Abstract
Background:
Previous studies on Helicobacter pylori infection in mice have contributed to better understanding of the pathogenesis of chronic gastritis and gastric carcinoma. The aim of this study was to evaluate H. pylori colonization and subsequent inflammatory responses in the stomachs of C57BL/6 mice depending on inoculation number and the presence of high-salt diet.
Methods:
Eighty-four female mice with 4 weeks age were used in this study. The infected mice were gavaged with H. pylori strain Sydney-1 (SS1), and the uninfected mice were dosed with vehicle. In each of these groups, half of the mice were fed ona basal diet (0.25% salt) and the other half were fed on a high-salt diet (7.5% salt). The infected mice were inoculated 4 or 5 times, and infection status and degree of inflammation were checked by culture and histopathology, respectively, after 4 weeks. Gastric mucosal myeloperoxidase and tumor necrosis factor-alpha were measured by ELISA.
Results:
The overall infection rate was 95.2%; the infection rate after 5 inoculations (100%) was greater than that after 4 inoculations (91.3%). However, no differences in the degree of inflammation were found between 2 groups. The bacterial density was also significantly increased in mice that were on the high-salt diet and had been inoculated 5 times, respectively. Mean neutrophil infiltration in the infected group was 1.7±0.6 (1, minimal; 2, mild; 3, moderate; 4, marked). However, the high-salt diet was not increase the inflammatory grade in the infected group. Gastric mucosal myeloperoxidase and tumor necrosis factor-alpha levels did not increased by the high-salt diet and increased the number of inoculation.
Conclusions:
In spite of well colonization of H. pylori in the stomachs of C57BL/6 mice, the degree of subsequent inflammation was irrelevant to high-salt diet and frequent (5 times) inoculations.
Keywords: Helicobacter pylori, Inflammation, Salt
INTRODUCTION
Helicobacter pylori, a gram-negative bacterium that colonizes the gastric epithelium, is the major cause of gastric carcinogenesis and other gastric diseases such as chronic gastritis, gastroduodenal ulcers, and gastric mucosa-associated lymphoid tissue lymphoma.1 H. pylori was classified as a “definite biological carcinogen” by the World Health Organization (WHO) in 1994.2 H. pylori infection has been examined in various experimental animals to elucidate underlying pathological and biochemical mechanisms.3 The mouse model is the best established animal model, and it was the first animal model of bacterial gastritis to show the progression from acute to persistent chronic active inflammation as is seen in human H. pylori infection.4 Thus, this model has been used to confirm host response,5 to examine the effects of environmental factors such as gastric acid levels on Helicobacter colonization,6 and to evaluate the virulence of various strains of H. pylori. Furthermore, the mouse model also contributed to development of vaccines that could be used to prevent H. pylori infection.7–9
High-salt ingestion has been proposed to increase the risk of stomach cancer.10,11 However, the associations between a high-salt diet and gastric tumors are less consistent and rarely raise the adjusted odds ratio in human epidemiologic studies.12 The hypothesis that high-salt ingestion promotes gastric cancer remains controversial and the WHO concluded that high-salt ingestion would “probably increase the risk of stomach cancer.”2 Experimental mouse models have been used to evaluate the relationship between high-salt intake and H. pylori infection.13,14 However, the previous experimental studies showed in consistent results regarding the effect of high-salt diet on carcinogenesis by enhancing colonization and increasing the impact of chronic H. pylori gastritis.14,15
The aim of this study was to establish an appropriate H. pylori mouse model and to clarify whether a high-salt diet can promote the H. pylori infection.
MATERIALS AND METHODS
1. Animals
Three-week-old female C57BL/6 mice (Orient Co., Ltd., Seoul, Korea) were housed in a cage maintained at 23°C with a 12/12-hour light/dark cycle under specific pathogen-free conditions. After 1 week of adaptation, 4-week-old mice weighing 10–15 g were used for the experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital (IACUC number: BA1004-059/016-01).
