Abstract
Objective
Silybin is a polyphenol with anti-oxidant and anti-cancer properties. The poor bioavailability of some polyphenols can be improved by binding to phosphatidylcholine. In recent years, studies have been conducted to evaluate the anti-cancer effect of silybin. We studied the effect of silybin and silybin-phosphatidylcholine on ESR1 and ESR2 gene expression and viability in the T47D breast cancer cell line.
Materials and Methods
In this experimental study, a 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide test (MTT test) was used to determine doses for cell treatment, and the gene expression was analyzed by real-time reverse transcriptase-polymerase chain reaction (real-time RT- PCR).
Results
Significant dose- and time-dependent cell growth inhibitory effects of silybin and silybin-phosphatidylcholine along with ESR1 down-regulation were observed in T47D cells. In contrast to ESR1, the T47D cell line showed negligible ESR2 expression.
Conclusion
This study suggests that silybin and silybin-phosphatidylcholine down-regulate ESR1 in ER+breast cancers. Results also show that in the T47D cell line, silybindown-regulation of ESR1 compared with silybin.
Keywords: Silybin, Silybin-Phosphatidylcholine, Breast Cancer, ESR1
Introduction
The estrogen receptor α (ERα) is frequently observed to be overexpressed in breast cancer (1), and has many functions, including tumor growth enhancement, and is also a prognostic and predictive factor (2). Estrogen receptor exists in two forms, ERα and ERβ, which have distinct tissue expression patterns. ERα and ERβ are encoded by ESR1 and ESR2 respectively, which are found at different chromosomes (6q25.1 and 14q22-25 respectively) (3). Stimulation of transcription by ERα occurs via a number of distinct molecular events in the nucleus. ERα homo- or heterodimerizes with other nuclear receptors such as estrogen receptor β (ERβ) or androgen receptor (AR) and binds, via the DNA-binding domain (DBD), to estrogen response elements (EREs) located on the promoters of estrogenresponsive genes (4).
Silybin (silibinin), the major component of milk thistle (Silybum marianum) is a natural polyphenol with high antioxidant and anti-cancer properties along with a few side effects (5- CELL JOURNAL(Yakhteh), Vol 16, No 3, Autumn 2014 300 12). Recent studies have shown the inhibitory effect of silybin in different cancers such as skin (13), colon (14), lung (15), prostate (16-17) and breast (18). Also, synergistic anti-cancer effects of silybin have been shown with other anti-cancer drugs such as doxorubicin (19), cisplatin and carboplatin (20) and mitoxantrone(21) in prostate cancers, and doxorubicin in MBA-MD-468 and MCF-7 breast cancer cell lines (22). The pharmacological activities assigned to silybin show that this phytochemical blocks VEGF, EGFR, COX-2 and TNF. Considering that a tumor cell uses multiple pathways to survive, drugs that intervene in a single pathway (e.g. Avastin) are unlikely to succeed. The advantage of plant-derived products, as described here, is that they intervene in multiple pathways. This characteristic supports the idea that they may have better anti-cancer potential. (23). However, the underlying mechanism of the inhibitory action of silybin in breast cancer has not yet been completely elucidated (5).
Also, recent in vivo studies on liver disease show that phosphatidylcholine bound to silybin is much more effective than silybin alone due to its bioavailability being 7 to 10 times more than silybin (24) Considering that bioavailability is influenced by a multitude of factors and has different levels including absorption, distribution (by the circulating blood), metabolism (by the liver), entry of the drug into specific body tissues, excretion and bioactivity, which in turn are governed by a large number of parameters (25, 26). However, in this in vitro study, certainly the bioavailability is only cell membrane absorption.
The absorption and therapeutic property of silybin is limited due to its poor water solubility (27) based on two factors. First, it is a multiplering molecule and too large to be absorbed by simple diffusion. Second, because it has poor miscibility with oils and other lipids of the membrane. Therefore the structure of silybin is limited in its ability to pass across the lipid-rich outer membranes of the enterocytes (intestinal absorptive cells) of the small intestine (28). Moreover, studies have shown that one of the multiple effects of silybin is the induction of growth inhibition and cell viability reduction in cancer cells (e.g. SHP-77 and A-549 lung carcinoma cell lines) (23). Hence, within this broader area, one specific research interest of ours was to evaluate cell viability reduction of T47D cancer cells by MTT, and to determin IC50 (half maximal inhibitory concentration) in order to estimate the comparative bioavailability of silybin with silybin-phosphatidylcholine.
