Abstract
The migration of immature neurons constitutes one of the major processes by which the central nervous system takes shape. Completing the migration at the final destination requires the loss of cell body motility, but little is known about the signaling mechanisms underlying this process. Here, we show that a loss of transient Ca2+ elevations triggers the completion of cerebellar granule cell migration. Simultaneous observation of the intracellular Ca2+ levels and cell movement in cerebellar slices of the early postnatal mice revealed that granule cells exhibit distinct frequencies of the transient Ca2+ elevations as they migrate in different cortical layers, and complete the migration only after the loss of Ca2+ elevations. The reduction of the Ca2+ elevation frequency by decreasing Ca2+ influx, or by inhibiting the activity of phospholipase C, PKC, or Ca2+/calmodulin, halted the granule cell movement prematurely. In contrast, increasing the Ca2+ elevation frequency by increasing Ca2+ release from internal stores, or by elevating intracellular cAMP levels, significantly delayed the completion of granule cell migration. The timing of the loss of Ca2+ elevations was intrinsically set in the granule cells and influenced by external cues. These results suggest that Ca2+ signaling, dictated by multiple signaling systems, functions as a mediator for completing the migration of immature neurons.
In the developing brain, the majority of postmitotic neurons migrate from the place of origin to the final destination (1, 2). This active movement of immature neurons is essential for the formation of ordered neuronal cytoarchitecture, synaptic connection, and neuronal differentiation (3, 4). In the cerebellar cortex, for example, granule cells migrate from the external granular layer (EGL) where they are generated, crossing the expanding molecular layer and the Purkinje cell layer to reach the internal granular layer (IGL), where they reside in the adult cerebellum (5, 6). The real-time observation of cell movement reveals that cerebellar granule cells display a distinct mode and rate of migration as they traverse different cortical layers (7–9). In the past several years, it became apparent that the migration of granule cells is controlled by the orchestrated activity of multiple molecular events, including the pathway selection, the activation of specific receptors and channels, and the assembly and disassembly of cytoskeletal components (10–14).
Although it has been expected that external cues and cell–cell contact are essential for the completion of neuronal migration at the right place (15, 16), signaling mechanisms underlying this process remain to be determined. We hypothesize that Ca2+ signaling is involved in controlling the completion of granule cell migration. In the microexplant cultures, granule cells exhibited transient elevations of intracellular Ca2+ levels, and the reduction of the amplitude and frequency components of the Ca2+ elevations resulted in a reversible retardation of cell movement (17). Furthermore, the rate of granule cell migration in the molecular layer was highly sensitive to the changes in the Ca2+ influx through the N-type Ca2+ channels or the N-methyl-d-aspartate subtype of glutamate receptors (18, 19). Moreover, the activation of somatostatin receptors, which reduces the Ca2+ channel activity, significantly slowed down the granule cell migration in the IGL (20). Taken together, these results suggest that Ca2+ signaling may play a critical role in completing the granule cell migration. To test this hypothesis, we used the acute cerebellar slice preparations, which maintain the neuronal cytoarchitecture and cellular milieu that are thought to be essential for the completion of neuronal migration in vivo. We determined whether the changes in Ca2+ signaling are responsible for the completion of granule cell migration at the final destination.
Methods
Ca2+ Measurements in Cerebellar Slices of 10-Day Postnatal Mouse Cerebellum. All procedures were approved by the Internal Animal Care and Use Committee of the Cleveland Clinic Foundation. Cerebella of postnatal 10-day-old mice (CD-1) were sectioned transversely or sagittally into 150- to 200-μm-thick slices on a vibrating blade microtome (7–9). To monitor the Ca2+ levels of granule cells, the cerebellar slices were incubated for 30 min with a cell-permeant acetoxymethyl ester form of 1 μM Oregon Green 488 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA-1) (Molecular Probes) diluted in the culture medium, which consisted of DMEM (GIBCO) supplemented with 1.8 mM glutamine and 24 mM NaHCO3. The slices were subsequently washed three times with the culture medium, and the dye was allowed to de-esterify for additional 30–60 min in the CO2 incubator. Cerebellar slices were transferred into the chamber of a microincubator (Medical Systems, Greenvale, NY) attached to the stage of a confocal microscope (TCS SP, Leica). Chamber temperature was kept at 37°C, and the cells were provided with a constant gas flow (95% O2/5% CO2). Slices loaded with Oregon Green 488 BAPTA-1 were illuminated with a 488-nm light from an argon laser, and the emission was detected at 530 ± 15 nm. Images of granule cells were collected every 1 to ≈15 s for up to 15 h. The changes in fluorescence intensity of each granule cell were normalized to its baseline fluorescence intensity.
