Abstract
Cognitive deficits associated with frontal lobe dysfunction are a determinant of long-term disability in schizophrenia and are not effectively treated with available medications. Clinical studies show that many aspects of these deficits are transiently induced in healthy individuals treated with N-methyl-d-aspartate (NMDA) antagonists. These findings and recent genetic linkage studies strongly implicate NMDA receptor deficiency in schizophrenia and suggest that reversing this deficiency is pertinent to treating the cognitive symptoms of schizophrenia. Despite the wealth of behavioral data on the effects of NMDA antagonist treatment in humans and laboratory animals, there is a fundamental lack of understanding about the mechanisms by which a general state of NMDA deficiency influences the function of cortical neurons. Using ensemble recording in freely moving rats, we found that NMDA antagonist treatment, at doses that impaired working memory, potentiated the firing rate of most prefrontal cortex neurons. This potentiation, which correlated with expression of behavioral stereotypy, resulted from an increased number of irregularly discharged single spikes. Concurrent with the increase in spike activity, there was a significant reduction in organized bursting activity. These results identify two distinct mechanisms by which NMDA receptor deficiency may disrupt frontal lobe function: an increase in disorganized spike activity, which may enhance cortical noise and transmission of disinformation; and a decrease in burst activity, which reduces transmission efficacy of cortical neurons. These findings provide a physiological basis for the NMDA receptor deficiency model of schizophrenia and may clarify the nature of cortical dysfunction in this disease.
N-methyl-d-aspartate (NMDA) receptors are increasingly implicated in schizophrenia. The majority of genes that have so far been linked to increased susceptibility to develop schizophrenia can modulate NMDA receptor-mediated signal transduction (1, 2), suggesting that NMDA receptors are a critical component of genetic vulnerability to develop schizophrenia. Recreational or investigator-administered exposure to drugs that have antagonist activity at NMDA receptors produces a transient state of psychosis and schizophrenia-like cognitive deficits (3–5). In fact, double-blind clinical studies suggest that the cognitive deficits produced by NMDA antagonists in healthy volunteers are nearly identical to the deficits observed in patients with schizophrenia (6). Based on these pharmacological and genetic linkage studies, the NMDA antagonist treatment is considered a mechanistically relevant model for cognitive deficits of schizophrenia (7–10). Understanding the impact of this treatment on cellular mechanisms that support the expression of abnormal cognition is critical for understanding the impact of an underlying NMDA dysfunction on the disease process and for defining better treatments. However, despite a large body of literature that has characterized the behavioral aspects of this model in laboratory animals, there remains a fundamental lack of understanding about the cellular mechanisms that lead to the disruption of cognitive functioning.
Functional imaging studies suggest that, after systemic administration of NMDA antagonists, the most affected brain region is the prefrontal cortex (PFC) (11, 12). Animal studies also suggest that the PFC is a key region responsible for the expression of behaviors that are relevant to schizophrenia, including working memory and behavioral stereotypy (13, 14). This is consistent with a large body of functional and postmortem literature implicating PFC abnormalities in schizophrenia (15–17) and the fact that the most prevalent cognitive dysfunctions reported in schizophrenia involve constructs that depend on the functional integrity of the PFC, such as working memory (18). The aim of the present study was to define the mechanisms by which behaviorally relevant reductions in NMDA receptor function may disrupt the pattern of activity of PFC neurons. We used ensemble recording in freely moving animals and post hoc analysis of spike activity to determine the effect of the use-dependent NMDA antagonist MK801, at doses that we found to impair working memory, on the firing characteristics of PFC neurons. Considering that behavioral stereotypy, which is induced by NMDA antagonist treatment (9, 19), is a critical component of cognitive and motor abnormalities in schizophrenia, we also quantified behavioral stereotypy during the recording sessions.
Experimental Procedures and Methods
General Animal Use and Electrophysiology Procedure. Male Sprague–Dawley rats (340–420 g) were used. All procedures were approved by the Institutional Animal Care and Use Committee. Arrays of eight stainless steel Teflon-insulated microwires (50-μm diameter, NB Labs, Denison, TX) were implanted under halothane anesthesia in the prelimbic PFC: 3.0–3.6 mm anterior and 0.5–0.8 lateral to Bregma, and 3.5 mm ventral to the dorsal surface of the brain (20). Animals were allowed to recover for 1 week. They were then acclimated to handling and recording procedures for 1 week before the first recording session. Recordings were performed in the animal's home cage (clear polycarbonate 44 × 22 × 42 cm), placed inside a Faraday cage. Animals were connected to an eight-channel connecter containing FET headstages (NB Labs) via a commutator (NB Labs) that permitted unrestricted movement during recording.
