Abstract
The instant blood-mediated inflammatory reaction (IBMIR) is a major obstacle to the engraftment of intraportal pig islet xenografts in primates. Higher expression of the galactose-α1,3-galactose (αGal) xenoantigen on neonatal islet cell clusters (NICC) than on adult pig islets may provoke a stronger reaction, but this has not been tested in the baboon model. Here, we report that WT pig NICC xenografts triggered profound IBMIR in baboons, with intravascular clotting and graft destruction occurring within hours, which was not prevented by anti-thrombin treatment. In contrast, IBMIR was minimal when recipients were immunosuppressed with a clinically relevant protocol and transplanted with NICC from αGal-deficient pigs transgenic for the human complement regulators CD55 and CD59. These genetically modified (GM) NICC were less susceptible to humoral injury in vitro than WT NICC, inducing significantly less complement activation and thrombin generation when incubated with baboon platelet-poor plasma. Recipients of GM NICC developed a variable anti-pig antibody response, and examination of the grafts 1 month after transplant revealed significant cell-mediated rejection, although scattered insulin-positive cells were still present. Our results indicate that IBMIR can be attenuated in this model, but long-term graft survival may require more effective immunosuppression or further donor genetic modification.
Keywords: Hyperacute rejection, instant blood-mediated inflammatory reaction, neonatal islet cell clusters, thrombosis, type 1 diabetes, xenotransplantation
Introduction
Islet allotransplantation has become an accepted treatment for a subset of patients with type 1 diabetes, but limited organ donor numbers prevent its more universal application. To overcome this shortage, xenotransplantation using pig islets has been proposed as a potential alternative. Both adult pig islets and neonatal islet cell clusters (NICC), which are easier to isolate 1, have been considered. While NICC have survived and functioned in the short to medium term in cynomolgus monkeys 2, rejection remains a major barrier to clinical application. Most studies have focused on preventing the T cell–mediated cellular immune response, using immunosuppressive protocols that are not clinically applicable 3. A more immediate problem is early islet loss due to inflammatory and thrombotic mechanisms. The preferred site for human islet allotransplantation is the liver via the portal circulation, where islets directly contact the blood 4. Bennet et al 5 demonstrated that contact of adult pig islets with human blood in vitro or cynomolgus monkey blood in vivo triggered an immediate inflammatory response that included activation of the complement and coagulation cascades and rapid clot formation with islet destruction. A similar reaction occurs when human islets were mixed with human blood, suggesting that nonspecific factors such as exposure of pro-coagulants contribute to this instant blood-mediated inflammatory reaction (IBMIR) 6,7. Complement activation is an initiating factor in IBMIR, and complement inhibitors are being trialed in islet allotransplantation 8. The potential for complement activation via the alternative pathway is probably greater in xenotransplantation 9.
If pig NICC are to become a viable alternative therapy for clinical islet replacement, they must survive IBMIR and provide long-term function. Previous observations of IBMIR in primates are limited to studies of adult pig islets transplanted into monkeys 5,10. Unlike adult islets, NICC strongly express the xenoantigen galactose-α1,3-galactose (αGal), making them susceptible to the binding of preformed anti-αGal antibodies. This is likely to amplify complement activation, promoting IBMIR and thrombosis 11–13. Balancing this is the fact that NICC preparations contain less contaminating prothrombotic material 13.
The use of donor pigs lacking αGal (GTKO) has been shown to improve engraftment of NICC in macaques 14. Another group reported long-term function of transgenic adult pig islets expressing the human complement regulator CD46 in cynomolgus monkeys 15. However, the combination of GTKO and transgenic complement regulation has not been examined. The initial aim of this study was to characterize IBMIR in baboons transplanted intraportally with WT pig NICC. We then examined whether NICC from GTKO pigs expressing human CD55 and CD59 were protected from IBMIR in recipient's immunosuppressed with a clinically relevant protocol. Finally, we monitored the survival of these genetically modified (GM) NICC xenografts up to 1 month after transplant, by histological analysis.
Materials and Methods
Recipient animals and ethics clearances
Baboons (Papio hamadryas) were supplied by the NH&MRC Australian National Baboon Colony, Sydney, Australia. All procedures were approved by Local Area Health Service Animal Ethics Committees and conducted in compliance with State Government legislation and NH&MRC Animal Research Guidelines.
Donor pigs
WT piglets were Large White/Landrace cross. GTKO piglets that co-expressed human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT) (GTKO/CD55-CD59-HT) were generated by mating GTKO/CD55-CD59-HT pigs with GTKO pigs. Piglets were screened by flow cytometry of peripheral blood mononuclear cells 16 for αGal and CD55 (data not shown); the level of expression in the transgenic line has been previously reported 17. Parental GTKO/CD55-CD59-HT pigs were generated by breeding heterozygous CD55-CD59-HT 17 onto the GTKO 18 background.
Preparation of NICC
Pancreata were obtained from 1- to 5-day-old piglets. NICC were produced using a modification of a previously described technique 1. Briefly, pancreata were finely chopped and digested with 2.5 mg/mL Collagenase Type V (Sigma–Aldrich, St. Louis, MO) at 37°C. Washed tissue was plated into Petri dishes containing Hams F-10 medium (Thermo Trace, Melbourne, Australia) and 1% pooled autologous pig sera. The cells were cultured for 6 days at 37°C and 5% CO2. Prior to transplantation, NICC purity was determined by quantitative image analysis.
