Abstract
Pancreatic cancer is one of the most aggressive, drug-resistant and lethal types of cancer with poor prognosis. Various factors including reactive oxygen species, cytokines, growth factors, and extracellular matrix proteins are reported to be involved in the development of pancreatic cancer. However, the pathogenesis of pancreatic cancer has not been completely elucidated. Oxidative stress has been shown to contribute to the development of pancreatic cancer. Evidences supporting the role of reactive oxygen species and cytokines as a risk for pancreatic cancer and the concept of antioxidant supplementation as a preventive approach for pancreatic cancer have been proposed. Here, we review the literature on oxidative stress, cytokine expression, inflammatory signaling, and natural antioxidant supplementation in relation to pancreatic cancer.
Keywords: Oxidative stress, Cytokines, Pancreatic cancer
INTRODUCTION
Reactive oxygen species (ROS) and cytokines are considered to be important factors in the pathogenesis of pancreatic cancer.1,2 As a source of ROS, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is involved in pancreatic cancer development.3 ROS activate signaling pathways mediated by p38 mitogen-activated protein kinases (MAPK), NF-κB, and janus kinase/signal transducer and activator of transcription (JAK/STAT),4–8 which inhibits cancer cell apoptosis9 and induces cytokine expression8 and epithelial-mesenchymal transition (EMT).10,11 High levels of fibronectin and laminin,10,11 cytokines (interleukin-1β [IL-1β], IL-6, IL-8, and tumor necrosis factor-β [TNF-β],12,13 and growth factors (insulin-like growth factor-1 [IGF-1] and transforming growth factor-β [TGF-β])14 are observed in pancreatic cancer. Growth factors,14 extracellular matrix (ECM) proteins,10 and cytokines (interferon-γ [IFN-γ] and TNF-α)14–17 have been shown to activate NOX in the pathogenesis of pancreatic cancer development. Bioactive compounds such as curcumin, genistein, and resveratrol have antioxidant18–20 and antitumor activities21–29 against pancreatic cancer. We will briefly review the role of ROS and cytokines in the pathogenesis of pancreatic cancer. In addition, bioactive compounds that may prevent the development of pancreatic cancer will also be discussed.
REACTIVE OXYGEN SPECIES AND NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE OXIDASE IN PANCREATIC CANCER
Since ROS and pro-inflammatory cytokines induce genetic instability, these factors may contribute to cancer development.1,30 Activation of NOX releases high amounts of ROS, which may contribute to development of cancers. Pro-inflammatory cytokine IFN-γ induces the activation of a family of NOX, dual oxidase 2 (DUOX2), to produce ROS in human pancreatic cancer cells.16
K-ras mutation is identified in 95% of pancreatic cancer, implying its critical role in the pathogenesis of pancreatic cancer. Recent study showed that NOX2 protein is present in pancreatic tumor cells and tumorigenic cells expressing K-ras while NOX2 was absent in the non-tumorigenic pancreatic ductal epithelial cells.31 In pancreatic cancer cells with K-ras, genetically silencing rac decreases ROS production and inhibits cell growth.31 The study demonstrates that NOX is important for ROS production and growth inhibition in K-ras-expressing cells since rac is a component of the active form of NOX.
Growth factors and ECM proteins mediate growth of pancreatic cancer cells. Growth factors such as IGF-1 and IGF-2 stimulate NOX4 and produce ROS, which protect pancreatic cancer cells (MIA PaCa-2 and PANC-1) from apoptosis.9,14 In addition, ROS induce activation of JAK2-STAT1/3 pathway, leading to suppression of apoptosis.5 ECM proteins fibronectin and laminin also activate NOX in pancreatic cancer cells.10 ROS have shown to mediate epithelial-mesenchymal transition (EMT) in pathophysiological condition.11 NOX4-generated ROS are involved in TGF-β-induced EMT in pancreatic cancer cells by activating p38 MAPK and inhibiting protein tyrosine phosphatase 1B (PTP1B).11 Since PTP1B inhibits EMT, inhibition of PTP1B by ROS contribute EMT in pancreatic cancer development.