Spontaneous unit activity was recorded by using multiple channel amplifiers with 500× gain and 220- to 5.9-kHz bandpass filters (Plexon, Dallas). The amplified signal from each electrode was digitized (30 kHz), and continuous data files were saved on a PC for spike sorting using off-line sorter software (Plexon). Neurons were selected as single units only if autocorrelograms and interspike interval histograms indicated there were no significant errors in sorting due to noise or similar waveforms. Typically, two to three neurons were isolated from each electrode.
All rats received, in random order, a maximum of four of five possible i.p. injections, which included saline and four doses of MK801 (0.01, 0.05, 0.1, and 0.3 mg/kg). Injections were made at least 1 week apart.
Analysis of Electrophysiology Data. General information. Electrophysiological data were imported into neuroexplorer (Plexon) for analysis. Only putative single units with autocorrelograms and interspike interval histograms consistent with an absolute refractory period of at least 1.1 msec were considered “single neurons.” Based on established criteria of firing rates and autocorrelogram statistics (21–23), we divided units into two groups that correspond to descriptions (24) of fast-spiking neurons (FS, putative interneurons) and regular spiking neurons (RS, putative pyramidal neurons). In general, FS cells had spike waveforms with large after-hyperpolarizations (unlike RS cells), high firing rates (>10 Hz), and autocorrelograms corresponding to a tonic firing pattern. RS neurons had lower firing rates (<5 Hz) and autocorrelograms consistent with a sporadic firing pattern.
Firing rate analysis. Firing rate statistics were calculated by using firing rate histograms (5-min bins) produced in neuroexplorer, and the results were imported into matlab (Math-Works, Natick, MA) for further analysis. For each unit, mean baseline spontaneous firing rates and 99% confidence intervals were calculated for the 30-min baseline period. Neurons were then grouped into one of three response categories depending on whether they had a significant increase (type 1), decrease (type 2), or no change (type 3) in spontaneous firing rate after systemic injection. Criterion for determining significant changes from baseline firing rates was two consecutive 5-min bins exceeding the 99% confidence intervals (greater than for type 1, less than for type 2). Criterion for terminating a significant change from baseline was two consecutive bins within the 99% confidence interval. χ2 tests were used to determine significant changes in the proportion of response types for different groups.
To compare neurons with different baseline firing rates, rate histograms were normalized by dividing each 5-min bin by the average of the bins during the 30-min baseline period for each individual neuron. Normalized rate histograms were compared across different groups by using one-way repeated-measures ANOVA with time as the repeated measure.
For neurons with type 1 or type 2 responses, we also measured the mean area of the normalized rate histogram for the duration of the response for each group. Changes in the mean area were compared across groups by a one-way ANOVA with the Bonferroni post hoc test.
Spontaneous burst analysis. The Poisson surprise method of Legendy et al. (25), as implemented in neuroexplorer was used to detect burst activity. The Poisson surprise method detects bursts by finding consecutive interspike intervals (ISI) that are less than half the mean ISI, and testing whether these ISI would be expected if the spike train were a Poisson process with random temporal patterning. Thus, the Poison surprise algorithm allows for differentiation between irregular, single spike patterns and bursting patterns contained within the same spike train.
Several parameters of bursts were measured for each spike train, including percentage of spikes that occur within bursts, mean number of bursts per minute, mean number of spikes per burst, and mean intraburst frequency. Changes in burst parameters were measured in two ways. First, burst parameters were measured in each individual spike train during the 2-h postinjection period. Comparisons of burst parameters between groups and response types were made by using ANOVA with the Bonferroni post hoc test. Second, a more detailed temporal analysis was done by measuring burst parameters for each neuron during the 30-min preinjection baseline period, and during four sequential 30-min postinjection periods. For each group, data pairs were produced for each neuron by using the value of one burst parameter during the baseline period (independent variable) and the value for the same parameter during one of the four postinjection periods (dependent variable). First-order linear regression was used to determine whether there were significant changes between baseline and postinjection burst parameters. The constant term was set to zero to force the regression line to pass through the origin. The quality of fit by linear regression was measured by Student's t test (P < 0.05). Changes in burst parameters relative to the baseline period were measured by changes in the regression coefficient (±SEM). A regression coefficient of 1.0 indicates that there is no change in that parameter compared to the baseline measure, whereas values <1.0 indicate a decrease, and values >1.0 indicate a significant increase. Regression coefficients were calculated during each of the four sequential 30-min postinjection periods relative to baseline so that the time course of response could be evaluated.
Recording and Analysis of Behavioral Stereotypy. Throughout the recording period, behavioral stereotypy was rated every 5-min. Animals received a score of “0” for absence or “1” for presence of each of the following behaviors: ambulation, freezing, turning, grooming, sniffing up, sniffing down, mouth movements, jaw tremor, bed digging, head wagging, and rearing. Behaviors were scored if they were expressed for >30 s. The rater was not blind to the experimental manipulations. Stereotypy scores were calculated by summing scores for individual behaviors during each 5-min block of time for each rat. Total stereotypy scores were calculated by summing scores across all time blocks. Differences between groups were analyzed by ANOVA with repeated measures. Stereotypy scores recorded during each 5-min bin were compared with firing rate changes during the same 5-min bins for each rat by using Pearson correlation with the Bartlett χ2 test for significance.
