observation that the fraction of patients who can walk without assistance substantially declined with older age in each study region (Table 1). It is however of interest that—in agreement with the general population—elderly dialysis patients were more likely to have high Center of Epidemiologic Studies Depression Rating Scale scores in Europe and in Japan, indicating the higher likelihood of possible clinical depression among elderly hemodialysis patients.
As expected, mortality rates were higher in elderly hemodialysis patients (28). Median survival for patients aged ≥75, 45 to 74, and <45 years was 3, 6, and 12 years, respectively. The relative risk of all-cause mortality increased linearly with each age group. It is important to note that acute factors (falls, fractures, orthopedic surgery, etc.), frequently observed in this frail population, may have contributed to increased mortality. However, advanced age is not the only predictive factor. Functional health status, comorbid conditions, caregivers' expertise, and social support also are determinant factors of survival (29,30). Interestingly, in Japan and North America diabetes was associated with a higher mortality risk in participants aged <75 but not in those ≥75 years. Elderly patients with diabetes may be overall healthier and therefore experience longer survival. Alternatively, late onset diabetes has a better prognosis in the elderly dialysis population than in the younger dialysis population. In addition, competing risks by other causes of death may substantially contribute to the observed relative ratio of death associated with diabetes among elderly hemodialysis patients. Cardiovascular disease was the most common cause of death among elderly patients but represented a lower percentage of events than in the younger age groups. Death due to discontinuation of dialysis was relatively common among elderly patients in North America (7.6%) and ANZ (14.3%) but not in Europe (3.5%) and Japan (0%), possibly reflecting cultural differences. It is worth noting that acute factors (e.g., stroke and surgery) may have precipitated the fatal event, leading nephrologists to voluntarily interrupt renal replacement therapy. There is ample debate in the international nephrology community surrounding the issues of denying or stopping hemodialysis treatment in elderly patients and use of alternative conservative therapeutic regimens (31–33).
The present study has some limitations that are mainly due to the observational nature of the DOPPS. The relationships that we have described between patient age groups, practices, and mortality are factual and not causal. The cross-sectional DOPPS sample may be biased by the fact that healthier patients will survive longer on dialysis. Despite adjustment for a wide variety of patient clinical characteristics, we cannot exclude that this may also affect our findings. Moreover, economic considerations and consequences concerning renal replacement therapy in elderly patients have not been evaluated. Although elderly patients have access to dialysis treatment in all DOPPS countries, it is possible that some restrictions in starting dialysis are applied for elderly patients with chronic kidney disease stage 5. Unfortunately, the DOPPS is not able to address this specific issue. Finally, the DOPPS includes only in-center hemodialysis patients; therefore, our findings are not applicable to ESRD patients receiving other modalities of renal replacement therapy.
On the basis of international DOPPS data, hemodialysis prescription in elderly patients was overall similar to that in younger age groups and tended to follow region-specific practices. Deterioration of quality of life affected the physical but not the mental component scores. Mortality risk was markedly higher in the elderly group but this was likely related to comorbid conditions and possibly to functionality at baseline.
Elderly dialysis patients represent a selected group of patients who can be considered survivors and are not representative of the general nonrenal population. Interventional studies on elderly dialysis patients comparing renal replacement therapy modalities (peritoneal dialysis, home hemodialysis, and hemodiafiltration) or introducing specific prescriptions such as longer treatment time, nutritional support, and a personalized treatment schedule should be evaluated (31). Social support for elderly patients must be considered as a determinant factor that affects dialysis outcomes. Further interventional studies in elderly dialysis patients deserve to be evaluated in the future to optimize the renal care and practice of this population.
Disclosures
None.