We examined the effect of loading of 1 μM Oregon Green 488 BAPTA-1 on granule cell migration. First, we labeled granule cells in the slices with a carbocyanine dye (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) (7.2 μg/ml) (Molecular Probes). The 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate staining at the concentration we used did not significantly affect the granule cell migration (unpublished results). Subsequently, the halves of the slices labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate were loaded with 1 μM Oregon Green 488 BAPTA-1. We found that the migration rate of granule cells labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate was 12.4 ± 1.5 μm/h(n = 45) in the molecular layer without Oregon Green 488 BAPTA-1, and 12.1 ± 1.4 μm/h (n = 42) with Oregon Green 488 BAPTA-1, indicating that the loading of 1 μM Oregon Green 488 BAPTA-1 does not cause any significant noxious effects on the granule cell migration.
The movement of granule cell somata to the outside of the focal plane often caused the slow changes in the baseline fluorescence intensity of Oregon Green 488 BAPTA-1. To determine whether the changes in baseline fluorescence intensity reflect the changes in Ca2+ levels or the deviation of granule cell somata from the focal plane, at the beginning and end of each recording session and when slow changes in baseline intensity were detected, the z axis positions of granule cells were determined by optical sectioning along the z axis. If the z axis position of granule cell somata changed by >1 μm from the initial position, the experimental data were excluded from this study.
Ca2+ Measurements in Microexplant Culture of 0- to 2-Day Postnatal Mouse Cerebellum. All procedures were approved by the Internal Animal Care and Use Committee of the Cleveland Clinic Foundation. Cerebella of postnatal day 0–2 mice (CD-1) were placed in ice-chilled Hanks' balanced salt solution and freed from meninges and choroid plexus (17). Cerebellar slices were then made with a surgical blade, from which white matter and deep cerebellar nuclei were removed. Rectangular pieces (50–100 μm) were dissected out from the remaining tissue, which mainly consisted of the cerebellar gray matter, under a dissecting microscope. These microexplants were placed on the 100 μg/ml poly-l-lysine/20 μg/ml laminin-coated glass coverslips with 50 μl of the culture medium (17). Each coverslip was transferred into a 35-mm Petri dish and put in a CO2 incubator. One hour after plating, we added 1 ml of the culture medium to the Petri dish. The culture medium consisted of MEM (GIBCO) supplemented with 10% FCS/30 mM glucose/1.8 mM glutamine/24 mM NaHCO3/90 units/ml penicillin/90 μg/ml streptomycin. In these cultures, >95% of migrating neurons were granule cells, which were easily distinguished from other neurons by the small size of their cell bodies (17, 21). Although granule cells were prepared from the EGL and the IGL of all lobules of the cerebellum, the vast majority of granule cells were derived from the EGL, because the IGL only contains very small numbers of postmigratory granule cells at postnatal day 0 (P0)–P2 (21). Therefore, the majority of granule cells were at the same developmental stage (21).
To minimize the effects of fluctuations in granule cell soma thickness on monitoring changes in Ca2+ levels, we used ratiometric Ca2+ measurements with two Ca2+ indicator dyes, Fluo-3 and Fura-Red (17). Four to 70 h after plating, the granule cells were coloaded with a cell-permeant acetoxymethyl ester form of 2 μM Fluo-3 and 2 μM Fura-Red (Molecular Probes) diluted in the culture medium for 30 min at 37°C. The loading of Fluo-3 and Fura-Red at these concentration did not cause any significant noxious effects on granule cell migration (20). A confocal microscope was used to examine the changes in the Ca2+ levels and cell movement. The granule cells were illuminated with 488-nm-wavelength light, and fluorescence images for ratiometric Ca2+ measurements (at 530 ± 15 nm for Fluo-3; at 660 ± 15 nm for Fura-Red) and transmitted images for monitoring cell movement were collected simultaneously every 1 to ≈15 s for up to 10 h. Fluo-3/Fura-Red ratio images were calculated by dividing pixel values of the Fluo-3 fluorescence intensity by pixel values of the Fura-Red fluorescence intensity.