Spontaneous Alternation Test. Animals were habituated by daily handling for 1 week. They were injected with vehicle or MK801 40 min before testing, which was conducted by placing the animal on the center platform of a plus maze and allowing 12 min of unimpeded exploration. The plus maze was constructed of Plexiglas (0.63 cm thick) and painted gray. It consisted of a central platform (14 cm × 14 cm) to which four arms (14.0 cm wide, 40.6 cm long, and 12.7 cm high) were joined with no space between adjacent arms. The number and sequence of arm entries was recorded for calculation of a percent alternation score. An alternation consisted of four different arm choices out of five consecutive arm entries. A 4/5 alternation score was computed by dividing the number of alternations in overlapping quintuplets by the total number of possible alternations, and multiplying the quotient by 100. These data were analyzed by one-way ANOVA with Bonferroni-adjusted post hoc tests.
Histology. Animals were anesthetized with chloral hydrate and perfused with saline followed by 10% buffered formalin. Brain sections (150 μm) were stained with cresyl violet for electrode placement verification. All recordings reported here were from layers V and VI of ventral prelimbic cortex.
Results
Baseline and Postinjection Characteristics of PFC Neurons. A total of 518 neurons were isolated from eight rats in 27 recording sessions. Neurons were separated into 510 RS neurons (putative pyramidal neurons) and eight FS neurons (putative interneurons), based on established criteria (21–23). The mean baseline firing rates were 1.32 ± 0.07 Hz for RS neurons and 13.6 ± 1.04 Hz for FS neurons. Because of the overall paucity of FS cells, we could not perform an accurate statistical analysis of their response to MK801. Therefore, only the results from the RS cells are depicted in figures.
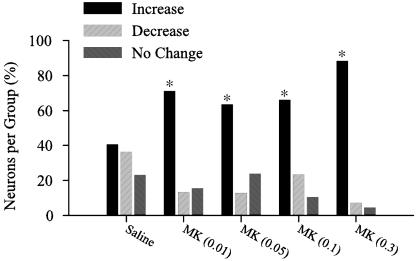
Three types of postinjection responses were identified in RS neurons: increased firing rate (type 1), decreased firing rate (type 2), or no change in firing rate (type 3). After saline injection, an approximately equal proportion of neurons responded with either an increase or decrease in firing rate, and 21% of the neurons had no significant change in firing rate (Fig. 1). However, after MK801 injection, there was a greater percentage of type 1 neurons (χ2 test = 61.08, df = 8, p < 0.00001). All FS cells had type 1 responses.
Fig. 1.
Percentage of neurons with increase, decrease, or no change in firing rate. There was a significant increase in the percentage of neurons with postinjection increases in firing rate after MK801 (0.01 mg/kg, 0.05 mg/kg, 0.1 mg/kg, and 0.3 mg/kg) compared to saline. Asterisks indicate significant difference by χ2 (P < 0.01) compared to saline. Note that this pattern of response did not generalize to other psychoactive drugs such as amphetamine (Fig. 7, which is published as supporting information on the PNAS web site).
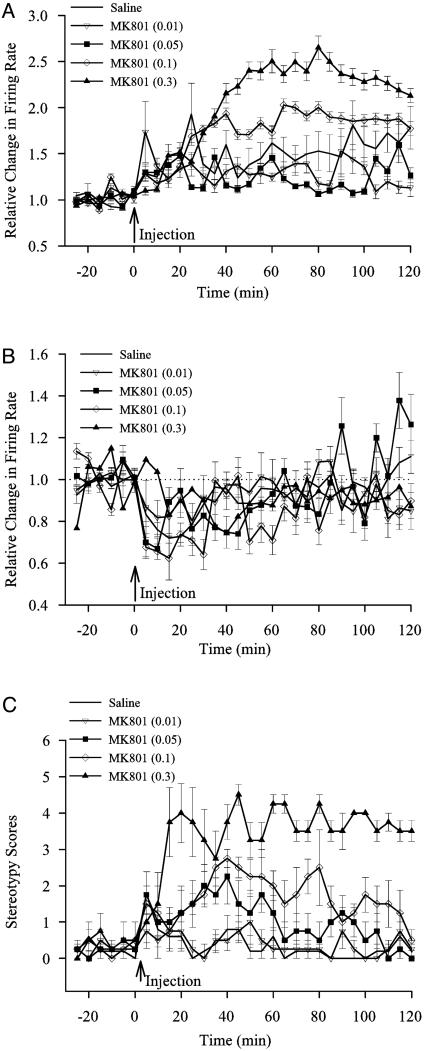
Temporal Profile of Firing Rate and Behavioral Changes After MK801. In neurons with type 1 responses (Fig. 2A), there was an overall effect of dose on firing rate (F4,312 = 26.9, P < 0.00001). When compared to saline, there was a significant sustained increase in firing rate after MK801 injection at 0.1 mg/kg (F1,123 = 4.46, P < 0.037) and 0.3 mg/kg (F1,144 = 18.89, P < 0.00003). Low doses of MK801 were not different from saline. Thus, although a greater number of neurons exhibited increased firing rate in low dose MK801 groups as compared to the saline group (see Fig. 1), the magnitude of this increase was similar in both groups. Neurons with type 2 responses were not significantly different from saline (Fig. 2B).