NICC transplantation into baboons
All surgical procedures were carried out under general anesthesia with 1–2% isoflurane in oxygen. Following midline incision, the portal and hepatic veins were identified. An arrow double-lumen venous cannula was inserted in the portal vein for NICC infusion under gravity.
Of four baboons receiving WT NICC, two were given 500 U/kg body weight recombinant human anti-thrombin (rhAT; GTC Biotherapeutics, Framingham, MA) infused over 20–25 min; 10–15 min prior to NICC infusion. This was expected to increase circulating AT activity to 15–20 U/mL 16. No immunosuppression was given to WT NICC recipients, but all received intravenous (IV) heparin (100 IU/kg recipient weight) prior to NICC infusion.
Five baboons transplanted with GTKO/CD55-CD59-HT NICC received heparin plus an immunosuppressive regimen based on our clinical islet transplant protocol 19. For the first 3 days, the animals were given 33 mg ATG (Fresenius Biotech GmbH, Bad Homburg, Germany) IV and twice daily 500 mg IV mycophenolate mofetil (MMF; CellCept®, Roche, Sydney, Australia). Thereafter, they received twice-daily oral doses of 500 mg MMF and 4.0 mg/kg Tacrolimus (Prograf, Janssen-Cilag Pty Limited, Sydney, Australia) with the dose adjusted to achieve a target trough concentration of 10–15 ng/mL.
Blood and plasma analysis
Total white and red cell counts and platelet and differential leukocyte counts of EDTA-treated blood were obtained using a cell counter (Advia 120 Hematology System; Siemens Healthcare, Erlangen, Germany). Complement activation product C3a in plasma was measured using sandwich ELISA kits (Quidel, San Diego, CA).
Measurement of non-Gal antibodies
Heat-inactivated serum samples were diluted 1/10 and incubated at room temperature with endothelial cells from GTKO and WT pigs. Cells were washed and incubated with either FITC conjugated goat (Fab') anti-human IgM (Southern Biotech, Birmingham, AL) or IgG (γ-chain specific) (Sigma, St. Louis, MO). Cells were then washed, resuspended in FACS buffer and analyzed on a FACSCalibur Flow Cytometer (Becton Dickinson, San Diego, CA) using FlowJo software (Tree Star, Ashland, OR).
Complement activation
The 1000 islet equivalent (IEQ) NICC in 500 µL Hanks Buffer Salt Solution without CaCl2 (HBSS) with 1% human serum albumin (HSA) (Albumex®20, CSL, Victoria, Australia) was added to 500 µL of platelet-poor plasma (PPP) in a nontissue culture-treated plate (BD Biosciences, San Jose, CA) agitated at 120 rpm at 37°C. A cytometric bead array kit for human anaphylatoxin was used (BD Biosciences) to analyze the supernatant.
Thrombin generation assay
Thrombin generation assay (TGA) was performed using a Calibrated Automated Thrombogram and fluorometer FLx800 with BIOTEK—Gen 5 software (BioTek Instruments, Winooski, VT). PPP was added to a microtiter plate with 250 IEQ NICC in 40 µL of HBSS/1% HSA or 40 µL of HBSS/1% HSA control. The reaction was started with 50 µL of 1 mM fluorogenic substrate (Z-GGR-AMC) and 15 mM CaCl2 (Technothrombin® TGA; Technoclone GmbH, Vienna, Austria). Fluorescence was measured for 120 min at 37°C (excitation 390 nm, emission 460 nm).
Liver biopsy for histology
In WT NICC transplanted baboons, slice biopsies were taken from different areas of the liver at 0, 10, 20 and 30 min and then at 30-min intervals until 300 min. Thereafter, wedge excision biopsies were taken at 60-min intervals until the liver had infarcted. Care was taken to ensure that traumatic artifact of the liver was avoided. Liver biopsies were taken from recipients of GM NICC at 60 min and 14, 21 and 30 days posttransplant, using a previously described method 20. At the end of the study period, baboons were re-anesthetized and the liver was removed for histology prior to euthanasia.
Histology and immunohistochemical staining
Baboon liver biopsy samples were divided and either mounted in OCT embedding medium (Tissue-Tek; Miles, Naperville, IL) and snap-frozen in liquid nitrogen, or placed in formalin fixative and paraffin-embedded. Sections were stained for platelet deposition using an anti-human platelet glycoprotein GPIIb/IIIa mAb (R&D Systems, Minneapolis, MN). For detection of infiltrating T and B cells, sections were stained using anti-human CD3 and CD20cy antibodies (Dako Australia, Botany, Australia), respectively. Staining for αGal was performed with an anti-αGal mAb 21.
Image analysis of liver biopsies
Stained liver biopsy sections were scanned with an Aperio Image-Scope Scanner (Leica Microsystems, Wetzlar, Germany). Sections were stained with Martius Scarlet Blue (MSB) trichrome, for clot analysis, with chromogranin A and hematoxylin for analysis of percent NICC present, and with anti-human CD3 and CD20cy and hematoxylin for analysis of percent T and B lymphocytes present. Eight 1 mm2 sections were randomly selected from each image and the automated image analysis algorithm Aperio Positive Pixel Count v9 was used to quantify the amount of clot (red blood cells [RBCs] + fibrin) and RBCs alone. This utilized inclusion exclusion analysis rather than gating of specific areas. Two annotations were performed on each square; the first with a hue value of 1.0 to quantify the total clot (red and yellow staining) and the second with a hue value of 0.25 to quantify the RBCs only (yellow staining). The annotations were used to determine the hue value between the positively stained areas (brown) and the hematoxylin-stained background. The algorithm counted the number and intensity sum in each intensity range and from this generated a positivity value (the total number of positive pixels divided by the total number of positive and negative pixels), which was used to determine the percentage of the 1 mm2 liver section detected as positive for either total clot or RBCs only staining. The percentage of fibrin was determined by subtracting the percentage RBC from the percentage total clot. NICC were calculated as number present per section analyzed; for T and B cells, the percentage of islet area staining positive was calculated.