In this study, we compared silybin with silybin- phosphatidylcholine in terms of cell membrane bioavailability, cytotoxicity and ESR expression (all by no serum starvation) in T47D human breast cancer cell line.
Materials and Methods
Tumor cell line and reagents
T47D is an ER+ human breast ductal carcinoma cell line. According to studies hitherto, it is not clear that T47D is a highly (29) or weakly (30) invasive (31- 33) or non-invasive (34, 35) cell line. A T47D cell line was purchased from the National Cell Bank, Pasteur Institute of Iran. The cell lines were cultured in RPMI1640 medium (Invitrogen) with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (all from PAA), 2 g/l sodium bicarbonate and 2.5 g/l HEPES (Sigma-Aldich, Missouri, USA). T47D cells were grown under standard culture conditions (37℃, 95% humidified air, and 5% CO2). For cell harvesting, 0.25% solution of trypsin (Sigma-Aldich, Missouri, USA) in PBS was used.
Chemical treatments and MTT assay
For the MTT assay, the cells were first seeded in three 96-well microplates. In each well containing 100 µl complete medium, 7x103 cells were seeded. The next day, the cells were treated with different doses of silybin (50, 75, 100, 150, 200, 250, 300, and 350 µM) or silybinphosphatidylcholine (50, 75, 100, and 150 µM) for 24, 48, and 72 hours. Our primary MTT tests showed that the cytotoxicity effects of silybinphosphatidylcholine are two or three times more than silybin, thus, some doses of silybin-phosphatidylcholine (i.e. 200, 250, 300, 350 µM) were not used. All doses were renewed every 24 hours. From the silybin (Sigma) stock solution, 100 mM was dissolved in dimethyl sulfoxide (DMSO). From the silybin-phoshphatidylcholine (Enzymatic Therapy, USA) stock solution, 10 mM was dissolved in DMSO: methanol at a ratio of 3:1. In all tests, the final concentration of DMSO did not exceed 0.1% (v/v).
After the 24, 48, and 72 hours treatments, the cells were incubated with 0.5 mg/ml microculture tetrazolium (Sigma) for about 3 hours. The optical density (OD) of formazan dye dissolved in DMSO was measured with an ELISA microplate reader (Gen5, Power Wave XS2, BioTek, USA) at 570 nm.
The percentage of cell viability at different doses was calculated by the following equation:
IC50 determination
The half maximal inhibitory concentration (IC50) of silybin and silybin-phosphatidylcholine was determined by using the Pharmacologic Calculation System statistical package (Pharm PCS) (Springer Verlag, USA) after 24, 48, and 72 hours in the T47D cell line.
After the MTT assay, and the determination of IC50, some doses were selected (75 μM and 150 μM for silybin, 25 μM and 50 μM for silybinphosphatidylcholine) for ESR1 and ESR2 gene expression analysis after 24, 48, and 72 hours. Each experiment had three individual samples (Error bars: ± SD).
RNA extraction and cDNA synthesis
Cells were seeded in three 6-well microplates. 2×105 cells were seeded in wells each containing 200 ml complete medium. After 24 hours, the cells were treated with 75 and 150 μM silybin and 25 and 50 μM silybin-phosphatidylcholine doses for 24, 48, and 72 hours.
Total RNA was isolated from the treated cells using the RNX Plus™ kit (CinnaGen, Tehran, Iran) according to manufacturer’s instruction.
For cDNA synthesis, 1000 ng of extracted RNA was reverse transcribed into cDNA according to the manufacturer’s protocol, using EDTA (CinnaGen), dNTP (CinnaGen), and random hexamer primer (Fermentas, Pittsburgh PA, USA), Reverse Transcriptase 10000 u (Fermentas), RiboLock RNase Inhibitor 2500 u (Fermentas), DEPC Water (CinnaGen).
Analysis of gene expression by real-time PCR
For ESR1, ESR2 and GAPDH (as a control), the following primer sets were purchased from Qiagen: ESR1 (QT00044492), ESR2 (QT00060641), and GAPDH (QT01192646).