Fig. 2.
Temporal profile of firing rate changes and behavioral activity after systemic MK801 injection. Peristimulus rate histograms (5-min bins) were normalized by dividing the postinjection firing rates by the baseline firing rate. (A) Neurons with type 1 responses had a sustained increase in firing rate for high doses of MK801. (B) Neurons with type 2 responses were not significantly different from saline. (C) MK801 at moderate and high doses significantly increased stereotypy scores.
MK801 at moderate doses is known to produce behavioral stereotypy in rats involving ambulation, rearing, head wag, and oral movements (Fig. 2C). A significant increase in stereotypy was observed in groups that received MK801 at 0.05 mg/kg (F1,7 = 5.46, P < 0.05), 0.1 mg/kg (F1,7 = 22.15, P < 0.002), and 0.3 mg/kg (F1,7 = 85.23, P < 0.00005).
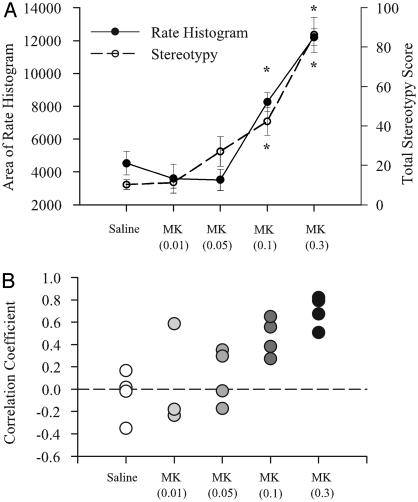
The mean area under the normalized postinjection rate histogram (Fig. 3A) significantly increased after the two highest doses of MK801 (F4,312 = 40.35, P < 0.00001). In addition, the total stereotypy score also increased at those doses (F4,16 = 26.76, P < 0.001). The correlation coefficients depicted in Fig. 3B indicate a poor correlation between behavior and firing rate for saline and MK801 at 0.01 mg/kg and 0.05 mg/kg, but a high correlation between firing rate and behavior at the two higher doses of MK801, suggesting an association between increases in PFC neural activity and increases in stereotypy.
Fig. 3.
Comparison of firing rate changes and behavioral changes after systemic MK801 injection. (A) The mean area under the normalized postinjection rate histogram for type 1 neurons in each group is plotted on the left vertical axis. The area gives an indication of the total neural activity during this period. Total stereotypy score is plotted on the right vertical axis. Increases in neural activity at the two highest doses of MK801 are highly correlated with increases in stereotypy. (B) Plot of correlation between firing rate increases and stereotypy scores for individual experiments. Stereotypy scores recorded in 5-min time bins were correlated with the averaged rate histogram (5-min bins) of all type 1 neurons recorded in that same animal. There were poor correlations between behavior and firing rate for saline and MK801 at 0.01 mg/kg and 0.05 mg/kg, but high correlations at the two higher doses of MK801. Asterisks indicate significant difference compared to saline by ANOVA with the Bonferroni post hoc test (P < 0.05).
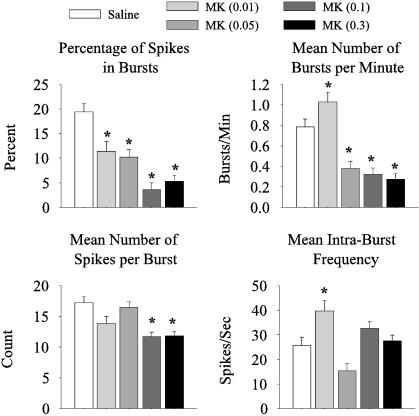
MK801-Induced Changes in Burst Patterns. As demonstrated on Fig. 4, in neurons with increased firing rate after MK801 injection, there was a dose-dependent decrease in the percentage of spikes that occurred within bursts (F4,312 = 16.4, P < 0.00001), a decrease in the mean number of bursts per min (F4,312 = 5.3, P < 0.00001), and a decrease in the mean number of spikes per burst (F4,312 = 9.96, P < 0.00001). These findings suggest that the increase in activity at the high doses of MK801 is caused by discharge of random spikes that are not clustered in bursts. In contrast, the 0.01 mg/kg MK801 group exhibited a significant increase in the mean number of bursts per min (Bonferroni post hoc test, P < 0.05) and mean intraburst frequency (F4,312 = 7.04, P < 0.00002, Bonferroni post hoc test, P < 0.05). Detailed burst analysis of the putative FS neurons in our sample could not be performed because of the small sample size, but they had similar trends.