Statistical analysis
The Linear Mixed Effects Model 22 was used to evaluate the hematological parameters, taking into account the variance of readings taken on the same blood donor for each variable. Generalized estimating equations were used to test for changes in hematological parameters over time, taking into account the dependence of readings taken on the same baboon. Mean values with standard deviation are shown unless otherwise indicated. Statistical software used for data analysis was S-Plus 6 for Windows (Insightful Corporation, Seattle, WA). p < 0.05 was considered significant.
Results
Production of porcine NICC
NICC were isolated from either WT piglets (n = 30) or from GTKO piglets co-expressing human CD55, CD59 and α1,2-fucosyltransferase (HT) (n = 53). There was no significant difference in the yield of NICC between WT and GTKO/CD55-CD59-HT piglets (Table 1). Purity was not significantly different between WT NICC (92 ± 1.9%) and GTKO/CD55-CD59-HT NICC (91 ± 2.8%).
Table 1.
Summary data for baboon transplants performed including the NICC yields from WT and GTKO/CD55-CD59-HT piglet pancreases used for transplantation
| Baboon | Weight (kg) | Donors | Total no. of NICC | NICC/g pancreas | Total no. of IEQ | IEQ/g pancreas | NICC/kg | IEQ/kg | Total no. of NICC/mm3 liver | rhAT (U) total dose |
|---|---|---|---|---|---|---|---|---|---|---|
| WT1 | 21 | WT | 547 500 | 39 360 | 185 432 | 13 330 | 26 071 | 8830 | 0.0276 | 10 500 |
| WT2 | 22 | WT | 547 500 | 39 360 | 185 432 | 13 330 | 24 886 | 8429 | 0.0260 | None |
| WT3 | 24 | WT | 508 750 | 24 261 | 156 396 | 7461 | 21 198 | 6517 | 0.0231 | 12 000 |
| WT4 | 23 | WT | 508 750 | 24 261 | 156 396 | 7461 | 22 120 | 6800 | 0.0241 | None |
| Mean | 528 125 | 31 811 | 170 914 | 10 396 | 23 569 | 7644 | 0.0252 | |||
| SD | 22 372 | 8717 | 16 764 | 3388 | 2289 | 1156 | 0.0020 | |||
| GM1 | 12 | GTKO/CD55-CD59-HT | 491 505 | 22 525 | 135 654 | 6217 | 40 959 | 11 305 | 0.0223 | None |
| GM2 | 12 | GTKO/CD55-CD59-HT | 445 500 | 25 766 | 111 927 | 6474 | 37 125 | 9327 | 0.0222 | None |
| GM3 | 10 | GTKO/CD55-CD59-HT | 318 750 | 12 340 | 53 875 | 2086 | 31 875 | 5388 | 0.0177 | None |
| GM4 | 10 | GTKO/CD55-CD59-HT | 805 000 | 23 802 | 224 124 | 6627 | 80 500 | 22 412 | 0.0447 | None |
| GM5 | 8 | GTKO/CD55-CD59-HT | 783 133 | 27 459 | 328 103 | 11 504 | 97 892 | 41 013 | 0.0502 | None |
| Mean | 568 778 | 22 378 | 170 737 | 6582 | 57 670 | 17 889 | 0.0314 | |||
| SD | 215 309 | 5918 | 107 192 | 3339 | 29 605 | 14 389 | 0.0148 | |||
GM, genetically modified; IEQ, islet equivalent; NICC, neonatal islet cell clusters; rhAT, recombinant human anti-thrombin; SD, standard deviation.
WT porcine NICC cause rapid thrombosis when transplanted intraportally in baboons
The 7644 ± 1156 IEQ WT pig NICC/kg of body weight were transplanted intraportally into four healthy nondiabetic baboons without (n = 2) or with (n = 2) treatment with 500 U rhAT/kg of body weight. Large baboons (21–24 kg) were used to enable multiple biopsies with minimal trauma to the liver. The dose of rhAT was based on our previous CD55-CD59-HT transgenic pig renal xenograft studies 16,17, and resulted in peak AT levels of 10–15 U/mL 17. There was substantial intraportal clot formation regardless of rhAT therapy, with biopsies showing NICC surrounded by clot containing fibrin, RBC and leukocytic infiltrate from as early as 1 h posttransplant (Figure 1). One of the rhAT-treated recipients developed a complete left portal vein thrombosis when the NICC were infused selectively toward the left lobe of the liver. A mechanical component cannot be excluded as being partially responsible for this. However, NICC were identified in the right liver lobe and hepatic vein in this recipient (Figure 1B), surrounded by intraportal clot (Figure 1C) despite the main portal vein to these liver segments remaining patent. Most NICC were surrounded by monocytes and neutrophils with areas staining for C3c (Figure 1D) in surrounding clot. Neutrophils were seen infiltrating NICC that stained positive for IgG deposition, leading to early destruction (Figure 1E–F). Quantification of NICC in end point biopsies demonstrated similar numbers in all recipients (Table 1).
Figure 1.