For each reaction, 1 μl cDNA was added to a 9 μl reaction mixture containing 1 μl of related primers and 5 μl SYBR Green I Master Mix (QuantiFast SYBR Green PCR, Q204054), and run on a Real Time Thermo cycler (RotorGene 6000, Corbett Life Science, USA). The real-time PCR program was as follows: initial denaturation 95˚C for 5 minutes, denaturation 95˚C for 15 seconds, annealing temperature optimized from 60 to 61˚Cfor 25 seconds, extension 72˚C for 25 seconds, 35 cycles. The specificity of the PCR product was assessed by verifying a single peak in melting curve analysis.
All measurements were taken twice in duplicate and the average was used for further analysis. GAPDH, a housekeeping gene, was used as a control; the fold change of each target gene relative to GAPDH was calculated based on relative quantitation using the ΔΔCT method, calculated by the 2 –ΔΔCT relative expression formula.
Statistical analysis
Data were analyzed using SPSS 18 software. One-way ANOVA and Dunnett’s two-tailed post hoc t test were employed to evaluate the statistical significance of differences between the control and all treatments. The data had normal distribution. The P values that were considered significant are displayed as *; p<0.05, **; p<0.01, ***; p<0.001 in figures 1, 2, and 4. Cell viability graphs were depicted by SPSS 18 (clustered bar, summaries for group of case). The IC50s were estimated using the Pharmacologic Calculation System statistical package (Pharm PCS, Springer Verlag, USA).
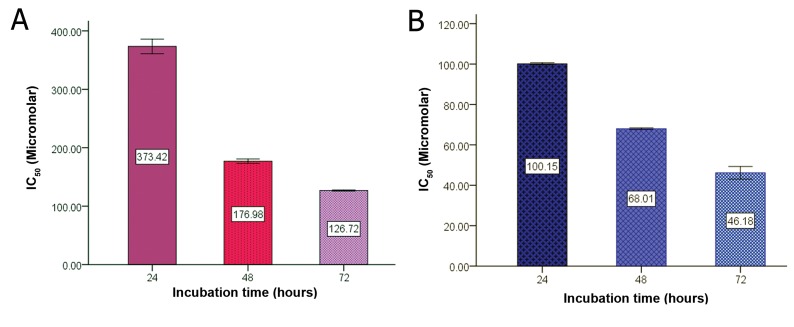
Fig 3.
Determination of IC50 of silybin (A), and silybin-phosphatidylcholine (B) during 24, 48, and 72 hours of incubation.
Fig 4.
Effect of Silybin and Silybin-phosphatidylcholine on ESR1 expression after 24 (A), 48 (B), and 72 hours (C) of treatment in the T47D breast cancer cell line. The reverse-transcribed RNA and amplified cDNA was normalized for GAPDH expression. Relative expression graphs were depicted by SPSS 18 (simple bar, summaries for group of case). Data were analyzed by the ΔΔCT relative expression method, and presented as two independent experiments. Each experiment had two individual samples (Error bars: ± SD). *; p< 0.05, **; p<0.01 and ***; p< 0.001.
Results
Proliferation and inhibitory effects of Silybin and Silybin-phosphatidylcholine on the T47D cell line
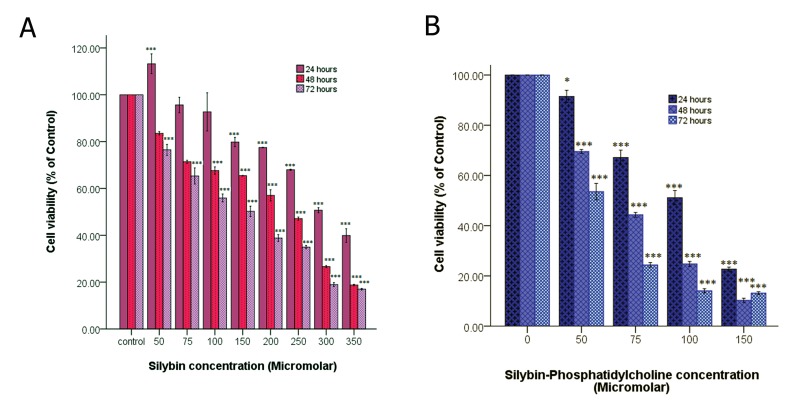
Briefly, 7×103 cells were seeded in 96 well plates for 24 hours and treated with different doses in a complete medium (no serum starvation). The cytotoxicity effects of silybin and silybin-phosphatidylcholine were evaluated by MTT assay in the T47D cell line in eight doses (50, 75, 100, 150, 200, 250, 300, 350 μM), and four doses (50, 75, 100, 150 μM) respectively for 24, 48 and 72 hours (Fig 1). Cell growth inhibition was observed after 24, 48, and 72 hours of treatment. Silybin and silybin-phosphatidylcholine treatments resulted in a dose and time-dependent decrease in cell viability. However, increasing cell proliferation was observed in 50 μM silybin (low doses) in the first 24 hours.