2. H. pylori culture and inoculation
The mouse-adapted H. pylori Sydney Strain 1 (SS1) established by Lee et al.16 was used for all experiments. Bacteria were grown on brain-heart infusion broth supplemented with 5% horse serum in a microaerophilic atmosphere (5% O2; 10% CO2; 85% N2) at 37°C for 72 hours. The organisms were positively identified on the basis of morphology and the presence of oxidase, catalase, and urease. The mice were inoculated orogastrically with >108 colony-forming units (CFU)/mL of H. pylori SS1 (0.25 mL) every other day over a period of 6–8 days (a total of 4 or 5 inoculations).
3. Experimental design (diet and bacterial inoculation schedule)
Four-week-old female mice of 84 were used in this study. The infected mice (n = 56) were gavaged with H. pylori SS1, and the uninfected mice (n = 28) were dosed with vehicle. Mice were gavaged with H. pylori SS1 or vehicle only (0.25 mL) every other day for a total of 4 or 5 times. The mice were then fed on either a basal diet (0.25% salt) or a high-salt diet (7.5% salt) resulting in the following 4 groups: (1) uninfected mice fed on the basal diet, (2) uninfected mice fed on the high-salt diet, (3) H. pylori-infected mice fed on the basal diet, and (4) H. pylori-infected mice fed on a high-salt diet. During the H. pylori inoculation period, all infected mice were fed a basal diet and then switched to a high-salt diet. All of the mice were sacrificed by CO2 inhalation at 4 weeks after inoculation (Fig. 1).
Figure 1.
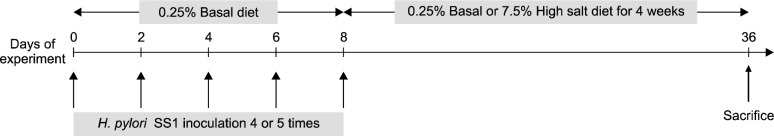
Schematic representation of the experimental design.
4. Histopathology
At necropsy, the stomach tissue was taken from the greater curvature beginning at the squamocolumnar junction and ending at the gastroduodenal junction. Linear gastric strips were fixed in 10% formalin solution, processed by standard methods, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). The stomach mucosa was examined histologically for inflammatory and epithelial changes and for the presence of H. pylori. The degree of neutrophil infiltration, mononuclear cell infiltration, atrophy, and metaplasia was assessed according to the updated Sydney classification as follows: 0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked.
5. The definition of a successful mouse model and Helicobacter pylori infection
A successful mouse model was defined as one in which all mice in the H. pylori SS1-inoculated group were positive for (1) H. pylori as determined by H&E stain, or (2) H. pylori culture, or (3) H. pylori-induced gastritis in histopathology. H. pylori infection was defined by the presence of H. pyloriin H&E stain or culture.
6. Measurement of mucosal myeloperoxidase and tumor necrosis factor alpha
Ten milligrams of scraped mucosa was homogenized for 30 seconds with a Polytron homogenizer in 200 μL of ice-cold lysis buffer (200 mM NaCl, 5 mM EDTA, 10 mM Tris [pH 7.4], 10% glycerin, 1 mM PMSF, 1 μg/mL leupeptin, and 28 μg/mL aprotinin). The cell suspensions were centrifuged at 13,000 rpm for 15 minutes, and the resulting supernatant was assayed using a myeloperoxidase (MPO) ELISA kit (HyCult Biotechnology, Uden, The Netherlands). For tumor necrosis factor alpha (TNF-α), the appropriate kits from R&D Systems (Minneapolis, MN, USA) were used following the manufacturer’s instructions. Protein concentration was measured using a Bio-Rad Protein Assay Kit (Bio-Rad Laboratories; Hercules, MA, USA). Concentration of each cytokine was presented as pictograms per milligram protein. All assays were performed in triplicate.
7. Statistical analysis
Data are expressed as mean ± SEM. Comparison of 2 groups was performed using the Mann-Whitney U test or Fisher’s exact test. P values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA).