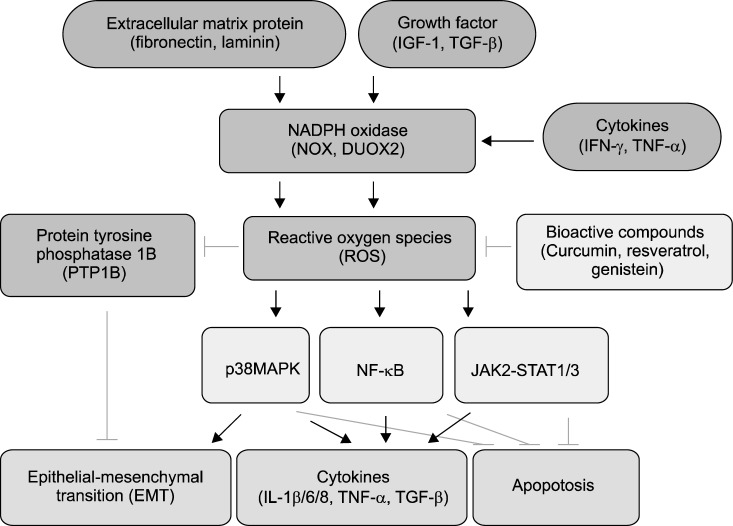
DUOX is one of the 7 members of the NOX family. The pro-inflammatory stimuli such as IFN-γ enhance the expression of DUOX2, leading to the production of substantial amounts of extracellular H2O2 in human pancreatic cancer BxPC-3 and AsPC-1 cells.16 H2O2 induces the activation of STAT1 and NF-κB signaling pathways in pancreatic cancer cells.16 Crosstalk of ROS and cytokines in cancer-related signaling is summarized in Figure. ROS are produced by NOX with stimulation of extracellular matrix (ECM) proteins,10,11 growth factors,14 and cytokines.15–17 ROS activates p38 MAPK,8 NF-κB,4 and JAK2-STAT1/3,5 which is believed to contribute to the carcinogenesis of pancreas since these signaling mediators induce ECM and expression of cytokines (IL-1β, IL-6, IL-8, TNF-α, and TGF-β)12–15,32–34 as well as inhibit apoptosis of cancer cells. Antioxidant compounds such as curcumin,18 resveratrol,19 and genistein20 may inhibit ROS-mediated signaling and thus prevent the development of pancreatic cancer.
Figure.
Crosstalk of reactive oxygen species (ROS) and cytokines for cancer-related signaling in pancreatic cancer. High levels of growth factors (insulin-like growth factor-1 [IGF-1], transforming growth factor-1 [TGF-β]), cytokines (interleukin-1β [IL-1β], IL-6, IL-8, and tumor necrosis factor-α [TNF-α]), and extracellular matrix (ECM) proteins (fibronectin and laminin) are observed in pancreatic cancer. Growth factors, cytokines (interferon-γ [IFN-γ], TNF-α) and ECM proteins activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and dual oxidase 2 (DUOX2) and produce ROS in pancreatic cancer cells. ROS activate signaling pathways mediated by p38 mitogen-activated protein kinase (MAPK), NF-κB, and janus kinase/signal transducer and activator of transcription (JAK/STAT), which inhibits cancer cell apoptosis and induces cytokine expression and epithelial-mesenchymal transition (EMT). ROS inhibit protein tyrosine phoshatase 1B (PTP 1B) which suppress EMT. Therefore, ROS induce phosphorylation of p38 MAPK and EMT in the course of pancreatic cancer development. Bioactive compounds, having antioxidant activities, such as curcumin, resveratrol, and genistein, reduce ROS levels and thus, may inhibit ROS-mediated signaling of p38 MAPK, NF-κB, and JAK/STAT. The compounds inhibit EMT and cytokine expression and induce cancer cell apoptosis.