Fig 1.
The effect of silybin (A) and silybin-phosphatidylcholine (B) on cell viability of the T47D breast cancer cell line. Data is presented as percentage of viability in three independent experiments. *; p< 0.05, **; p<0.01 and ***; p< 0.001.
The comparison of four doses of silybin and silybin-phosphatidylcholine (50, 75, 100, 150 μ higher M) after 24 hours of treatment shows that each silybin-phosphatidylcholine dose had a much higher inhibitory effect on cell growth than the same silybin dose (Fig 2A). As indicated, all doses except silybin 50 μM reduced cell proliferation, and all doses except 75 μM and 100 μM silybin were considered statistically significant (p<0.05 or p<0.001).
Fig 2.
Comparison of different doses of silybin, and silybin-phosphatidylcholine after 24 (A), 48 (B), and 72 hours (C) of treatment. Data is presented as percentage of viability in three independent experiments. *; p< 0.05, **; p<0.01 and ***; p< 0.001.
All doses after 48 and 72 hours of treatment decreased cell viability and were statistically significant (p<0.001). Figure 2B (after 48 hours of treatment) and figure 2C (after 72 hours of treatment) show that, each silybin-phosphatidylcholine dose had a much higher inhibitory effect on cell growth than the same silybin dose, and this difference was more significant in the 72 hours treatment than that of 48 hours.
Figure 3 shows the IC50s of silybin and silybin- phosphatidylcholine after 24, 48 and 72 hours of treatment. Data from three independent experiments are presented. The IC50 comparison of silybin and silybin-phosphatidylcholine indicated that the bioavailability of silybinphosphatidylcholine is 2.5-3 times more than silybin.
Down regulation of ESR1 gene expression after 24, 48, and 72 hours of treatment with Silybin and Silybin-phosphatidylcholine in the T47D cell line
According to the MTT assay results, silybinphosphatidylcholine is more effective than silybin on ESR1 down-regulation. Thus, to compare the effect of these two compounds on ESR1 and ESR2 gene expression, the same doses were not used. Considering that silybin-phosphatidylcholine bioavailability in T47D cell line is 2.5-3 times greater than that of silybin, silybin doses were selected three times more than the silybinphosphatidylcholine doses. On the other hand, the aim of this step of the study was to analyze ESR1 and ESR2 gene expression by real-time RT-PCR (no cell mortality). Hence, all selected doses were less than the IC50s. Therefore, silybin at concentrations of 75 and 150 μM corresponded to 25 and 50 μM of silybin-phosphatidylcholine respectively.
Figure 4A shows that all silybin and silybinphosphatidylcholine doses down-regulate ESR1 but not significant after 24 hours.
As shown in figure 4B, the level of ESR1 down regulation of 25 μM silybin-phosphatidylcholine after 48 hours is nearly the same of its corresponding dose of silybin (75 μM). After 48 hours, 150 μM silybin seems more effective than its corresponding dose (50 μM silybinphosphatidylcholine) (p< 0.01, p<0.001).
Figure 4C indicates that after 72 hours of treatment, only the high doses of silybin (150 μM) and silybin-phosphatidylcholine (50 μM) showed significant ESR1down-regulation. Overall, the most down-regulation was observed using 50 μM of silybin-phosphatidylcholine. The T47D cell line showed negligible ESR2 expression.
Discussion
Considering the aim of the study, to obtain reliable results, the MTT assays and cell treatments were not done by serum starvation, and the medium were exchanged every 24 hours. The comparison of silybin and silybin-phosphatidylcholine by MTT assay (by no serum starvation) indicates that all silybin-phosphatidylcholine doses had a much larger inhibitory effect on cell growth (2.5-3 times more) than the same silybin doses in the T47D cell line. This difference became more significant as the duration of treatment increased. The results reported in this study show significant dose- and time-dependent cell growth inhibitory effects of silybin and silybin-phosphatidylcholine in T47D cells after 48 and 72 hours of treatment at all doses. Also, in our latest studies on silybin cytotoxicity on MDA-MB-231 breast (36), and PC-3 prostate cancer (37) cell lines, all silybin doses had growth inhibitory effects after 24, 48 and 72 hours of treatment.