Fig. 4.
Changes in burst parameters after MK801 injection. For neurons that had an increase in firing rate after MK801 injection, there was a dose-dependent decrease in the percentage of spikes in bursts, mean number of bursts per min, and mean number of spikes per burst. However, for neurons in the 0.01 mg/kg MK801 group, there was a significant increase in the mean number of bursts per min and mean intraburst frequency. Asterisks indicate significant difference compared to saline by ANOVA with the Bonferroni post hoc test (P < 0.05).
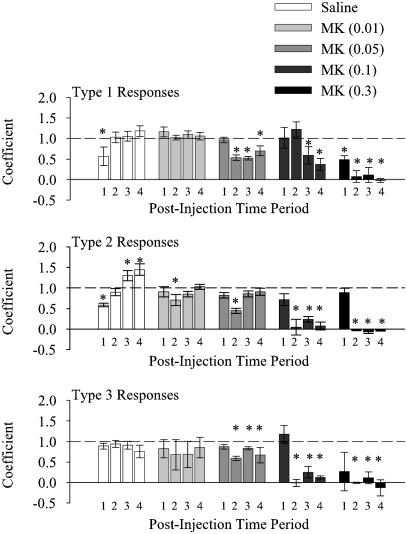
Dose-dependent analysis of burst activity using ANOVA could not be performed reliably for neurons that had a decrease or no change in firing rate because too few neurons fell into these categories at the higher doses of MK801. Thus, we used linear regression to analyze the changes in the percentage of spikes that occur in bursts for the three response types (Fig. 5). This method of analysis also allowed us to compare the time course of changes in individual spike trains from baseline activity by determining regression coefficients during sequential 30-min periods. At higher doses of MK801 (0.05, 0.1, 0.3 mg/kg), there was a significant reduction in the percentage of spikes in bursts for each of the three time periods beginning 30 min after injection. Thus, MK801, at doses of 0.05 mg/kg and greater, caused a decrease in burst activity regardless of whether the neuron had an increase, decrease, or no change in firing rate during the same period (Fig. 5). Multiple linear regression showed no significant effect of changes in mean firing rate or of response type, but there was a significant effect of group (t = -9.148, P < 0.0001).
Fig. 5.
The percentage of spikes contained within bursts decreased with high doses of MK801. The percentage of spikes contained within bursts was calculated for each neuron during a 30-min baseline period and during four sequential 30-min postinjection periods. Data for all groups and response types is summarized by plotting the linear regression coefficients. Numbers under each bar indicate the postinjection time period as follows: lanes 1, 0–30 min; lanes 2, 30–60 min; lanes 3, 60–90 min; and lanes 4, 90–120 min. Higher doses of MK801 caused a significant decrease in the percentage of neurons in bursts, regardless of the response type. Asterisks indicate significant difference from a regression coefficient of 1.0, which indicates no change from baseline.
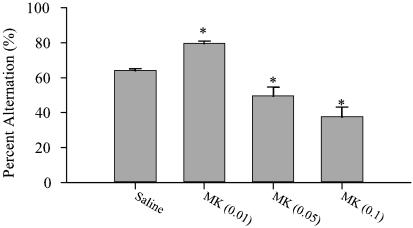
Dose-Dependent Effect of MK801 on Spontaneous Alternation. To assess the relevance of doses of MK801 used in the electrophysiology recordings to cognitive functioning, we examined the dose-dependent effect of MK801 on spontaneous alternation, a test of spatial working memory in rodents (26). MK801 at 0.05 and 0.1 mg/kg produced a significant decrease in percent alternation (ANOVA, F3,30 = 21.6, P < 0.0001), indicating impaired working memory (Fig. 6). However, the low dose of MK801 (0.01 mg/kg) significantly improved performance. The decreased alternation rate at the high doses was not associated with decreased mobility because the total number of arm entries at these doses did not decrease and, in fact, increased after the highest dose of MK801 (ANOVA, F3,30 = 6.32, P < 0.002) total arm entries. (The profound motor stereotypy produced by the highest dose of MK801 precluded animals from completing the task.)
Fig. 6.
Effect of MK801 on spontaneous alternation performance. (A) There was a differential dose-dependent effect of MK801 on percent alternation. The lowest dose of MK801 (0.01 mg/kg) improved performance, whereas higher doses (0.5 and 0.1 mg/kg) impaired performance. Asterisk indicates significant difference by ANOVA with the Bonferroni post hoc test (P < 0.05).