Results
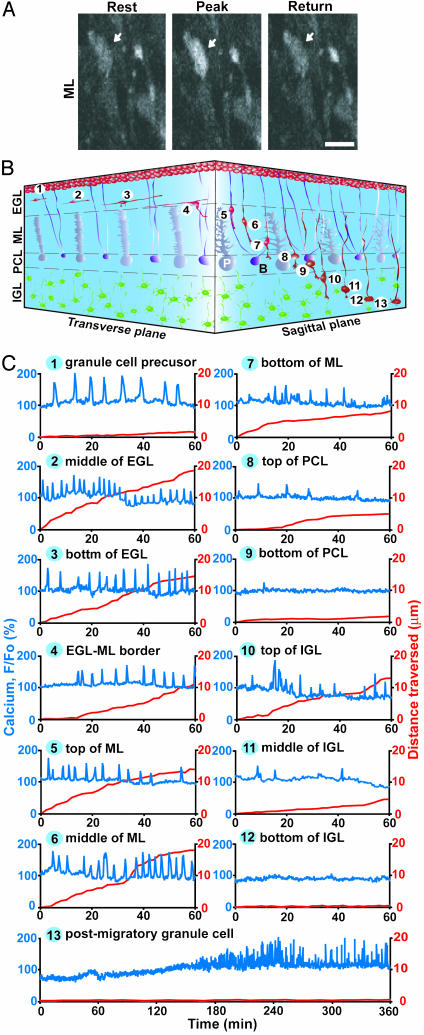
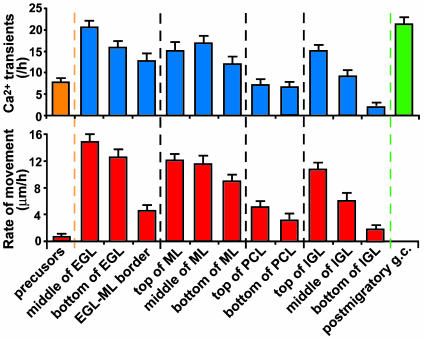
Distinct Pattern of Ca2+ Elevations of Granule Cells Along the Migratory Pathway. The combined use of confocal microscopy and Ca2+ indicator dye revealed that in the cerebellar slices of P10 mouse, migrating granule cells exhibit spontaneous Ca2+ elevations of their somata (Fig. 1A). Because granule cells sequentially alter the direction, mode, and tempo of migration (7–9) as schematically presented in Fig. 1B, we examined the alterations of transient Ca2+ elevations in their somata along the migratory pathway. We found that the granule cells exhibit the distinct pattern of transient Ca2+ elevations as they migrated in different cortical layers (Figs. 1C and 2). The frequency of Ca2+ transients depended on the position of granule cells along the migratory pathway and positively correlated with the rate of cell movement (correlation coefficient, 0.85) (Fig. 2). Furthermore, granule cells significantly reduced the frequency of Ca2+ elevations and the rate of cell movement at each boundary between cerebellar cortical layers, implying the presence of external cues, which induce the changes in the migratory behavior at these points on the migratory pathway. These results, as a whole, suggest that the frequency of Ca2+ transients may be one of the factors that control the alterations of migratory behavior of granule cells in vivo.
Fig. 1.
Dynamic changes in Ca2+ transient frequency of granule cells along the migratory pathway. (A) Typical example of a migrating granule cell in the molecular layer showing transient elevations of the intracellular Ca2+ levels in its soma (indicated by white arrows). (Bar = 7 μm.) (B) Schematic diagram showing the sequence of cerebellar granule cell migration from the birthplace at the top of the EGL to the final destination at the bottom of the IGL. The numbers 1–13 represent the position of granule cells along the migratory pathway and the stage of the differentiation in order: 1, granule cell precursor at the top of the EGL; 2–4, tangential migration at the middle and bottom of the EGL; 5–7, radial migration along the Bergmann glial process in the molecular layer; 8 and 9, stationary period in the Purkinje cell layer; 10 and 11, radial migration at the top and middle of the IGL; 12, completion of migration at the bottom of the IGL; and 13, postmigratory granule cell at the bottom of the IGL. B, Bergmann glial cell; P, Purkinje cell. (C) Sequential changes in the Ca2+ transients (blue lines) and the distance traversed by granule cells (red lines) over time. The number at the top of each graph corresponds to the number in B. Upward deflections in blue lines represent elevations of Ca2+ levels, and downward deflections indicate decreases of Ca2+ levels.
Fig. 2.
Relationship between the Ca2+ transient frequency and the granule cell position along the migratory pathway (Upper) and between the migration rate and the granule cell position (Lower). Each column represents the average values and SD obtained from >30 granule cells.