According to the 24 hours MTT assay results in T47D cells, silybin and silybin-phosphatidylcholine cytotoxicity were effective at most (but not all) doses. Since an increase in cell proliferation was observed at 50 μM of silybin (low dose) in the first 24 hours, choosing very low doses of this compound (depending on the type of the cell line) can cause opposite results (cell growth is stimulated at low concentration) and, thus, may result in misleading conclusions.
Moreover, many researchers use serum starvation because it commonly leads to cell cycle arrest in the G0/G1 phase, and also has been used to arrest the G1 phase in cancer cells (38).
In our latest research, the comparison of silybin IC50s, using complete medium and serum starvation procedures in MDA-MB-453 or BT474 cell line, indicates that IC50 reported doses by serum starvation method are less than the complete medium method (data not shown). Therefore, for studies that are not focused on cell cycle arrest, to obtain reliable results, serum starvation method should not be used for cell treatments.
Breast cancer is a major public health problem worldwide and about 70% of primary breast tumors in women are ER-positive (ERα) (39). Phytochemicals such as flavonoids have good potential as anti-cancer agents because of their anti-proliferative activity against human tumor cell lines, safety and ability to target multiple cell-signaling pathways (40-41). Silybin is a flavonoid antioxidant that has been used as both an antihepatotoxic and an anti-carcinogenic agent (42). More importantly, it has been reported that silybin has no significant effect on the growth of normal human prostate epithelial cells (43). Siliphos was shown to be well tolerated in acute and long-term toxicity tests in rodents and primates up to oral doses of 2000 mg/ kg (as silybin). The excellent tolerability of this complex was confirmed in volunteers at doses up to 360 mg p.o. (as silybin) for three weeks (28). Phytosomes such as silybin-phosphatidylcholine are advanced forms of herbal formulations that are better absorbed, and as a result produce better bioavailability and therapeutic action than the conventional herbal extracts such as silybin (44).
We examined the effect of silybin and silybin- phosphatidylcholine on ESR1 expression in T47D breast cancer cells by RT-PCR. In the first 24 hours, all doses showed no significant down- regulation in ESR1 expression, perhaps demonstrating that for optimum effects of silybin and silybin-phosphatidylcholine on ESR1 regulation, more than 24 hours of treatment is required. The results for 48 hours indicated all doses significantly down-regulated ESR1 (p<0.01 or p<0.001). The results also showed that 75 μM silybin and 25μM silybin-phosphatidylcholine almost down-regulated ESR1 as the same level, indicating that, the 25 μM silybin-phosphatidylcholine is as effective as 75 μM silybin which it is 3 times greater (three fold). The 72 hours treatment demonstrates 50 μM silybin-phosphatidylcholine down-regulates ESR1 more than 75 and 150 μM silybin which are higher concentrations than the silybin- phosphatidylcholine dose. On the contrary, the T47D cell line showed negligible ESR2 expression by real time RT-PCR, suggesting that this cell line is ESR2 negative.
Some evidence has shown that anti-cancer drugs are not effective enough to treat all cases of cancers and may also show resistance (45, 46). For many years, tamoxifen was the mainstay of endocrine treatment for ER+ breast cancer (47), but recently the third-generation aromatase inhibitors (AIs) called estrogen receptor down- regulators (ERDs) such as fulvestrant (Faslodex) have started to be used ahead of tamoxifen in the first-line advanced (48) and adjuvant (49) settings because of their superior efficacy and tolerability profiles. Fulvestrant is an ER antagonist with no estrogen agonist effects and a novel mode of action; it binds, blocks, and increases degradation of ER (50).
Since cancer has different causes, and more than one mutation, sometimes blocking a receptor is not sufficient to silence the related cell signals (51, 52). Thus, flavonoids, such as silybin in contrast to some anti-cancer drugs (e.g. tamoxifen, fulvestrant) have more extensive effects on multiple cell signals, and may be used for different types of breast or other cancers. It can be used for a set period either singly or in combination with anti- cancer drugs to downregulate ESR1.
Conclusion
This study suggests that silybin and silybinphosphatidylcholine down regulate ESR1 in ER+ breast cancers. Results show that in T47D cells, silybin-phosphatidylcholine has a much higher inhibitory effect and down-regulated ESR1 more significantly than silybin. However, systematic clinical trials are required to test silybin- phosphatidylcholine in order to fully understand its potential.