Acknowledgments
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications. We are grateful to Hal Morgenstern for his scientific contribution. Editorial assistance was provided by Shauna Leighton from Arbor Research Collaborative for Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hamer RA, El Nahas AM: The burden of chronic kidney disease. BMJ 332: 563–564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Li S, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis 49 [Suppl]: A6–A7, S1–S296, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Jager KJ, van Dijk PC, Dekker FW, Stengel B, Simpson K, Briggs JD: The epidemic of aging in renal replacement therapy: An update on elderly patients and their outcomes. Clin Nephrol 60: 352–360, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Kurella M, Covinsky KE, Collins AJ, Chertow GM: Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 146: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 5.ERA-EDTA Registry: ERA-EDTA Registry 2005 Annual Report. Amsterdam, The Netherlands, Academic Medical Center, Department of Medical Informatics, June 2007 [Google Scholar]
- 6.Gitto LSV, Santoro D, Bellinghieri G, Biagio Di Iorio, Li Vecchi M, De Santo NG: Survival in octogenarian dialysis patients: Analysis in two Southern Italian regions. J Nephrol 21: 118–123, 2008 [PubMed] [Google Scholar]
- 7.Tazza L, Di Napoli A, Bossola M, Valle S, Pezzotti P, Luciani G, Di Lallo D; Lazio Dialysis Registry: Ageing of patients on chronic dialysis: Effects on mortality–a 12-year study. Nephrol Dial Transplant 24: 940–947, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Fang W, Kothari J, Khandelwal M, Naimark D, Jassal SV, Bargman JM, Oreopoulos DG: Clinical outcomes of elderly patients undergoing chronic peritoneal dialysis: Experiences from one center and a review of the literature. Int Urol Nephrol 39: 1295–1302, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 44: 7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57: S74–S81, 2000 [Google Scholar]
- 11.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339–349, 2003 [DOI] [PubMed] [Google Scholar]
- 12.SAS/STAT User's Guide Version 8, Cary, NC, SAS Institute Inc., 2000 [Google Scholar]
- 13.Klein JP, Moeschberger ML: Survival analysis techniques for censored and truncated data, New York, Springer, 1997 [Google Scholar]
- 14.Allison PD: Survival Analysis Using SAS: A Practical Guide, Second Edition, Cary, NC, SAS Institute Inc., 2010 [Google Scholar]
- 15.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48 [Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Wei M, Bargman JM, Oreopoulos DG: Factors related to erythropoietin hypo-responsiveness in patients on chronic peritoneal dialysis. Int Urol Nephrol 39: 935–940, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 35: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 18.U.S. Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 19.Luke RG, Beck LH: Gerontologizing nephrology. J Am Soc Nephrol 10: 1824–1827, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Oreopoulos DG, Dimkovic N: Geriatric nephrology is coming of age. J Am Soc Nephrol 14: 1099–1101, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Nakai S, Masakane I, Akiba T, Iseki K, Watanabe Y, Itami N, Kimata N, Shigematsu T, Shinoda T, Syoji T, Syoji T, Suzuki K, Tsuchida K, Nakamoto H, Hamano T, Marubayashi S, Morita O, Morozumi K, Yamagata K, Yamashita A, Wakai K, Wada A, Tsubakihara Y: Overview of regular dialysis treatment in Japan (as of 31 December 2005). Ther Apher Dial 11: 411–441, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 287: 356–359, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Mokdad AH, Marks JS, Stroup DF, Gerberding JL: Actual causes of death in the United States, 2000. JAMA 291: 1238–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Bedani PL, Verzola A, Bergami M, Stabellini G, Gilli P: Erythropoietin and cardiocirculatory condition in aged patients with chronic renal failure. Nephron 89: 350–353, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Iseki K, Kohagura K: Anemia as a risk factor for chronic kidney disease. Kidney Int Suppl (107): S4–S9, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Moreno F, Aracil FJ, Perez R, Valderrabano F: Controlled study on the improvement of quality of life in elderly hemodialysis patients after correcting end-stage renal disease-related anemia with erythropoietin. Am J Kidney Dis 27: 548–556, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara S, Lopes AA, Bragg-Gresham JL, Kurokawa K, Mapes DL, Akizawa T, Bommer J, Canaud BJ, Port FK, Held PJ; Worldwide Dialysis Outcomes and Practice Patterns Study: Health-related quality of life among dialysis patients on three continents: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 64: 1903–1910, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Munshi SK, Vijayakumar N, Taub NA, Bhullar H, Lo TC, Warwick G: Outcome of renal replacement therapy in the very elderly. Nephrol Dial Transplant 16: 128–133, 2001 [DOI] [PubMed] [Google Scholar]
- 29.DeOreo PB: Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 30: 204–212, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Peri UN, Fenves AZ, Middleton JP: Improving survival of octogenarian patients selected for haemodialysis. Nephrol Dial Transplant 16: 2201–2206, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Burns A: Conservative management of end-stage renal failure: Masterly inactivity or benign neglect? See Smith et al., pp. c40–c46. Nephron Clin Pract 95: c37–c39, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grünfeld JP, Jungers P: Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 14: 1012–1021, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]