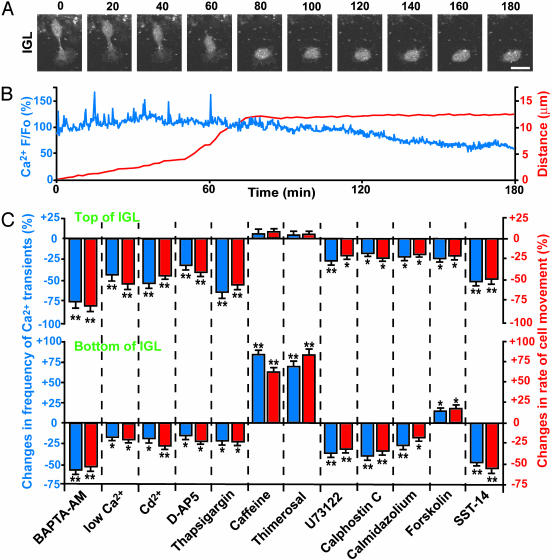
Loss of Ca2+ Transients Before Completion of Migration. We noticed that at the bottom of the IGL, where the majority of granule cells terminated their migration (8), the Ca2+ transients disappeared or significantly reduced in frequency (Figs. 1C and 2). The loss (or reduction) of Ca2+ transients is not caused by physiological deterioration after a prolonged period of observation, because postmigratory (stationary) granule cells resumed spontaneous Ca2+ transients 2–5 h later (Figs. 1C and 2). Next, we determined the sequence of the loss of Ca2+ transients and the completion of cell migration. In the bottom of the IGL, a granule cell initially migrated with variable amplitudes of Ca2+ transients, but completely lost Ca2+ transients ≈18 min before becoming permanently stationary (Fig. 3 A and B). Among 52 granule cells we examined in the IGL, all cells completed the migration only after the loss of Ca2+ transients. The average time lag between the loss of Ca2+ transients and the cessation of migration was 16.8 ± 4.3 min (mean ± SD, n = 52), with a range of 5–27 min. These results suggest that the loss of Ca2+ transients may be prerequisite for completing the granule cell migration at their final destination.
Fig. 3.
Completion of granule cell migration triggered by loss of Ca2+ transients. (A) Time-lapse images showing a typical example of the completion of granule cell migration at the bottom of the IGL. Elapsed time (in minutes) is indicated on the top of each photograph. (Bar = 10 μm.) (B) Alterations of spontaneous Ca2+ transients in the granule cell soma before and after the completion of migration. Changes in Ca2+ levels (blue line) and distance (red line) traversed by the granule cell shown in A are plotted as a function of elapsed time. (C) Effects of the changes in the Ca2+ transient frequency on the migration rate of granule cells at the top and bottom of the IGL. We chose the granule cells that migrated at the upper part of the top and bottom area, where the majority of the cells moved toward the IGL–white matter border at the average rates of ≈11 μm/h (top) and ≈4 μm/h (bottom). BAPTA-AM (10 μM), CdCl2 (500 μM), d-2-amino-5-phosphonopentanoic acid (100 μM), thapsigargin (1 μM), caffeine (10 mM), thimerosal (5 μM), U73122 (1 μM), calphostin C (100 nM), calmidazolium (5 μM), forskolin (30 μM), or somatostatin-14 (SST-14, 1 μM) was added to the medium in separate experiments, or extracellular Ca2+ concentrations were lowered from 1.8 mM to 0.1 mM after 60-min control observations. Here and in Fig. 4D, the effects of each treatment were evaluated by dividing the number of Ca2+ transients and the distance traveled during the 60 min after the application of each reagent by the number of Ca2+ transients and the distance traveled during the first 60 min in the absence of each reagent. Each column represents the average changes (obtained from >30 cells) in the number of Ca2+ transients (blue) or the migration rate (red). *, P < 0.05; **, P < 0.01.