RESULTS
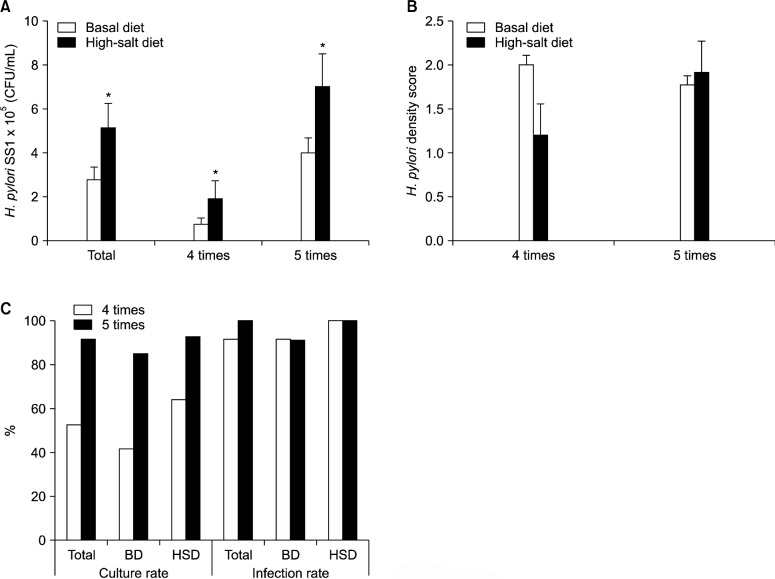
1. The high-salt diet increased the level of Helicobacter pylori colonization in quantitative culture
H. pylori-infected mice on the high-salt diet showed significantly higher gastric bacterial density (CFU/mL) than did mice on the basal diet. The mean H. pylori colonization in mice on the basal diet was 2.80×105 CFU/mL, whereas bacterial density in mice on the high-salt diet was 5.14 × 105 CFU/mL (P < 0.05). The bacterial density was also significantly increased in mice that were on the high-salt diet and inoculated 5 times (Fig. 2A). However, histopathological H. pylori density score was not increased significantly by repeated inoculation and high-salt diet (Fig. 2B).
Figure 2.
Helicobacter pylori (H. pylori) colonization of the gastric mucosa as determined by quantitative culture in mice on a basal or high-salt diet (A). The histopathological H. pylori density score on a basal or high-salt diet (B). Culture and infection rate of H. pylori according to the number of inoculations (C). BD, basal diet; HSD, high-salt diet. *P<0.05 vs. basal diet.
2. Helicobacter culture rate increased by inoculation times
The H. pylori culture rate was significantly higher in the group that had been inoculated 5 times than in the group that had been inoculated 4 times. The H. pylori infection rate (positive culture or positive H&E stain) was high in infected mice that had been fed on either the basal diet or the high-salt diet (91.3% in the group that had been inoculated 4 times and 100% in the group that had been inoculated 5 times; Fig. 2C). H&E stain indicated that H. pylori was located in the gastric pit (Fig. 3).
Figure 3.
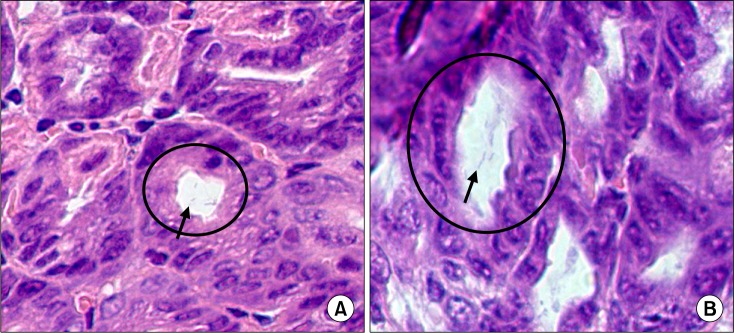
Helicobacter pylori colonization on gastric mucosa in C57BL/6 mice (H&E stain); ×200 (A), ×1000 (B). The spiral bacteria were observed in gastric pits at week 4 after inoculation (arrow).