Discussion
The NMDA antagonist treatment model has excellent face validity for cognitive symptoms of schizophrenia because a single exposure to these drugs impairs PFC-dependent cognitive functions in a manner that is similar to schizophrenia (3, 5, 6, 10, 27). Recent postmortem and genetic linkage studies, which implicate NMDA receptor dysfunction in schizophrenia, suggest that this model also has construct validity for schizophrenia (1, 2, 7, 8). Thus, understanding the mechanisms that subserve NMDA antagonist-mediated disruption of PFC function is critical for understanding the neuronal basis of cognitive deficits of schizophrenia, and for the design of treatments for these deficits. Toward this aim, this study presents analysis of the dose-dependent effect of an NMDA antagonist on the firing characteristics of PFC neurons in freely moving animals. We found that the NMDA antagonist MK801, at doses that impaired working memory performance, produced a sustained firing rate potentiation in the majority of PFC neurons. However, this potentiation was not caused by an increase in the number of action potentials discharged in bursts but instead resulted from an increase in the number of randomly distributed single spikes. Administration of MK801, in fact, produced a profound reduction in burst activity as evident by a dose-dependent reduction in the total number of bursts, mean number of bursts per minute, and the mean number of spikes within each burst.
These findings define possible mechanisms that may underlie the psychotomimetic and cognitive impairing effects of NMDA antagonists, and cortical dysfunction in schizophrenia. Specifically, bursts are more effective than single spikes at enhancing neurotransmitter release and information processing (28–30). On the other hand, single spikes may not merely produce a weaker signal but have been proposed to produce “noise” that impairs filtering of irrelevant information and transmission of misinformation (31). Thus, the concomitant increase in random spike activity and decrease in burst activity would be expected to profoundly disrupt PFC function. Specifically, reduced burst activity attenuates the signal transmission efficiency of PFC neurons and diminishes cortical mediation of normal behavior, whereas at the same time increased random spike activity may impair filtering of irrelevant information and lead to expression of abnormal behavior. The latter mechanism is supported by our observation that behavioral stereotypy correlates with the increase in random spike activity.
The sustained firing rate potentiation of PFC neurons by an NMDA antagonist is somewhat unexpected because this effect contradicts the anticipated outcome that blocking one of the major excitatory receptors of the brain would lead to postsynaptic inhibition and reduction in spontaneous firing. NMDA antagonists, in fact, reduce spontaneous activity in anesthetized animals (32). However, the significance of awake animal studies is that the impact of physiologically active circuits on the behavior of neurons can be revealed. We have previously reported that systemic administration of NMDA antagonists increases extracellular levels of glutamate in the PFC of awake animals (33, 34). A disinhibition process, i.e., NMDA antagonist-induced blockade of γ-aminobutyric acid (GABA) input to cortical glutamate afferents, is thought to result in this increase (10, 33, 35, 36). In agreement with an enhancement in cortical glutamate release, functional imaging studies have shown an increase in metabolic activity in the human PFC after exposure to the NMDA antagonist ketamine (11, 37). The increase in glutamate efflux by NMDA antagonists can stimulate cortical α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which would lead to the general increase in spike activity that was observed here. The notion that cortical AMPA receptors are activated by systemic NMDA antagonist treatment is supported by previous studies demonstrating that application of an AMPA antagonist into the PFC blocks the behavioral and neurotoxic effects of NMDA antagonists (10, 14, 33).
Activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and the resulting increase in spike activity, however, would not be expected to increase burst activity if NMDA receptor channels are blocked. It is generally thought that bursting arises from stimulation of NMDA receptors and opening of l-type calcium channels, which leads to sustained depolarization and increased probability of action potential generation in response to subsequent synaptic events (24, 38). Thus, the lack of sustained NMDA receptor-mediated membrane depolarization would decrease the probability of successive action potential generation, and reduce the number of closely spaced spikes and burst activity (39–42).
It should be emphasized that, in general, the term “burst” is not uniquely defined and can be described on many time scales (24, 25, 43, 44). The mechanisms that govern burst firing are likely to be a combination of intrinsic neural properties and local network connectivity (24, 38, 45, 46). The bursting that we describe here is a period in a spike train that has a higher discharge rate than other periods in the same spike train. This type of bursting, which most likely results from coordinated excitatory inputs to a neuron, is thought to be critical for organization of behavior because it is more effective than single spikes in releasing neurotransmitters (28–31, 47). Recordings in behaving primates further suggest that switching from single spiking mode to bursting may support the ability of the PFC to predict reward values, to bring memories on-line, and to support decision making and behavioral flexibility (29, 30, 43, 48–50).
Although the focus of our study has been on the effect of MK801 at doses that produce cognitive deficits, an interesting finding of the present study was that a very low dose of MK801 improved working memory performance. This behavioral effect is consistent with the cognitive enhancing properties of the weak NMDA antagonist memantine (51). The low dose of MK801 did not significantly change the spontaneous spike activity of PFC neurons, but in contrast to the effects of higher doses of MK801, it increased the mean number of bursts per min and the intraburst frequency of these neurons. It may be hypothesized that this pattern of response enhances the signal to noise ratio of cortical neurons and improves working memory. Although further studies are necessary to elucidate the mechanisms that support the cognitive enhancing effects of low doses of NMDA antagonists, these findings further suggest that influencing burst activity of PFC neurons is a critical mechanism by which NMDA receptors influence cognitive functioning.