Completion of Migration Regulated by Loss of Ca2+ Transients. To determine the role of the Ca2+ transients in the completion of neuronal migration, we induced the changes in the Ca2+ transient frequency at two different positions (top and bottom) of the IGL. At the top of the IGL (within 50 μm of the Purkinje cell layer–IGL border), granule cells migrated at an average rate of 11.3 ± 2.4 μm/h(n = 38), with average Ca2+ transients of 15.1 ± 1.6 μm/h(n = 38), and were expected to move for an additional 8–13 h before completing the migration (8). In contrast, at the bottom of the IGL (>100 μm from the Purkinje cell layer–IGL border), granule cells migrated at a significantly reduced rate (an average rate of 4.3 ± 0.8 μm/h, n = 41) with fewer numbers of Ca2+ transients (an average frequency of 2.4 ± 0.5/h, n = 41) and were expected to cease migration within 3–5 h (8). Because the Ca2+ levels of the granule cell somata are influenced by the amount of Ca2+ influx through the voltage-gated Ca2+ channels (18) and neurotransmitter-coupled ion channels (19) and the Ca2+ release from the internal stores (17), we stimulated or inhibited these potential Ca2+ sources with the use of pharmacological tools. Chelating the intracellular Ca2+ levels with 10 μM BAPTA-AM (17), reducing Ca2+ influx by lowering the extracellular Ca2+ concentrations from 1.8 mM to 0.1 mM, blocking the voltage-dependent Ca2+ channels with 500 μM CdCl2 (18), inhibiting the N-methyl-d-aspartate subtype of glutamate receptors with 100 μM d-2-amino-5-phosphonopentanoic acid (19), or decreasing the internal Ca2+ release with 1 μM thapsigargin (17), in separate experiments, resulted in a significant reduction of the Ca2+ transient frequency and a slowdown of the cell movement regardless of the position of the granule cells in the IGL (Fig. 3C). The stimulation of the internal Ca2+ release through the ryanodine receptors with 10 mM caffeine (22) or the inositol 1,4,5-trisphosphate receptors with 5 μM thimerosal (23) significantly increased the Ca2+ transient frequency and accelerated the cell movement at the bottom of the IGL (Fig. 3C). Interestingly, upon the stimulation of the internal Ca2+ release, small numbers (16%) of granule cells changed the direction of migration and exhibited backward movement of their somata (toward the Purkinje cell layer–IGL border), whereas the majority (84%) of cells continuously migrated toward the IGL–white matter border. Neither caffeine nor thimerosal changed the Ca2+ transient frequency and the cell motility at the top of the IGL (Fig. 3C). Taken together, these results demonstrate that the reduction of the Ca2+ transient frequency is always accompanied by the slowdown of granule cell movement, implying that the loss of Ca2+ transients may provide an intracellular signal for triggering molecular cascades leading to the completion of migration. The comparison of the effects of caffeine and thimerosal on the migration rate between the top and bottom of the IGL suggests that the basic levels (or amount) of spontaneous Ca2+ release from the internal stores may be reduced in the granule cells at the bottom of the IGL. Such reduction of internal Ca2+ release may be responsible, at least in part, for the slowdown (or termination) of granule cell migration.
To further examine the role of Ca2+ signaling in the cessation of neuronal migration, we modified the activity of the Ca2+ signaling upstream and downstream (24). Inhibiting the Ca2+-signaling upstream by blocking phospholipase C with 1 μM U73122 significantly decreased the Ca2+ transient frequency and slowed down the cell movement regardless of the position of the granule cells in the IGL (Fig. 3C). Likewise, inhibiting the Ca2+-signaling downstream by blocking PKC with 100 nM calphostin C or Ca2+/calmodulin with 5 μM calmidazolium, in separate experiments, resulted in a significant reduction of the Ca2+ transient frequency and migration rate at the top and bottom of the IGL (Fig. 3C). Furthermore, we examined whether the alteration of cAMP levels is involved in the loss of the Ca2+ transients and the completion of movement, as the changes in the intracellular Ca2+ levels can influence cAMP levels and vice versa (25). Increasing cAMP levels with 30 μM forskolin decreased the Ca2+ transient frequency and the migration rate at the top of the IGL but increased both of them at the bottom (Fig. 3C). Finally, we determined whether somatostatin, which is an external cue for controlling granule cell migration (20), modifies the Ca2+ transient frequency at the final destination. Application of 1 μM somatostatin-14 significantly reduced the Ca2+ transient frequency and the cell motility regardless of the position of granule cells in the IGL (Fig. 3C), suggesting that somatostatin also participates in controlling the termination of granule cell migration by decreasing the Ca2+ transient frequency. These results suggest that changes in the Ca2+ signaling upstream and downstream, such as phospholipase C, PKC, and Ca2+/calmodulin, influence the completion of granule cell migration. Furthermore, the different effects of cAMP elevations on the Ca2+ transient frequency and the cell motility between the top and bottom of the IGL may be critical for the completion of granule cell migration.