3. The high-salt diet and repeated inoculation did not increase inflammation scores
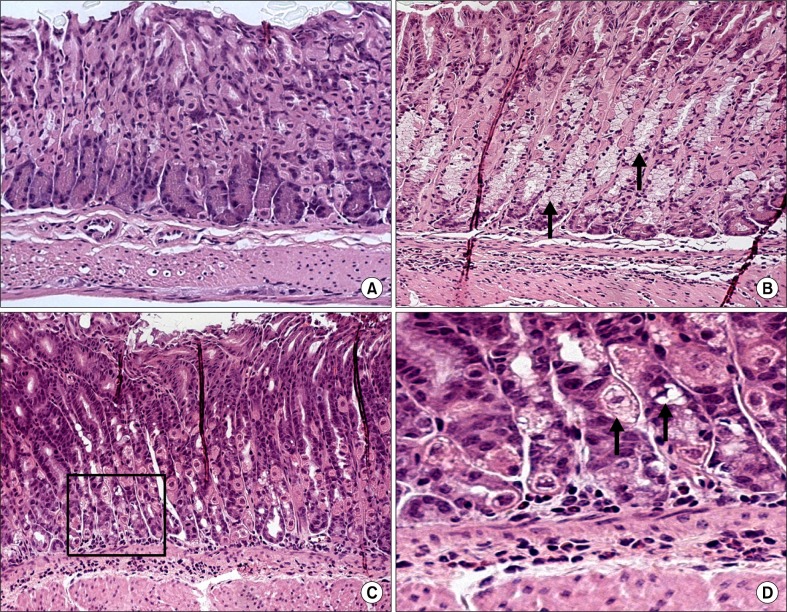
In histopathological scores, the neutrophil grade of mice fed on a high-salt diet was not significantly different from that of mice fed on the basal diet, for both uninfected and infected mice (Fig. 4A). However, the monocyte grade was lower in the infected, high-salt diet group than in the infected, basal diet group, although this result was not statistically significant (Fig. 4B). The group that had been inoculated 4 times had a slightly elevated neutrophil grade, although the difference was not statistically significant (Fig. 5A), and the monocyte grade was reduced in mice that had been inoculated 5 times and fed on a high-salt diet (Fig. 5B). Compared with histologically normal mice (Fig. 6A), H. pylori-infected mice on both diets developed chronic active gastritis with mucous metaplasia (Fig. 6B–D).
Figure 4.
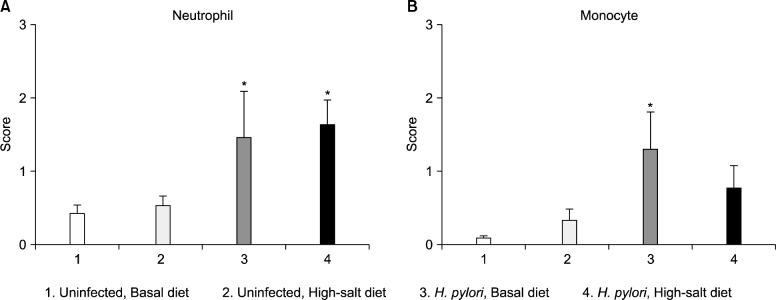
Gastric histopathology scores according to diet and Helicobacter pylori (H. pylori) infection status regardless of inoculation times. The degree of neutrophil infiltration (A), mononuclear cell infiltration (B) (y-axis) was assessed according to the updated Sydney classification as follows: 0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked. Mice were divided into 4 groups according to the diet and H. pylori infection status (x-axis). *P<0.05 vs. uninfected group.
Figure 5.