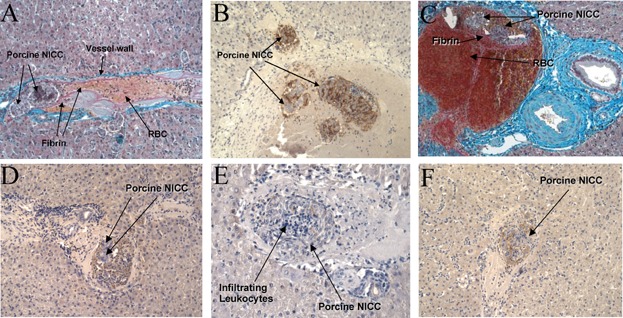
Evidence of instant blood-mediated inflammatory reaction in baboons transplanted intraportally with WT pig NICC. One-hour posttransplant baboon liver biopsies were stained with Martius Scarlet Blue (A, C) or H&E (E), or for αGal (B), complement C3c (D) or IgG (F). Massive clotting can be seen around the αGal-positive (B) NICC, along with leukocyte infiltration (E) and deposition of C3c (D) and IgG (F). All images ×20 magnification except C (×10). NICC, neonatal islet cell clusters.
Intraportal GTKO/CD55-CD59-HT NICC xenografts do not cause thrombosis in immunosuppressed recipients
GTKO/CD55-CD59-HT NICC were transplanted intraportally into five nondiabetic baboons. Expression of human CD55 and CD59 on the NICC prior to transplantation was confirmed by immunohistochemistry (Figure S1). Two modifications were made to the protocol to allow for the possibility that these NICC would survive beyond IBMIR and could be monitored by histological analysis. First, the number of early biopsies was reduced to one (at 1 h), permitting the use of smaller recipients (8–12 kg) and as a consequence approximately doubling the relative number of NICC transplanted (17 889 ± 14 389 IEQ/kg). Second, the recipients were treated with a clinically relevant immunosuppressive protocol.
None of the recipients showed evidence of thrombosis. Analysis of 1-h biopsies showed intact NICC surrounded by normal vasculature, with some erythrocytes but no platelets, fibrin or clot. A representative example is shown in Figure 2. Although most of the NICC observed appeared to be free floating, it is more likely that the images represent tangential sections of NICC in contact with the vessel wall. An example of the less frequently observed case of an NICC clearly lodged against the vessel wall, but again with no evidence of thrombus, is shown in Figure S2. No lymphocytic infiltrate was seen. The xenografts showed strong expression of CD55 and CD59 (Figure 2C and D) and absence of αGal (Figure 2E).
Figure 2.
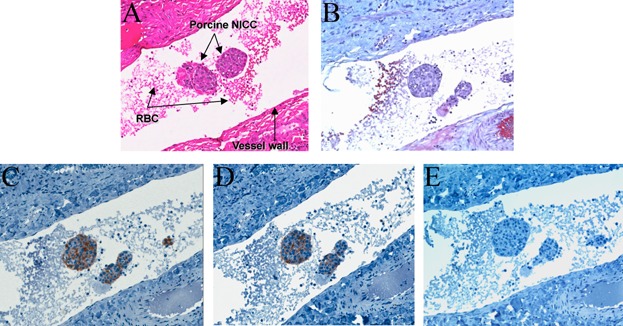
Absence of early thrombosis in immunosuppressed baboons transplanted intraportally with GTKO/CD55-CD59-HT NICC. One-hour posttransplant liver biopsies stained with hematoxylin and eosin (A) or Martius Scarlet Blue (B) showed intact NICC surrounded by normal vasculature, with some erythrocytes but no platelets, fibrin or clot present in either the micro- or macro-vasculature. Staining confirmed that the NICC expressed human CD55 (C) and CD59 (D) but not αGal (E). All images ×20 magnification. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Analysis of end point (1 month) biopsies demonstrated NICC in similar numbers (Table 1) in all lobes. There was no evidence of thrombosis (Figure 3A and B), and scattered cells staining for chromogranin A, insulin, glucagon or somatostatin were observed (Figure 3C–F). However, most NICC contained a significant infiltrate including T and B cells (Figure 3G and H). Image analysis (percent of NICC area staining for CD3 or CD20) revealed a significant increase in T and B cells infiltrating the grafts over time, with more T cells at 2 weeks and similar numbers of T and B cells at 1 month (Figure 4). Two-week biopsies confirmed the absence of thrombosis and the early signs of cellular rejection at this time (Figure S3). Porcine C-peptide was not detected at either 2 weeks or 1 month, presumably due to the immaturity of the NICC, ongoing cellular rejection and the use of nondiabetic recipients.
Figure 3.
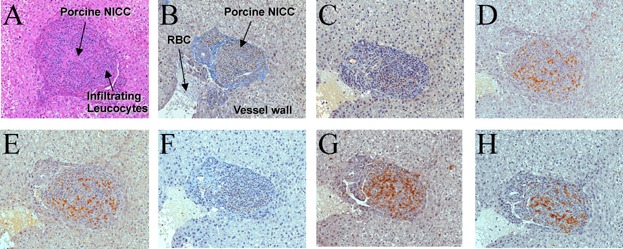
Lack of thrombosis and evidence of cellular rejection of GTKO/CD55-CD59-HT NICC xenografts 1 month after transplant. Baboon liver sections were stained with hematoxylin and eosin (A), Martius Scarlet Blue (B), or antibodies for chromogranin A (C), insulin (D), glucagon (E), somatostatin (F), CD3 (G) or CD20 (H). All images ×20 magnification. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Figure 4.