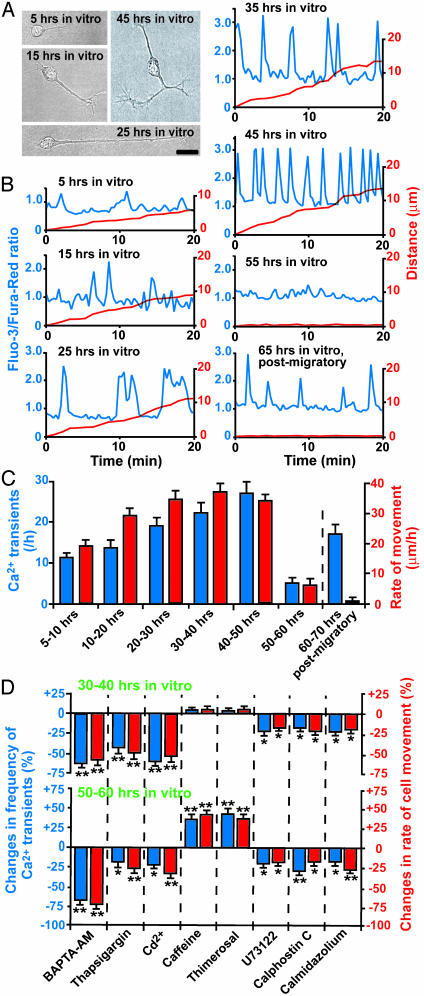
Timing of Loss of Ca2+ Transients Set by Intrinsic Programs. The loss of Ca2+ transients may be induced by external stop signals or contact with other cells and processes, but intrinsic programs may also be responsible. To test this, we examined the Ca2+ transients of isolated granule cells in the microexplant culture of P0–P2 mouse cerebella (Fig. 4A). In this culture, isolated granule cells ceased migrating without external stop signals and cell–cell contact within 2–3 days after culture (21). We found that isolated granule cells exhibit transient Ca2+ elevations in their somata during the cell movement (Fig. 4B). The Ca2+ transient frequency depended on the elapsed time after plating (Fig. 4C) and positively correlated with the migration rate (correlation coefficient, 0.81). Interestingly, the Ca2+ transients disappeared or had significantly reduced occurrences when isolated granule cells stopped migrating at 50–60 h in vitro (Fig. 4 B and C), although 1–3 h later, the postmigratory granule cells resumed generating Ca2+ transients (Fig. 4 B and C). The loss of the Ca2+ transients always preceded the completion of migration. The average time lag between the loss of Ca2+ transients and the cessation of migration was 11.6 ± 2.4 min (n = 46) with a range of 3–21 min. We also examined whether the changes in Ca2+ transient frequency affect the completion of migration of isolated granule cells. We found that the reduction of the Ca2+ transient frequency is always accompanied by the slowdown of granule cell movement regardless of the elapsed time after plating (Fig. 4D). The prevention of the loss of Ca2+ transients by stimulating the internal Ca2+ release significantly increased the migration rate at the final phase of migration (50–60 h in vitro), leading to the delay in the completion of migration (Fig. 4D). Taken together, these results suggested that intrinsic programs may set the timing of the loss of Ca2+ transients in isolated granule cells at ≈50–60 h in vitro, and may trigger the completion of the migration. This 50- to 60-h term set by the internal clock is comparable to the time required for granule cells to complete the migration in the early postnatal cerebellum in vivo (13, 14), suggesting that internal programs are involved in controlling the completion of granule cell migration in vivo.
Fig. 4.
Loss of the Ca2+ transients in isolated granule cells set by intrinsic programs. (A) Photomicrographs of isolated granule cells in the microexplant culture of the early postnatal cerebellum. (Bar = 12 μm.) (B) Changes in the Ca2+ transients and the movement of isolated granule cells as elapse time in vitro goes by. The Ca2+ levels (blue lines) and the distance (red lines) traversed are plotted as a function of elapsed time. Upward deflections in blue lines represent the elevations of the intracellular Ca2+ levels, and downward deflections indicate the decreases of the intracellular Ca2+ levels. (C) Sequential changes in the Ca2+ transient frequency and the migration rate of isolated granule cells in the microexplant culture. Each column represents the average values (obtained from >30 granule cells) of Ca2+ transients (blue) and the migration rate (red). (D) Effects of the changes in the Ca2+ transient frequency on the migration rate of isolated granule cells at 30–40 h in vitro and 50–60 h in vitro. BAPTA-AM (10 μM), thapsigargin (1 μM), CdCl2 (500 μM), caffeine (10 mM), thimerosal (5 μM), U73122 (1 μM), calphostin C (100 nM), and calmidazolium (5 μM) were added to the culture medium in separate experiments after 60-min control observations. Each column represents the average changes (obtained from >30 cells) in the Ca2+ transient frequency (blue) or the migration rate (red). *, P < 0.05; **, P < 0.01.