INFLAMMATORY CYTOKINES IN PANCREATIC CANCER
Cytokines promote pancreatic tumor cell progression by modulating tumor microenvironment as well as directly acting on cancer cells for growth, invasion and metastasis. Cytokines are produced from the leukocytes and stellate cells which are infiltrated into the damaged tissues. In the serum of pancreatic cancer patients, pro-inflammatory cytokines IL-6, IL-8, TGF-β, TNF-α, and IL-1β were increased compared to healthy controls.2,12–15,32–34
IL-6 induces the expression of vascular endothelial growth factor (VEGF) in pancreatic cancer cells and stimulates angiogenesis and tumor vascularization.35 IL-6 induced phosphorylation of STAT3, which in turn, mitochondrial localization of pSTAT3 promotes pancreatic tumorigenesis by activating cell proliferation and inhibiting autophagy.36
IL-1β promotes pancreatic cancer cell invasiveness.37 IL-1β induces constitutive activation of NF-κB and expression of cyclooxygenase-2 of pancreatic cancer cells and these cancer cells become chemoresistant.38,39 Patients with both a high IL-6 level and a high IL-1β level exhibited shortened overall and progression-free survival, a reduction in the tumor control rate, and a high dose intensity of gemcitabine monotherapy compared with pancreatic cancer patients with low levels of both IL-6 and IL-1β.40 Therefore, the serum levels of IL-6 and IL-1β predict the efficacy of gemcitabine monotherapy in patients with advanced pancreatic cancer.
IL-8 stimulates the expression of VEGF, neuropilin-2, and VEGF receptor, which are key molecules in angiogenesis. In addition, IL-8 increases the activation of MAPK pathway for cell growth, survival, and tumorigenesis.12 It has been reported that IL-8 promotes tumor aggressiveness and invasiveness in pancreatic cancer.32 In addition, IL-8 enhances the invasiveness by regulating matrix metalloproteinase-2 activity in human pancreatic cancer cells.41 Both IL-8 and chemokine CXCL12 promote migration and invasion of pancreatic cancer cells.32
TNF-α promotes pancreatic cancer cell proliferation.14 TNF-α induces the invasiveness of human pancreatic cancer cells and promotes tumor growth and metastasis in mice.42 Treatment of anti-TNF-α suppresses tumor growth and metastasis.42
BIOACTIVE COMPOUNDS IN PANCREATIC CANCER
Treatments for pancreatic cancer patients are surgery, chemotherapy, radiation, and targeted therapy. However, low survival rate of patients with pancreatic cancer needs improved therapeutic and chemopreventive strategies. Since ROS mediate cancer-associated signaling in pancreatic cancer development as described above, supplementation of antioxidant compounds may prevent pancreatic cancer development. Curcumin, resveratrol, and genistein have antioxidant activities18–20 and showed anti-cancer effects against pancreatic cancer in vitro and in vivo experiments.21–29
Curcumin (diferuloylmethane) inhibits the proliferation of pancreatic cancer cells and suppresses the activation of NF-κB.43,44 Soluble curcumin analogue inhibits NF-κB-DNA binding activity and induces apoptotic cell death in pancreatic cancer cells.45 Moreover, curcumin inhibits cell growth and induces apoptosis through down-regulating the Notch signaling pathway in pancreatic cancer BxPC-3 and PANC-1 cells.24,25 Using orthotopic xenograft model of human pancreatic cancer Mia PaCa-2 cells, curcumin enhanced the antitumor activity of gemcitabine by inhibiting cell proliferation and NF-κB-regulated cyclin D1, Bcl-xL and survivin genes.46 In addition, curcumin inhibits constitutive STAT3 phosphorylation and down-regulation of survivin expression in human pancreatic cancer cells.47 Regarding EMT in carcinogenesis, curcumin reverses EMT of TGF-β-stimulated pancreatic PANC-1 cancer cells by inhibiting the Hedgehog signaling.48
Resveratrol plays dual roles in pancreatic cancer: as a tumor suppressor via the up-regulation of Bax; as a tumor activator via the up-regulation of VEGF-B; and the anticancer effect of resveratrol is much stronger than its cancer promotion effect. Since resveratrol shows chemosensitization effect on tumor cells, the combination of resveratrol with pharmacological inhibitor of VEGF-B has been suggested as a promising modality for clinical pancreatic cancer therapy.29 The chemosensitization of tumor cells by resveratrol appears to be mediated through its ability to modulate multiple cell-signaling molecules, including drug transporters, cell survival proteins, cell proliferative proteins, and members of the NF-κB and STAT3 signaling pathways.28 Besides, resveratrol inhibits EMT of pancreatic BxPC-3 and PANC-1 cancer cells through the PI-3K/AKT/NF-kB signaling.49
Genistein stimulates apoptotic effects of the chemotherapeutic drugs cisplatin21 and gemcitabine22 by inhibiting NF-κB activity. Treatment of genistein inhibits pancreatic cancer cell growth and induces apoptosis through the down-regulation of NF-κB and Notch-1 signaling.23 Genistein reverses the epithelial to mesenchymal transition (EMT) of FoxM1-overexpressing pancreatic AsPC-1 cancer cells.50