Acknowledgments
This research was supported by University of Tehran. The authors declare no conflict of interest.
References
- 1.Hayashi SI, Eguchi H, Tanimoto K, Yoshida T, Omoto Y, Inoue A, et al. The expression and function of estrogen receptor and in human breast cancer and its clinical application. Endocr Relat Cancer. 2003;10(2):193–202. doi: 10.1677/erc.0.0100193. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua SAW. Hormone receptors in breast cancer, cancer treatment and research. USA: Springer Science Business Media; 2009. pp. 1–1. [Google Scholar]
- 3.Deroo BJ, Korach KS. Estrogen receptor and human disease. J Clin Invest. 2006;116(3):561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167(1-2):139–150. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 5.Noh EM, Yi MS, Youn HJ, Lee BK, Lee YR, Han JH, et al. Silibinin enhances ultraviolet B-induced apoptosis in MCF-7 human breast cancer cells. J Breast Cancer. 2011;14(1):8–13. doi: 10.4048/jbc.2011.14.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CJ, Sukarieh R, Pelletier J. Silibinin inhibits translation initiation: implications for anticancer therapy. Mol Cancer Ther. 2009;8(6):1606–1612. doi: 10.1158/1535-7163.MCT-08-1152. [DOI] [PubMed] [Google Scholar]
- 7.Chinni SR, Li Y, Upadhyay S, Koppuiu PK, Sarkar FH. Indole-3- carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogen. 2001;20(23):2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 8.Lippaman SM, Hong WK. Cancer prevention science and practice. Cancer Res. 2002;62(18):5119–5125. [PubMed] [Google Scholar]
- 9.Clinton SK, Giovannucci E. Diet, nutrition and prostate cancer. Annu Rev Nutr. 1998;18:413–440. doi: 10.1146/annurev.nutr.18.1.413. [DOI] [PubMed] [Google Scholar]
- 10.Siess MH, Le Bon AM, Canivenc MC, Suschete M. Mechanisms involved in the chemoprevention of flavonoids. Biofactors. 2000;12(1-4):193–199. doi: 10.1002/biof.5520120131. [DOI] [PubMed] [Google Scholar]
- 11.Singh R P, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and down regulation of surviving, Akt and NF-kappaB: implication for angioprevention and antiangiogenic therapy. Oncogene. 2005;24(7):1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 12.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008;14(1):300–308. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF- 7 and MDA-MB468 cells. Oncol Rep. 2004;11(2):493–499. [PubMed] [Google Scholar]
- 14.Sangeetha N, Aranganathan S, Nalini N. Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1, 2 dimethylhydrazine induced rat colon cancer. Invest News Drugs. 2009;28(3):225–233. doi: 10.1007/s10637-009-9237-5. [DOI] [PubMed] [Google Scholar]
- 15.Sharma GG, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649–2655. [PubMed] [Google Scholar]
- 16.Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferation and apoptosis effects of silibinin in rat prostate cancer cells. Prostate. 2002;53(3):211–217. doi: 10.1002/pros.10146. [DOI] [PubMed] [Google Scholar]
- 17.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45(6):436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 18.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cycline-dependent kinases and associate cycles. Clin Cancer Res. 1998;4:1055–1064. [PubMed] [Google Scholar]
- 19.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Cancer Res. 2002;8(11):3512–3519. [PubMed] [Google Scholar]
- 20.Dhanalakshmi S, Agarwal P, Glode LM, Agarwal R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatinand carboplatin-induced growth inhibition and apoptotic death. Int J Cancer. 2003;106(5):699–705. doi: 10.1002/ijc.11299. [DOI] [PubMed] [Google Scholar]
- 21.Flaig TW, Su LJ, Harrison G, Agarwal R, Glode LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int J Cancer. 2007;120(9):2028–2033. doi: 10.1002/ijc.22465. [DOI] [PubMed] [Google Scholar]
- 22.Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDAMB468 cells. Oncol Rep. 2004;11(2):493–499. [PubMed] [Google Scholar]
- 23.Agarwal R, Agarwal C, Ichikawa H, Singh RS, Agarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26(6B):4457–4498. [PubMed] [Google Scholar]
- 24.Mahmoodi N, Besharati-Seidani T, Motamed N, Mahmoodi N. Anti-cancerous effect of 4,4'-dihydroxychalcone ((2E,2'E)-3,3'-(1,4- phenylene) bis (1-(4-hydroxyphenyl) prop-2-en- 1-one)) on T47D breast cancer cell line. Annual Research & Review in Biology. 2014;4(12):2045–2052. [Google Scholar]