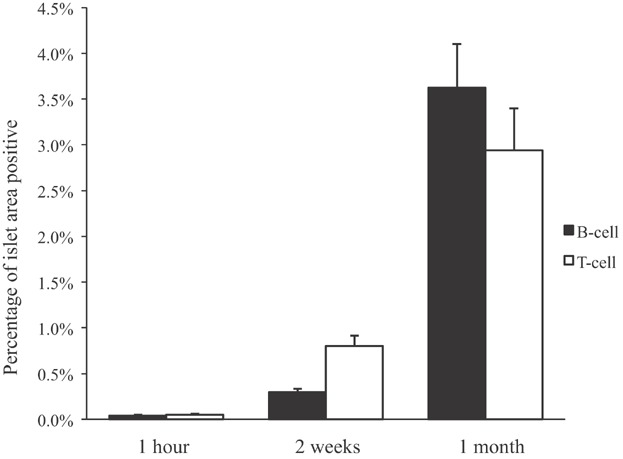
Degree of infiltration of GTKO/CD55-CD59-HT NICC xenografts by T and B cells at 1 h, 2 weeks and 1 month posttransplant. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Quantitative analysis demonstrated heavy red cell sequestration and fibrin deposition in clots in the liver of baboons transplanted with WT NICC within hours of transplant. The mean percentage of clot was 2.5 ± 0.36% of the total biopsy area (Figure 5A), with RBC comprising 1.76 ± 0.79% (Figure 5B) and fibrin 0.75 ± 0.10% (Figure 5C). In contrast, early biopsies of baboons transplanted with GTKO/CD55-CD59-HT NICC demonstrated minimal clotting, red cell sequestration and fibrin deposition; the mean percentage of clot was significantly lower (0.79 ± 0.12%, p < 0.05 vs. WT) (Figure 5A), with significantly fewer RBC (0.79 ± 0.13%, p < 0.05 vs. WT) (Figure 5B) and less fibrin (0.07 ± 0.02%, p < 0.05 vs. WT) (Figure 5C). By 8 h posttransplant, the mean percentage of clot in WT NICC xenograft biopsies was 3.95 ± 1.18% of the total biopsy area (Figure 5D), with RBC 3.39 ± 1.19% (Figure 5E) and fibrin 0.65 ± 0.08% (Figure 5F). This was significantly greater than that observed in GTKO/CD55-CD59-HT NICC xenograft biopsies (mean percentage of clot 0.5 ± 0.10%, p < 0.05 vs. WT, Figure 5D; RBC 0.3 ± 0.10%, p < 0.05 vs. WT, Figure 5E; fibrin 0.2 ± 0.02%, p < 0.05 vs. WT, Figure 5F).
Figure 5.
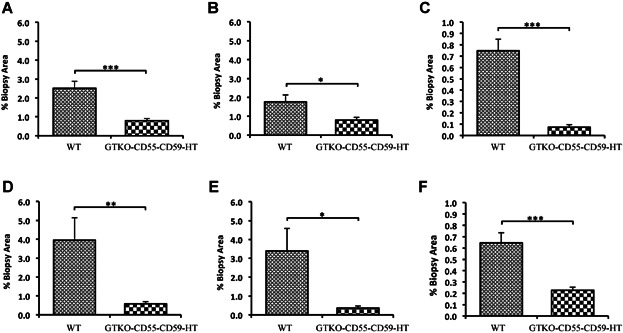
Reduction in total clot formation (A and D), including both red blood cell sequestration (B and E) and fibrin deposition (C and F), in GTKO/CD55-CD59-HT NICC versus WT NICC xenografts. Quantitative computer image analysis was used to quantify the degree of thrombosis around the grafts at 1–3 h (A–C) and up to 8 h (D–F) posttransplant. Data are shown as mean ± SEM of the total percentage of the biopsy area. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Mechanism of clotting
Comparison of the fold change in the systemic platelet count demonstrated greater early consumption in baboons transplanted with WT NICC than in those transplanted with GTKO/CD55-CD59-HT NICC (Figure 6A). There was a significant decrease in platelet count in blood sampled from both the hepatic and portal veins within 20 min following transplantation of WT NICC. This consumption of platelets continued to 6 h posttransplant (Figure 6B).
Figure 6.
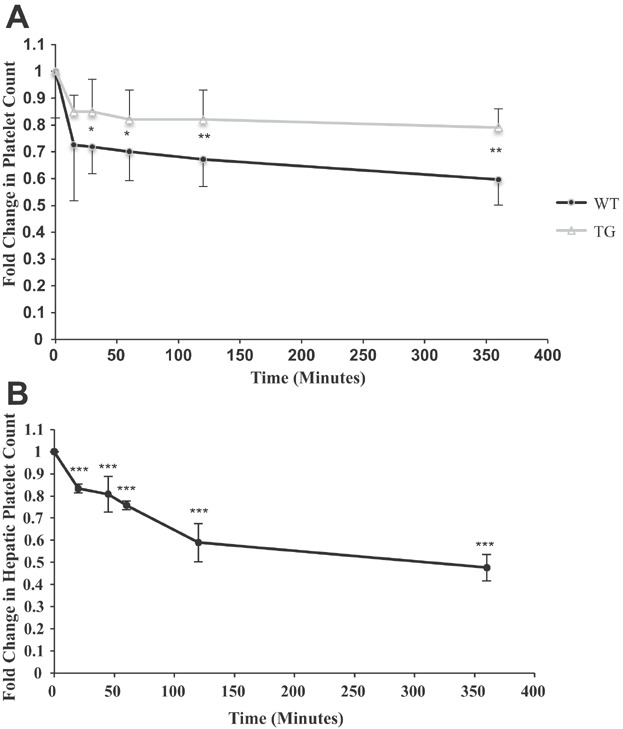
Changes in systemic and portal platelet count early after intraportal NICC xenotransplantation. Recipients of WT NICC (n = 4) had significantly lower systemic platelet counts than recipients of GTKO/CD55-CD59-HT NICC (n = 5) (A). The reduction in platelets was particularly pronounced in the hepatic blood samples of the WT NICC recipients (B). Data are shown as mean ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Complement activation and thrombin generation were also measured in vitro by incubating NICC with baboon PPP. GTKO/CD55-CD59-HT NICC triggered significantly less complement activation than WT NICC (Figure 7A), and generated significantly less thrombin than WT NICC, as demonstrated by reduced endogenous thrombin potential (Figure 7B).