CONCLUSIONS
ROS are produced by the activated NOX in pancreatic cancer. Growth factors, ECM proteins, and cytokines protect pancreatic cancer cells from apoptosis. ROS induce activation of p38 MAPK, NF-κB, and JAK2-STAT1/3 pathway, leading to suppression of apoptosis as well as induction of EMT and cytokine expression which amplify ROS-mediated signaling in pancreatic cancer cells. Cytokines promote pancreatic tumor cell progression, angiogenesis, and the activation of MAPK pathway, which is important for cell growth, survival, and tumorigenesis. STAT3 contributes to tumor initiation by promoting the dedifferentiation of the acinar cells to cancer cells. Since ROS activate STAT3 and MAPK as well as induction of cytokines, targeting ROS may contribute to the prevention of cancer progression in pancreatic tissues. Curcumin inhibits the proliferation of pancreatic cancer cells and suppresses the activation of NF-κB and expression of cyclooxygenase-2 in pancreatic cancer cells. Resveratrol inhibit proliferation of pancreatic cancer. Genistein stimulates apoptotic effects of the chemotherapeutic drugs, and reverses EMT of pancreatic cancer cells. Taken together, supplement of antioxidant compounds may be beneficial for preventing the development and/or initiation of pancreatic cancer.
Acknowledgments
This work was supported by the NRF of Korea grant, which is funded by the Korean government (MSIP) (2007-0056092).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Chiera F, Meccia E, Degan P, Aquilina G, Pietraforte D, Minetti M, et al. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med. 2008;44:332–42. doi: 10.1016/j.freeradbiomed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Miron N, Miron MM, Milea VG, Cristea V. Proinflammatory cytokines: an insight into pancreatic oncogenesis. Roum Arch Microbiol Immunol. 2010;69:183–9. [PubMed] [Google Scholar]
- 3.Wu Y, Antony S, Juhasz A, Lu J, Ge Y, Jiang G, et al. Up-regulation and sustained activation of Stat1 are essential for interferon-gamma (IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2 (DuoxA2) expression in human pancreatic cancer cell lines. J Biol Chem. 2011;286:12245–56. doi: 10.1074/jbc.M110.191031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DR. Death and NF-kappaB in T cell activation: life at the edge. Mol Cell. 2003;11:551–2. doi: 10.1016/s1097-2765(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–48. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda A, Wang SC, Morris JP, 4th, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Ju KD, Lim JW, Kim KH, Kim H. Potential role of NADPH oxidase -mediated activation of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-b1 in the pathophysiology of acute pancreatitis. Inflamm Res. 2011;60:791–800. doi: 10.1007/s00011-011-0335-4. [DOI] [PubMed] [Google Scholar]
- 9.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–54. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 10.Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–47. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431–8. [PubMed] [Google Scholar]
- 12.Li M, Zhang Y, Feurino LW, Wang H, Fisher WE, Brunicardi FC, et al. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008;99:733–7. doi: 10.1111/j.1349-7006.2008.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41:1001–7. doi: 10.1097/MPA.0b013e3182546e13. [DOI] [PubMed] [Google Scholar]
- 14.Friess H, Guo XZ, Nan BC, Kleeff J, Büchler MW. Growth factors and cytokines in pancreatic carcinogenesis. Ann N Y Acad Sci. 1999;880:110–21. doi: 10.1111/j.1749-6632.1999.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 15.Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E. Tumor necrosis factor alpha and interferon gamma genes polymorphisms and serum levels in pancreatic adenocarcinoma. Neoplasma. 2009;56:56–62. doi: 10.4149/neo_2009_01_56. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G, et al. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-gamma and lipopolysaccharide in human pancreatic cancer cell lines. J Immunol. 2013;190:1859–72. doi: 10.4049/jimmunol.1201725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah Z, Bayraktutan U. NADPH oxidase mediates TNF-a-evoked in vitro brain barrier dysfunction: roles of apoptosis and time. Mol Cell Neurosci. 2014;61C:72–84. doi: 10.1016/j.mcn.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: From bedside to bench and back [published online ahead of print April 30, 2014] Biotechnol Adv. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Feng L, Xing Y, Wang Y, Du L, Xu C, et al. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. Int J Mol Sci. 2014;15:5928–39. doi: 10.3390/ijms15045928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong DK, Liu BH, Tan XH. Genistein prevents cadmium-induced neurotoxic effects through its antioxidant mechanisms [published online ahead of print June 11, 2014] Drug Res(Stuttg) doi: 10.1055/s-0034-1372595. [DOI] [PubMed] [Google Scholar]