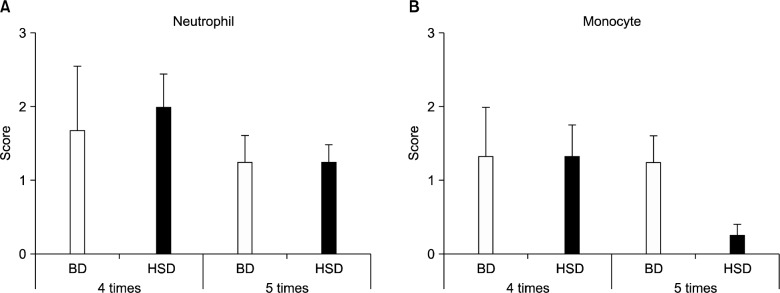
Gastric histopathology scores according to the number of inoculations. The degree of neutrophil infiltration (A), mononuclear cell infiltration (B) (y-axis) was assessed according to the updated Sydney classification as follows: 0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked. BD, basal diet; HSD, high-salt diet; 4 times, 4 times inoculation; 5 times, 5 times inoculation.
Figure 6.
Normal gastric mucosa (A) and mucous metaplasia and lymphoid aggregation in the gastric mucosa of Helicobacter pylori (H. pylori) infected C57BL/6 mice on a basal diet (B) and a high-salt diet (C, D) 4 weeks after inoculation (H&E stain); ×100 (A–C), ×200 (D). The glandular epithelium has been replaced by a hyperplastic, hypertrophic mucous epithelium (arrow and black square).
4. The high-salt diet and repeated inoculation did not correlate with inflammatory markers
The gastric mucosal MPO level was significantly increased in the group that had been inoculated 4 times as compared with uninfected mice. However, the use of 5 inoculations and a high-salt diet did not increase MPO levels (Fig. 7A). In addition, there was no correlation between the use of repeated inoculations with a high-salt diet and gastric mucosal TNFα levels (Fig. 7B).
Figure 7.
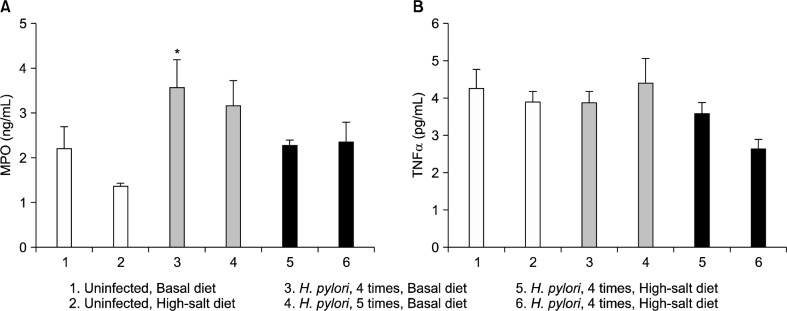
Gastric mucosal myeloperoxidase (MPO, ng/mL) (A) and tumor necrosis factor alpha (TNFα, pg/mL) (B) levels according to the diet, Helicobacter pylori (H. pylori) infection status and the number of inoculations. *P<0.05 vs. uninfected group.
DISCUSSION
This study demonstrated the development of H. pylori-induced gastric inflammation in response to various stimuli, including repeated inoculation and administration of a high-salt diet, in an attempt to establish an experimental mouse model for H. pylori infection using C57BL/6 mice. Before the main experiment, we conducted several preliminary tests (data not shown) to find the best mouse conditions. First, we compared 4-week-old BALB/c mice with 4-week old C57BL/6 mice and concluded that H. pylori was well colonized and the resulting inflammatory reaction was well established in C57BL/6 mice. Secondly, we compared 7-week-old with 4-week-old C57BL/6 mice. The neutrophil grade was almost the same in both groups, although the monocyte grade was significantly higher in the 4-week-old mice group. Third, we observed 4-week-old C57BL/6 mice for 4, 8, and 12 weeks after inoculation, respectively, to determine the appropriate infection period, and observed that inflammation decreased after 4 weeks. Fourth, we found that feeding infected mice with the basal diet during the inoculation period and then switching them to the high-salt diet was the best method because when the period of the high-salt diet was long, the body weight of the mice markedly decreased. In addition, we found that H. pylori SS1 strain was better colonized than the other 2 strains (TN2GF4 and ATCC43504) compared in C57BL/6 mouse stomach. From these results, we concluded that 4-week -old C57BL/6 mice sacrificed 4 weeks after inoculation were best for the H. pylori infection mouse model.