Figure 7.
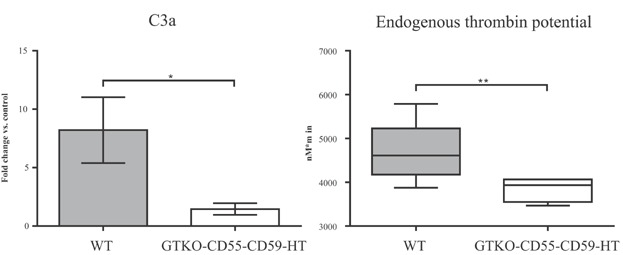
GTKO/CD55-CD59-HT NICC trigger significantly less complement activation (A) and thrombin generation (B) than WT NICC when incubated in vitro with baboon platelet-poor plasma. Data are shown as mean ± SD of 10 independent experiments. * p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Development of xenoreactive antibodies
The antibody response to GTKO/CD55-CD59-HT NICC was investigated by measuring serum IgM and IgG binding to GTKO pig aortic endothelial cells by flow cytometry. Marked increases in anti-pig IgM and IgG were observed at or after day 14 in three and two of the five recipients, respectively (Figure 8). The reason for the inconsistent antibody response is unclear, although it may be due to inter-individual variability between recipients, for example, in the absorption of immunosuppressive drugs.
Figure 8.
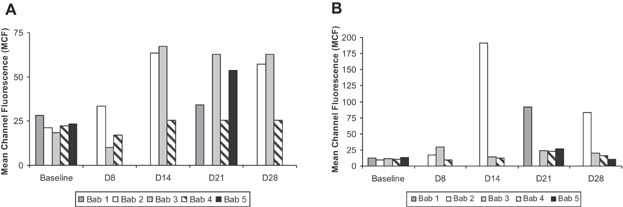
The immunosuppressive protocol was insufficient to prevent an anti-pig antibody response in most baboons transplanted with GTKO/CD55-CD59-HT NICC xenografts. (A) anti-pig IgM; (B) anti-pig IgG. GTKO/CD55-CD59-HT, GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT); NICC, neonatal islet cell clusters.
Discussion
This is the first study reporting the fate of neonatal pig islets after intraportal transplantation into baboons. WT NICC induced a profound and rapid thrombotic response, more severe than IBMIR observed after human islet allotransplantation 6. Despite the use of prophylactic anti-coagulant therapy, human islet allotransplantation can cause portal vein thrombosis 23,24. Pig islet xenografts are likely to be more thrombogenic because of the multiple antigenic differences and receptor-ligand incompatibilities between pig and human coagulation pathways 25. Two previous studies have examined adult pig islet intraportal xenografts in baboons; in both cases, the islets were lost early to rejection despite heavy immunosuppression, suggesting that destruction was due to activation of the innate rather than the acquired immune response and consistent with our findings of a strong and widespread IBMIR 26,27. WT NICC have been transplanted intraportally into macaques 14,28,29, but IBMIR was not specifically examined in those studies. Baboons, which are closer in size to humans, may have subtle but important differences in their coagulation response compared to macaques. Furthermore, most of the data describing coagulation abnormalities following vascularized organ xenotransplantation have been obtained in baboon recipients 17,30.
Liver biopsies taken within 60 min of transplantation showed WT NICC within the portal vasculature surrounded by a dense fibrin clot and heavily infiltrated by neutrophils. This intimate interlinking of the inflammatory and coagulation pathways is similar to other innate responses seen in mammals, in particular the response to sepsis 31. Excessive coagulation activates the inflammatory response via G protein-coupled protease-activated receptors, which can lead to up-regulation of tissue factor in monocytes and neutrophils, providing a molecular link between coagulation and inflammation 32,33. Monocytes and neutrophils have high levels of inducible tissue factor, which can be up-regulated rapidly to drive a positive feedback loop for coagulation 32–34.
rhAT, previously shown to be effective at inhibiting coagulopathy after renal xenotransplantation in baboons 17, was ineffective at preventing the intraportal clotting induced by WT NICC. This suggests that antibody binding and complement activation were sufficient to overcome the anti-thrombotic effects of anti-thrombin. Surprisingly, analysis of 1-h liver biopsies revealed that most NICC remained in the venules of the portal system. The relatively low portal pressure (<5 mmHg) may be insufficient to force the NICC into the sinusoids, and there may be stasis upstream due to localized obstruction. This is commonly seen in reduced wedged venous hepatic pressures in our clinical islet transplant patients.