- 21.Mohammad RM, Banerjee S, Li Y, Aboukameel A, Kucuk O, Sarkar FH. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer. 2006;106:1260–8. doi: 10.1002/cncr.21731. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–72. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of NF-kB activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006;118:1930–6. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- 24.Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, Greif J, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–30. [PubMed] [Google Scholar]
- 25.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 26.Lev-Ari S, Zinger H, Kazanov D, Yona D, Ben-Yosef R, Starr A, et al. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells Biomed Pharmacother 200559(suppl 2)S276–80. [DOI] [PubMed] [Google Scholar]
- 27.Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28:166–71. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150–60. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Yang L, Tian W, Li J, Liu J, Zhu M, et al. Resveratrol plays dual roles in pancreatic cancer cells. J Cancer Res Clin Oncol. 2014;140:749–55. doi: 10.1007/s00432-014-1624-4. [DOI] [PubMed] [Google Scholar]
- 30.Du J, Nelson ES, Simons AL, Olney KE, Moser JC, Schrock HE, et al. Regulation of pancreatic cancer growth by superoxide. Mol Carcinog. 2013;52:555–67. doi: 10.1002/mc.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du J, Liu J, Smith BJ, Tsao MS, Cullen JJ. Role of Rac1-dependent NADPH oxidase in the growth of pancreatic cancer. Cancer Gene Ther. 2011;18:135–43. doi: 10.1038/cgt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, et al. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer Int J Cancer 2009124853-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wigmore SJ, Fearon KC, Sangster K, Maingay JP, Garden OJ, Ross JA. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int J Oncol. 2002;21:881–6. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- 34.Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, et al. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2006;55:684–98. doi: 10.1007/s00262-005-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang RF, Wang SX, Zhang FR, Peng L, Wang SX, Xiao Y, et al. Interleukin-1alpha, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2005;4:460–3. [PubMed] [Google Scholar]
- 36.Kang R, Tang D, Lotze MT, Zeh HJ., 3rd AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 2012;8:989–91. doi: 10.4161/auto.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greco E, Basso D, Fogar P, Mazza S, Navaglia F, Zambon CF, et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1beta not by transforming growth factor-beta1. Int J Biol Markers. 2005;20:235–41. doi: 10.1177/172460080502000406. [DOI] [PubMed] [Google Scholar]
- 38.Arlt A, Vorndamm J, Muerköster S, Yu H, Schmidt WE, Fölsch UR, et al. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 2002;62:910–6. [PubMed] [Google Scholar]
- 39.Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144:57–65. doi: 10.1016/j.surg.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, et al. Serum levels of IL-6 and IL-1b can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013;108:2063–9. doi: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuwada Y, Sasaki T, Morinaka K, Kitadai Y, Mukaida N, Chayama K. Potential involvement of IL-8 and its receptors in the invasiveness of pancreatic cancer cells. Int J Oncol. 2003;22:765–71. [PubMed] [Google Scholar]
- 42.Egberts JH, Cloosters V, Noack A, Schniewind B, Thon L, Klose S, et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68:1443–50. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 43.Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, et al.Curcumin analog GO-Y030 is a novel inhibitor of IKKb that suppresses NF-kB signaling and induces apoptosis Cancer Sci 20111021045–51. [DOI] [PubMed] [Google Scholar]
- 44.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 45.Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, et al. Curcumin analog GO-Y030 is a novel inhibitor of IKKb that suppresses NF-kB signaling and induces apoptosis. Cancer Sci. 2011;102:1045–51. doi: 10.1111/j.1349-7006.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 46.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 47.Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28:166–71. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 48.Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29:2401–7. doi: 10.3892/or.2013.2385. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Ma J, Ma Q, Li B, Han L, Liu J, et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kB pathway. Curr Med Chem. 2013;20:4185–94. doi: 10.2174/09298673113209990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A, et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]