Taken together, the results of this study describe two concomitant mechanisms whereby a state of NMDA deficiency, which we elicited via NMDA antagonist treatment, and which may exist in schizophrenia as a result of active expression of any number of implicated genes (1, 2), might impair PFC function. These mechanisms are: (i) a profound potentiation of disorganized spike activity, which could lead to increased cortical noise and transmission of disinformation, and (ii) a reduction in organized burst activity, which attenuates signal transmission efficiency of the PFC, thus diminishing its role in organization of behavior. These findings have important implications for elucidating the underlying mechanisms that lead to cortical dysfunction in schizophrenia. Moreover, by defining a physiologic basis for NMDA antagonist treatment in behaving animals, these findings will be valuable for the design and testing of treatments for schizophrenia.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NMDA, N-methyl-d-aspartate; PFC, prefrontal cortex; FS, fast spiking; RS, regular spiking.
References
- 1.Harrison, P. J. & Owen, M. J. (2003) Lancet 361, 417-419. [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam, B. (2003) Neuron 40, 881-884. [DOI] [PubMed] [Google Scholar]
- 3.Krystal, J. H., Karper, L. P., Seibyl, J. P., Freeman, G. K., Delaney, R., Bremner, J. D., Heninger, G. R., Bowers, M., Jr., & Charney, D. S. (1994) Arch. Gen. Psychiatry 51, 199-214. [DOI] [PubMed] [Google Scholar]
- 4.Lahti, A. C., Koffel, B., LaPorte, D. & Tamminga, C. A. (1995) Neuropsychopharmacology 13, 9-19. [DOI] [PubMed] [Google Scholar]
- 5.Newcomer, J. W., Farber, N. B., Jevtovic-Todorovic, V., Selke, G., Melson, A. K., Hershey, T., Craft, S. & Olney, J. W. (1999) Neuropsychopharmacology 20, 106-118. [DOI] [PubMed] [Google Scholar]
- 6.Adler, C. M., Goldberg, T. E., Malhotra, A. K., Pickar, D. & Breier, A. (1998) Biol. Psychiatry 43, 811-816. [DOI] [PubMed] [Google Scholar]
- 7.Konradi, C. & Heckers, S. (2003) Pharmacol. Ther. 97, 153-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff, D. C. & Coyle, J. T. (2001) Am. J. Psychiatry 158, 1367-1377. [DOI] [PubMed] [Google Scholar]
- 9.Geyer, M. & Moghaddam, B. (2002) in Psychopharmacology: The Fifth Generation of Progress, eds. Davis, K. L., Charney, C., Coyle, J. T. & Nemeroff, C. (Lippincott Williams and Wilkins, Philadelphia), pp. 689-702.
- 10.Olney, J. & Farber, N. (1995) Arch. Gen. Psychiatry 52, 998-1007. [DOI] [PubMed] [Google Scholar]
- 11.Lahti, A. C., Holocomb, H. H., Medoff, D. R. & Tammings, C. A. (1995) NeuroReport 6, 869-872. [DOI] [PubMed] [Google Scholar]
- 12.Breier, A., Malhotra, A. K., Pinals, D. A., Weisenfeld, N. I. & Pickar, D. (1997) Am. J. Psychiatry 154, 805-811. [DOI] [PubMed] [Google Scholar]
- 13.Jentsch, J. D. & Roth, R. H. (1999) Neuropsychopharmacology 20, 201-225. [DOI] [PubMed] [Google Scholar]
- 14.Takahata, R. & Moghaddam, B. (2003) Neuropsychopharmacology 28, 1117-1124. [DOI] [PubMed] [Google Scholar]
- 15.Goldman-Rakic, P. (1999) Biol. Psychiatry 46, 650-661. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger, D. R., Egan, M. F., Bertolino, A., Callicott, J. H., Mattay, V. S., Lipska, B. K., Berman, K. F. & Goldberg, T. E. (2001) Biol. Psychiatry 50, 825-844. [DOI] [PubMed] [Google Scholar]
- 17.Robbins, T. (1996) Philos. Trans. R. Soc. London B 351, 1463-1470. [DOI] [PubMed] [Google Scholar]
- 18.Goldman-Rakic, P. (1995) Neuron 14, 477-485. [DOI] [PubMed] [Google Scholar]
- 19.Steinpreis, R. (1996) Behav. Brain Res. 74, 45-55. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos, G. & Watson, C. (1986) The Rat Brain in Stereotaxic Coordinates (Academic, San Diego).