Discussion
Present results demonstrate that the loss of Ca2+ transients during the final phase of granule cell migration, which is set by intrinsic programs and influenced by external cues, provide an internal signal triggering cellular cascades responsible for the completion of granule cell migration. Transient Ca2+ elevations are essential for maintaining the movement of cells ranging from fibroblast to immature neurons (26–29). Although it has been reported that buffering of intracellular Ca2+ levels blocks Ca2+ transients and reduces or inhibits the movement of neutrophils (30), it has never been shown that the loss of Ca2+ transients plays a critical role for the completion of neuronal migration in the developing brain. Neuronal precursors and postmigratory neurons in the fetal cerebrum (31–33) and the early postnatal cerebellum exhibit spontaneous Ca2+ transients, suggesting that the role of Ca2+ transients in development may be constantly remodeled by internal programs and extracellular cues (34–36). At present, little is known about how the loss of Ca2+ transients induces the cessation of migration. One possibility is that the loss of Ca2+ transients might cause the changes in the Ca2+-dependent activation of specific enzymes (24), which in turn affects the phosphorylation state or extent of proteolysis of large numbers of proteins (24, 37). These changes could induce the rearrangement of cytoskeletal components, which are required for the completion of neuronal migration (11, 38). Intrinsic programs might set the timing of the loss of Ca2+ transients by adding and/or removing Ca2+ signaling components, or modifying their sensitivity (24), and may contribute to completing migration at the right time. Stopping migration at the right place may be induced by various external cues. One of them may be somatostatin, which suppresses the generation of Ca2+ transients (20). Netrin-1 may also participate because the desensitization of netrin-1 receptors is accompanied by a reduction of Ca2+ signaling (39), and the mutation of this receptor gene (Unc5h3) causes an abnormal allocation of granule cells within the white matter (40). The Ca2+ transient frequency and the rate of cell movement observed in isolated granule cells in the microexplant culture are higher than those observed in granule cells in acute cerebellar slices, suggesting that external cues present on the migratory pathway may act to reduce the generation of the Ca2+ transients and the migration rate. In the present study, we cannot exclude the possibility that changes in steady-state Ca2+ levels also play a role in stopping granule cell migration (41).
Acknowledgments
We thank Grahame Kidd, Jacqueline Morris, and Carol Haney for critically reading the manuscript. H.K. was supported by National Institutes of Health Grant AA 13613 and Whitehall Foundation Grant 2001-12-35.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EGL, external granular layer; IGL, internal granular layer; Pn, postnatal day n; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate.
References
- 1.Rakic, P. (1990) Experientia 46, 882-891. [DOI] [PubMed] [Google Scholar]
- 2.Hatten, M. E. (1999) Annu. Rev. Neurosci. 22, 511-539. [DOI] [PubMed] [Google Scholar]
- 3.Caviness, V. S., Jr., & Rakic, P. (1978) Annu. Rev. Neurosci. 1, 297-326. [DOI] [PubMed] [Google Scholar]
- 4.Ross, M. E. & Walsh, C. A. (2001) Annu. Rev. Neurosci. 24, 1041-1070. [DOI] [PubMed] [Google Scholar]
- 5.Rakic, P. (1971) J. Comp. Neurol. 141, 283-312. [DOI] [PubMed] [Google Scholar]
- 6.Komuro, H. & Rakic, P. (1998) J. Neurobiol. 37, 110-130. [PubMed] [Google Scholar]
- 7.Komuro, H. & Rakic, P. (1995) J. Neurosci. 15, 1110-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komuro, H. & Rakic, P. (1998) J. Neurosci. 18, 1478-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komuro, H., Yacubova, E., Yacubova, E. & Rakic, P. (2001) J. Neurosci. 21, 527-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakic, P. & Komuro, H. (1995) J. Neurobiol. 26, 299-315. [DOI] [PubMed] [Google Scholar]