The effectiveness of genetic modifications that do not directly target coagulation might not be expected to have a major impact on IBMIR, given that thrombosis predominates. However, there is significant cross-talk between complement activation—a very early component of IBMIR—and coagulation, and it has been shown previously that inhibition of complement suppresses thrombin generation associated with intraportal islet transplantation in rats 35. Furthermore, complement inhibition significantly inhibits the expression of active tissue factor by neutrophils 36.
We recognize the potential limitations associated with the experimental design. Although the increased resistance of GM NICC to humoral immunity in vitro suggested that the genetic modifications contributed to the protection against IBMIR, we cannot rule out a role for other differences between the groups, in particular the use of immunosuppression and smaller recipients. On the latter point, although the recipients of GM NICC received more NICC per kilogram body weight, one might anticipate a greater propensity for thrombosis in these smaller recipients due to a higher risk of stasis induced by mechanical obstruction. Despite this, they showed markedly less thrombosis, suggesting that stasis due to obstruction was not the major contributor to clotting in this model.
Another caveat is that we used nondiabetic baboons and therefore could not monitor the maturation and function of the transplanted NICC. Our rationale was that we did not require diabetic animals to obtain proof of inhibition of IBMIR and short-term survival of the GM NICC. Furthermore, we were not expecting the grafts to mature sufficiently to control blood glucose within the time frame of the experiments, and the study was designed to assess histological evidence of survival. Our results provide the basis for future functional studies using diabetic recipients. Finally, the data do not allow us to establish which of the three genetic modifications provide an anti-IBMIR effect. Cardiac xenotransplantation studies suggest that CD55 adds benefit to the GTKO background 37, but there are no corresponding data from islet xenotransplantation. Transgenic expression of human CD46 improved survival of long-term adult pig islet xenografts in cynomolgus monkeys, but had little impact on IBMIR 15. However, differences between that study and the current study (e.g. recipients, islet maturity, presence/absence of αGal) make it difficult to directly compare the two.
Even though the GTKO/CD55-CD59-HT NICC avoided IBMIR, presumably allowing more NICC to survive the initial transplant period, they were destroyed within 1 month by T cell–mediated cellular mechanisms despite the use of an immunosuppressive protocol that we have demonstrated to be safe and effective in clinical human islet allotransplantation 19. The long-term aim is to focus on genetic modification of the donor NICC to achieve better survival rather than rely on unacceptably high and toxic immunosuppressive protocols. Histology of the xenografts 2–4 weeks after transplantation showed progressive infiltration by T and B cells. In addition, most recipients had elevated anti-pig antibody titers, confirming a T cell–mediated B cell response. This suggests that GM pig NICC provoked strong T cell–mediated rejection and additional strategies will be required to promote long-term islet xenograft survival. These might include less conventional immunosuppressive regimens including costimulation blockade, which has met with some success in the pig-to-macaque and pig-to-cynomolgus monkey models 14,15,28,29. Another attractive possibility is further genetic manipulation of the donor pig to suppress the T cell–mediated response, for example by the transgenic, graft-mediated local expression of immunosuppressive agents such as CTLA4-Ig 38 or anti-CD2 mAb 39. This inherent advantage needs to be utilized further if islet xenotransplantation is to be brought successfully to the clinic.
Acknowledgments
The authors wish to thank the staff of the Australian National Baboon Colony, in particular Scott Heffernan, Dr. Penny Farrell and Professor Annemarie Hennessy, for their ongoing support of this project. We thank Matt Borg for his dedicated care of the pigs used in this study and Dr. Ross Matthews for veterinary supervision. We also thank staff of the Experimental Medical Surgical Unit, St. Vincent's Hospital, Melbourne, for their assistance. We thank GTC Biotherapeutics Corporation (Framingham, MA) for the donation of recombinant human anti-thrombin. This work was supported by the National Health and Medical Research Council of Australia and the Juvenile Diabetes Research Foundation.
Glossary
- αGal
galactose-α1,3-galactose
- GM
genetically modified
- GTKO
GGTA1 gene knockout
- GTKO/CD55-CD59-HT
GTKO pigs expressing human CD55, CD59 and α1,2-fucosyltransferase (H-transferase, HT)
- HBBS
Hanks Buffer Salt Solution
- HSA
human serum albumin
- IBMIR
instant blood-mediated inflammatory reaction
- IEQ
islet equivalent
- MMF
mycophenolate mofetil
- MSB
Martius Scarlet Blue
- NICC
neonatal islet cell clusters
- PPP
platelet-poor plasma
- RBC
red blood cell
- rhAT
recombinant human anti-thrombin
- TGA
thrombin generation assay
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Figure S1: Staining of isolated NICC for human CD55 (A) and CD59 (B) (×40 magnification).
Figure S2: One-hour liver biopsy of GTKO/CD55-CD59-HT NICC recipient, showing absence of thrombosis and proximity of an NICC to the vessel wall.
Figure S3: Two-week liver biopsy of GTKO/CD55-CD59-HT NICC recipient, stained for chromogranin A (A), insulin (B), glucagon (C), somatostatin (D), CD3 (E) and CD20 (F).