- 21.McCormick, D. A., Connors, B. W., Lighthall, J. W. & Prince, D. A. (1985) J. Neurophysiol 54, 782-806. [DOI] [PubMed] [Google Scholar]
- 22.Jung, M., Qin, Y., McNaughton, B. & Barnes, C. (1998) Cereb. Cortex 8, 437-450. [DOI] [PubMed] [Google Scholar]
- 23.Baeg, E. H., Kim, Y. B., Jang, J., Kim, H. T., Mook-Jung, I. & Jung, M. W. (2001) Cereb. Cortex 11, 441-451. [DOI] [PubMed] [Google Scholar]
- 24.Connors, B. W. & Gutnick, M. J. (1990) Trends Neurosci. 13, 99-104. [DOI] [PubMed] [Google Scholar]
- 25.Legendy, C. R. & Salcman, M. (1985) J. Neurophysiol. 53, 926-939. [DOI] [PubMed] [Google Scholar]
- 26.Ragozzino, M. E., Unick, K. E. & Gold, P. E. (1996) Proc. Natl. Acad. Sci. USA 93, 4693-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javitt, D. C. & Zukin, S. R. (1991) Am. J. Psychiatr. 148, 1301-1308. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell, P. & Grace, A. A. (1995) J. Neurosci. 15, 3622-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, E. K. & Cohen, J. D. (2001) Annu. Rev. Neurosci. 24, 167-202. [DOI] [PubMed] [Google Scholar]
- 30.Cooper, D. C. (2002) Neurochem. Int. 41, 333-340. [DOI] [PubMed] [Google Scholar]
- 31.Lisman, J. E. (1997) Trends Neurosci. 20, 38-43. [DOI] [PubMed] [Google Scholar]
- 32.Gratton, A., Hoffer, B. J. & Freedman, R. (1987) Neuropharmacology 26, 1275-1283. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam, B., Adams, B., Verma, A. & Daly, D. (1997) J. Neurosci. 17, 2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moghaddam, B. & Adams, B. (1998) Science 281, 1349-1352. [DOI] [PubMed] [Google Scholar]
- 35.Lorrain, D. S., Baccei, C. S., Bristow, L. J., Anderson, J. J. & Varney, M. A. (2003) Neuroscience 117, 697-706. [DOI] [PubMed] [Google Scholar]
- 36.Greene, R. (2001) Hippocampus 11, 569-577. [DOI] [PubMed] [Google Scholar]
- 37.Abel, K. M., Allin, M. P., Kucharska-Pietura, K., David, A., Andrew, C., Williams, S., Brammer, M. J. & Phillips, M. L. (2003) NeuroReport 14, 387-389. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi, T., Nagao, T., Fukuda, H., Hicks, T. P. & Oka, J. I. (1993) NeuroReport 4, 735-738. [DOI] [PubMed] [Google Scholar]
- 39.Grubb, B. D., Riley, R. C., Hope, P. J., Pubols, L. & Duggan, A. W. (1996) Neuroscience 74, 1077-1086. [DOI] [PubMed] [Google Scholar]
- 40.Poulain, P. (2001) Eur. J. Neurosci. 14, 657-665. [DOI] [PubMed] [Google Scholar]
- 41.Harsch, A. & Robinson, H. P. (2000) J. Neurosci. 20, 6181-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi, W. X. & Zhang, X. X. (2003) J. Pharmacol. Exp. Ther. 305, 680-687. [DOI] [PubMed] [Google Scholar]
- 43.Eggermont, J. J., Smith, G. M. & Bowman, D. (1993) J. Neurophysiol. 69, 1292-1313. [DOI] [PubMed] [Google Scholar]
- 44.Bair, W., Koch, C., Newsome, W. & Britten, K. (1994) J. Neurosci. 14, 2870-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman, A. & Gutnick, M. J. (1987) Neurosci. Lett. 81, 117-122. [DOI] [PubMed] [Google Scholar]
- 46.Steriade, M., Amzica, F. & Contreras, D. (1994) Electroencephalogr. Clin. Neurophysiol. 90, 1-16. [DOI] [PubMed] [Google Scholar]
- 47.Lisman, J., Fellous, J. & Wang, X. (1998) Nat. Neurosci. 1, 273-275. [DOI] [PubMed] [Google Scholar]
- 48.Schultz, W. & Dickinson, A. (2000) Annu. Rev. Neurosci. 23, 473-500. [DOI] [PubMed] [Google Scholar]
- 49.Valentine, P. A. & Eggermont, J. J. (2001) Hearing Res. 154, 146-157. [DOI] [PubMed] [Google Scholar]
- 50.Kaneoke, Y. & Vitek, J. L. (1996) J. Neurosci. Methods 68, 211-223. [DOI] [PubMed] [Google Scholar]
- 51.Scarpini, E., Scheltens, P. & Feldman, H. (2003) Lancet Neurol. 2, 539-547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.