- 11.Rakic, P., Knyihar-Csillik, E. & Csillik, B. (1996) Proc. Natl. Acad. Sci. USA 93, 9218-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams, N. C., Tomoda, T., Cooper, M., Dietz, G. & Hatten, M. E. (2002) Development (Cambridge, U.K.) 129, 965-972. [DOI] [PubMed] [Google Scholar]
- 13.Komuro, H. & Yacubova, E. (2003) Cell. Mol. Life Sci. 60, 1084-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yacubova, E. & Komuro, H. (2003) Cell Biochem. Biophys. 37, 213-234. [DOI] [PubMed] [Google Scholar]
- 15.D'Arcangelo, G., Miao, G. G., Chen, S. C., Soares, H. D., Morgan, J. I. & Curran, T. (1995) Nature 374, 675-676. [DOI] [PubMed] [Google Scholar]
- 16.Gongidi, V., Ring, C., Moody, M., Brekken, R., Sage, E. H., Rakic, P. & Anton, E. S. (2004) Neuron 41, 57-69. [DOI] [PubMed] [Google Scholar]
- 17.Komuro, H. & Rakic, P. (1996) Neuron 17, 275-285. [DOI] [PubMed] [Google Scholar]
- 18.Komuro, H. & Rakic, P. (1992) Science 257, 806-809. [DOI] [PubMed] [Google Scholar]
- 19.Komuro, H. & Rakic, P. (1993) Science 260, 95-97. [DOI] [PubMed] [Google Scholar]
- 20.Yacubova, E. & Komuro, H. (2002) Nature 415, 77-81. [DOI] [PubMed] [Google Scholar]
- 21.Yacubova, E. & Komuro, H. (2002) J. Neurosci. 22, 5966-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens, D. F., Flint, A. C., Dammerman, R. S. & Kriegstein, A. R. (2000) Dev. Neurosci. 22, 25-33. [DOI] [PubMed] [Google Scholar]
- 23.Elferink, J. G. (1999) Gen. Pharmacol. 33, 1-6. [DOI] [PubMed] [Google Scholar]
- 24.Berridge, M. J., Bootman, M. D. & Roderick, H. L. (2003) Nat. Rev. Mol. Cell. Biol. 4, 517-529. [DOI] [PubMed] [Google Scholar]
- 25.Gorbunova, Y. V. & Spitzer, N. C. (2002) Nature 418, 93-96. [DOI] [PubMed] [Google Scholar]
- 26.Jaconi, M. E. E., Theler, J. M., Schlegel, W., Appel, R. D., Wright, S. D. & Lew, P. D. (1991) J. Cell Biol. 112, 1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brundage, R. A., Fogarty, K. E., Tuft, R. A. & Fay, F. S. (1993) Am. J. Physiol. 265, C1527-C1543. [DOI] [PubMed] [Google Scholar]
- 28.Anton, E., Hadjiargyrou. M., Patterson, P. H. & Matthew, W. D. (1995) J. Neurosci. 15, 584-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez, T. M. & Spitzer, N. C. (1999) Nature 397, 350-355. [DOI] [PubMed] [Google Scholar]
- 30.Marks, P. W. & Maxfield, F. R. (1990) J. Cell Biol. 110, 43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens, D. F. & Kriegstein, A. R. (1998) J. Neurosci. 18, 5374-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciccolini, F., Collins, T. J., Sudhoelter, J., Lipp, P., Berridge, M. J. & Bootman, M. D. (2003) J. Neurosci. 23, 103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scemes, E., Duval, N. & Meda, P. (2003) J. Neurosci. 23, 11444-11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer, N. C., Lautermilch, N. J., Smith, R. D. & Gomez, T. M. (2000) BioEssays 22, 811-817. [DOI] [PubMed] [Google Scholar]
- 35.Ducibella, T., Huneau, D., Angelichio, E., Xu, Z., Schultz, R. M., Kopf, G. S., Fissore, R., Madoux, S. & Ozil, J. P. (2002) Dev. Biol. 250, 280-291. [PubMed] [Google Scholar]
- 36.Webb, S. E. & Miller, A. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 539-551. [DOI] [PubMed] [Google Scholar]
- 37.Carafoli, E., Santella, L., Brance, D. & Brisi, M. (2001) Crit. Rev. Biochem. Mol. Biol. 36, 107-260. [DOI] [PubMed] [Google Scholar]
- 38.Feng, Y. & Walsh, C. A. (2001) Nat. Rev. Neurosci. 2, 408-416. [DOI] [PubMed] [Google Scholar]
- 39.Ming, G. L., Wong, S. T., Henley, J., Yuan, X. B., Song, H. J., Spitzer, N. C. & Poo, M. M. (2002) Nature 417, 411-418. [DOI] [PubMed] [Google Scholar]
- 40.Goldowitz, D., Hamre, K. M., Przyborski, S. A. & Ackerman, S. L. (2000) J. Neurosci. 20, 4129-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kater, S. B. & Mills, L. R. (1991) J. Neurosci. 11, 891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]