H. pylori SS1 infection of C57BL/6 mice has been regarded as the standard model for the study of chronic infection and host specificity.16,17 The C57BL/6 mouse is the best established and most preferred animal species and can be infected with Helicobacter felis as well as with other different H. pylori strains, which results in a strong Th1 response. BALB/c mice exhibit a higher bacterial burden like C57BL/6, but they tend to polarize toward Th2 response and reveal less epithelial damage.18 In fact, a previous study revealed that although BALB/c mice develop mild duodenitis and gastritis during H. pylori infection, they are not susceptible to developing peptic ulcers.19 When H. pylori SS1 was inoculated in BALB/c mice, only 5% of bacteria was colonized in antrum and the BALB/c mice developed chronic gastritis or atrophy less frequently than the C57BL/6 mice.4 Therefore, the C57BL/6 mice seem to be more suitable as an H. pylori animal model, with a high colonization rate and similar pathological results as in human gastric experiments.20,21
Although Ferrero et al.22 revealed that even a single inoculation of SS1 with 1.2 × 107 CFU/mL could colonize in 100% of mice stomachs, most of previous studies had inoculated H. pylori SS1 for 3 times at 2 days intervals.14,15,19–21 However in the present study we tried to inoculate H. pylori 4 or 5 times to enhance bacterial colonization and 5 times inoculation produced higher bacterial density than 3 or 4 times inoculation and the culture rate was more than 90% when inoculated 5 times. To prove successful infection, H. pylori culture from the scratched mice stomach was performed in this study and satisfactory amounts of colonies were founded. However, considering the possibility of ineffective growth of H. pylori by another microorganism contamination or insufficient mucosal specimen, additional H&E staining was done. A positive H. pylori infection was confirmed by use of any of these 2 methods and the infection rate was revealed to be 91.3% and 100% in 4 and 5 times inoculated group, respectively.
Salt-associated inflammation and carcinogenesis studies have been performed in rats and mice, and the concentrations of sodium varied from 0.7% to 20%. Mice fed on a diet containing 7% sodium developed both acute and chronic gastritis. Another study showed that mice fed on excessive salt diet over a period of time developed significant glandular atrophy.23 It has been demonstrated that a sodium-deficient diet restricts tumor growth and that the antineoplastic activity of certain anticancer agents decreases in mice treated with a salted vehicle.24 In another study, C57BL/6 mice infected with H. pylori SS1 for 4 months, and maintained on a high-salt diet exhibited higher gastric urease activity, serum gastrin, foveolar hyperplasia, and colonization levels than did those on a basal diet. However, there was no meaningful effect of high-salt diet on gastric inflammation or oxyntic atrophy scores.14 In this study, H. pylori was well colonized in the stomachs of the mice, especially of those in the high-salt diet group; however, the inflammation scores did not increase accordingly. The increased colonization density of H. pylori in high-salt diet-fed mice could be due to several possible mechanisms. Gastrin appears to be a H. pylori-specific growth factor in vitro. Fox et al.14 demonstrated that serum gastrin concentrations were significantly increased in H. pylori-infected mice and further elevated in mice fed on high-salt diets. Another factor in the increase of H. pylori colonization may be the induction of foveolar hyperplasia, which is observed in mice fed high-salt diets. Thus, high-salt diets may synergize with gastric H. pylori infections through expansion of cells in the area where H. pylori colonizes. Further experimental studies should be needed to clarify the association among high-salt diet, H. pylori infection and gastric carcinogenesis.