References
- 1.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97:2119–2129. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 3.Ekser B, Cooper DK. Overcoming the barriers to xenotransplantation: Prospects for the future. Expert Rev Clin Immunol. 2010;6:219–230. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Bennet W, Sundberg B, Lundgren T, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: Protective effects of sCR1 and heparin. Transplantation. 2000;69:711–719. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 7.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: Possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 8.Spirig R, Gajanayake T, Korsgren O, Nilsson B, Rieben R. Low molecular weight dextran sulfate as complement inhibitor and cytoprotectant in solid organ and islet transplantation. Mol Immunol. 2008;45:4084–4094. doi: 10.1016/j.molimm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Miyagawa S, Yamamoto A, Matsunami K, et al. Complement regulation in the GalT KO era. Xenotransplantation. 2010;17:11–25. doi: 10.1111/j.1399-3089.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof N, Shibata S, Wijkstrom M, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11:396–407. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie IFC, Xing P-X, Sandrin MS, Koulmanda M, Mandel TE. Pig-to-human xenotransplantation: The expression of Galα(l–3)Gal epitopes on pig islet cells. Xenotransplantation. 1995;2:1–7. [Google Scholar]
- 12.Rayat GR, Rajotte RV, Elliott JF, Korbutt GS. Expression of Gal alpha(1,3)gal on neonatal porcine islet beta-cells and susceptibility to human antibody/complement lysis. Diabetes. 1998;47:1406–1411. doi: 10.2337/diabetes.47.9.1406. [DOI] [PubMed] [Google Scholar]
- 13.Strokan V, Bennet W, Molne J, Korsgren O, Breimer ME. Distribution of the Galalpha1-3Gal antigen in cultured adult and fetal porcine pancreatic islet cells: An immunoelectron microscopic study. Transplantation. 2000;70:846–851. doi: 10.1097/00007890-200009150-00024. [DOI] [PubMed] [Google Scholar]
- 14.Thompson P, Badell IR, Lowe M, et al. Islet xenotransplantation using Gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11:2593–2602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 16.Cowan PJ, Aminian A, Barlow H, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002;2:520–525. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 17.Cowan PJ, Aminian A, Barlow H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69:2504–2515. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 18.Nottle MB, Beebe LF, Harrison SJ, et al. Production of homozygous alpha-1,3-galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation. 2007;14:339–344. doi: 10.1111/j.1399-3089.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell PJ, Holmes-Walker DJ, Goodman D, et al. Multicenter Australian trial of islet transplantation: Improving accessibility and outcomes. Am J Transplant. 2013;13:1850–1858. doi: 10.1111/ajt.12250. [DOI] [PubMed] [Google Scholar]
- 20.Hawthorne WJ, Cachia AR, Walters SN, et al. A large-animal model to evaluate the clinical potential of fetal pig pancreas fragment transplantation. Cell Transplant. 2000;9:867–875. doi: 10.1177/096368970000900613. [DOI] [PubMed] [Google Scholar]
- 21.Gock H, Murray-Segal L, Salvaris E, Cowan PJ, D'Apice AJ. Gal mismatch alone causes skin graft rejection in mice. Transplantation. 2002;74:637–645. doi: 10.1097/00007890-200209150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Tu XM, Kowalski J, Zhang J, Lynch KG, Crits-Christoph P. Power analyses for longitudinal trials and other clustered designs. Stat Med. 2004;23:2799–2815. doi: 10.1002/sim.1869. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell PJ, Hawthorne WJ, Holmes-Walker DJ, et al. Clinical islet transplantation in type 1 diabetes mellitus: Results of Australia's first trial. Med J Aust. 2006;184:221–225. doi: 10.5694/j.1326-5377.2006.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro AM, Lakey JR, Rajotte RV, et al. Portal vein thrombosis after transplantation of partially purified pancreatic islets in a combined human liver/islet allograft. Transplantation. 1995;59:1060–1063. doi: 10.1097/00007890-199504150-00027. [DOI] [PubMed] [Google Scholar]
- 25.Robson SC, Cooper DK, d'Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 26.Buhler L, Deng S, O'Neil J, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9:3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 27.Cantarovich D, Blancho G, Potiron N, et al. Rapid failure of pig islet transplantation in non human primates. Xenotransplantation. 2002;9:25–35. doi: 10.1034/j.1399-3089.2002.0o144.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson P, Badell IR, Lowe M, et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am J Transplant. 2012;12:1765–1775. doi: 10.1111/j.1600-6143.2012.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11:947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ierino FL, Kozlowski T, Siegel JB, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66:1439–1450. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25:536–542. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111:25–27. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeliger S, Derian CK, Vergnolle N, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003;17:1871–1885. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- 35.Tokodai K, Goto M, Inagaki A, et al. Attenuation of cross-talk between the complement and coagulation cascades by C5a blockade improves early outcomes after intraportal islet transplantation. Transplantation. 2010;90:1358–1365. doi: 10.1097/tp.0b013e3181ffb9f5. [DOI] [PubMed] [Google Scholar]
- 36.Kourtzelis I, Markiewski MM, Doumas M, et al. Complement anaphylotoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGregor CG, Ricci D, Miyagi N, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klymiuk N, van Buerck L, Bahr A, et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes. 2012;61:1527–1532. doi: 10.2337/db11-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brady JL, Sutherland RM, Hancock M, et al. Anti-CD2 producing pig xenografts effect localized depletion of human T cells in a huSCID model. Xenotransplantation. 2013;20:100–109. doi: 10.1111/xen.12025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Staining of isolated NICC for human CD55 (A) and CD59 (B) (×40 magnification).
Figure S2: One-hour liver biopsy of GTKO/CD55-CD59-HT NICC recipient, showing absence of thrombosis and proximity of an NICC to the vessel wall.
Figure S3: Two-week liver biopsy of GTKO/CD55-CD59-HT NICC recipient, stained for chromogranin A (A), insulin (B), glucagon (C), somatostatin (D), CD3 (E) and CD20 (F).