The strengths of this study are as follows: through a long period including preliminary studies, various conditions were evaluated and we were able to find the best inflammation condition. Another strong point is the classification of H. pylori-induced mouse gastritis with an update Sydney System by an experienced pathologist.
In conclusion, H. pylori was found to be relatively well colonized in the stomachs of C57BL/6 mice, but the inflammation was not aggravated despite the use of various methods such as the administration of a high-salt diet or increasing the number of inoculations. To develop methods to increase the level of gastric inflammation, further trials are planned for this mouse model of H. pylori infection.
Acknowledgments
This work was supported by grant no 06-2011-167 from the Seoul National University Bundang Hospital Research fund.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukamoto T, Toyoda T, Mizoshita T, Tatematsu M. Helicobacter pylori infection and gastric carcinogenesis in rodent models. Semin Immunopathol. 2013;35:177–90. doi: 10.1007/s00281-012-0357-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–23. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 5.Sakagami T, Dixon M, O’Rourke J, Howlett R, Alderuccio F, Vella J, et al. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–48. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–45. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Rourke JL, Lee A. Animal models of Helicobacter pylori infection and disease. Microbes Infect. 2003;5:741–8. doi: 10.1016/s1286-4579(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Lee A, Hazell S. Immunisation against gastric Helicobacter infection in a mouse/Helicobacter felis model. Lancet. 1992;339:1120–1. doi: 10.1016/0140-6736(92)90720-n. [DOI] [PubMed] [Google Scholar]
- 9.Czinn SJ, Nedrud JG. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59:2359–63. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuyns AJ. Salt and gastrointestinal cancer. Nutr Cancer. 1988;11:229–32. doi: 10.1080/01635588809513992. [DOI] [PubMed] [Google Scholar]
- 11.Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res. 1985;76:705–16. [PubMed] [Google Scholar]
- 12.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, et al. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer. 2004;7:46–53. doi: 10.1007/s10120-004-0268-5. [DOI] [PubMed] [Google Scholar]
- 13.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, et al. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942–50. [PubMed] [Google Scholar]
- 14.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–8. [PubMed] [Google Scholar]
- 15.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 16.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF.A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain Gastroenterology 1997; 1121386–97. [DOI] [PubMed] [Google Scholar]
- 17.Krueger S, Roessner A, Kuester D.Murine models of H. pylori-induced gastritis and gastric adenocarcinoma Pathol Res Pract 2011; 207599–607. [DOI] [PubMed] [Google Scholar]
- 18.Rogers AB, Fox JG. Inflammation and Cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol. 2004;286:G361–6. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- 19.Rabelo-Goncalves EM, Nishimura NF, Zeitune JM. Development of a BALB/c mouse model of Helicobacter pylori infection with fresh and frozen bacteria. Biol Res. 2005;38:101–9. doi: 10.4067/s0716-97602005000100012. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Kim SW, Song YJ, Oh TY, Han SU, Kim YB, et al. Long-term evaluation of mice model infected with Helicobacter pylori: focus on gastric pathology including gastric cancer. Aliment Pharmacol Ther. 2003;18(Suppl 1):14–23. doi: 10.1046/j.1365-2036.18.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor NS, Fox JG. Animal models of Helicobacter-induced disease: methods to successfully infect the mouse. Methods Mol Biol. 2012;921:131–42. doi: 10.1007/978-1-62703-005-2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrero RL, Thiberge JM, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–55. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama M, Kodama T, Suzuki H, Kondo K. Effect of rice and salty rice diets on the structure of mouse stomach. Nutr Cancer. 1984;6:135–47. doi: 10.1080/01635588509513817. [DOI] [PubMed] [Google Scholar]
- 24.Fine BP, Ponzio NM, Denny TN, Maher E, Walters TR. Restriction of tumor growth in mice by sodium-deficient diet. Cancer Res. 1988;48:3445–8. [PubMed] [Google Scholar]