- 25.Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. 2014;58(3):516–527. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 26.Espy M. Bioavailability: How the nutrients in food become available to our bodies. "Health guidance". Available from:http://www.healthguidance.org/entry/6265/1/ Bioavailability-How-the-Nutrients-in-Food-Become- Available-to-Our-Bodies.html. (7 Jul 2013)
- 27.Gazaka R, Purchartovaa K, Marhola P, Zivnaa L, Sedmeraa P, Valentovab K, et al. Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur J Med Chem. 2010;45(3):1059–1067. doi: 10.1016/j.ejmech.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 28.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybinphosphatidylcholine complex(siliphos) Altern Med Rev. 2005;10(3):193–203. [PubMed] [Google Scholar]
- 29.Eiseler T, Doppler H, Yan IK, Goodison S, Storz P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009;11(1):1–12. doi: 10.1186/bcr2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis AL, Tran NT, Chatigny JM, Charlton N, Vu H, Brown SA, et al. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2- positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res. 2008;6(5):725–34. doi: 10.1158/1541-7786.MCR-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao J, Salari K, Bocanegra M, Choi Y, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. Plos One. 2009;4(7):1–16. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AW, Paulson QX, Hong J, Stubbins RE, Poh K, Schrader E, et al. Alcohol promotes breast cancer cell invasion by regulating the Nm23-ITGA5 pathway. J Exp Clin Cancer Res. 2011;30:75–75. doi: 10.1186/1756-9966-30-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goicoechea SM, Bednarski B, Garcia-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28(4):587–598. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF. Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer. 2009;125(2):318–327. doi: 10.1002/ijc.24308. [DOI] [PubMed] [Google Scholar]
- 36.Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, et al. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of b1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol. 2011;29(4):2512–2518. doi: 10.1007/s12032-011-0113-8. [DOI] [PubMed] [Google Scholar]
- 37.Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol Int. 2008;32(8):888–892. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Shin JS, Hong SW, Oom lee SL, Kim TH, Park IC, An SK, et al. Serum starvation induces G1 arrest through suppression of Skp2-CDK2 and CDK4 in SK-OV-3 cells. Int J Oncol. 2008;32(2):435–439. [PubMed] [Google Scholar]
- 39.Ford CHJ, Al-Bader M, Al-Ayadhi B, Francis I. Reassessment of estrogen receptor expression in human breast cancer cell lines. Anticancer Res. 2011;31(2):521–527. [PubMed] [Google Scholar]
- 40.Morley KL, Ferguson PJ, Koropatnick J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007;251(1):168–178. doi: 10.1016/j.canlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Kim SH, Hur SM, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cells. J Ethnopharmacol. 2009;126(2):252–257. doi: 10.1016/j.jep.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Singh RP, Agarwal R. Prostate cancer prevention by silibinin. Curr Cancer Drug Targets. 2004;4(1):1–11. doi: 10.2174/1568009043481605. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharya S. Phytosomes: emerging strategy in delivery of herbal drugs and nutraceuticals. Pharma Times. 2009;41(3):9–12. [Google Scholar]
- 45.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of Disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3(5):269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia A, Kumar R, Katare OP. Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J Pharm Pharm Sci. 2004;7(2):252–259. [PubMed] [Google Scholar]
- 47.Wickerham L. Tamoxifen-an update on current data and where it can now be used. Breast Cancer Res Treat. 2002;75(Suppl 1):S7–S12. doi: 10.1023/a:1020353530963. [DOI] [PubMed] [Google Scholar]
- 48.Sainsbury R. Aromatase inhibition in the treatment of advanced breast cancer: is there a relationship between potency and clinical efficacy? Br J Cancer. 2004;90(9):1733–1739. doi: 10.1038/sj.bjc.6601731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: Status report 2004. J Clin Oncol. 2005;23(3):619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 50.Robertson JFR. Fulvestrant (Faslodex)-how to make a good drug better. Oncologist. 2007;12(7):774–784. doi: 10.1634/theoncologist.12-7-774. [DOI] [PubMed] [Google Scholar]
- 51.Sibilia M, Zielinski CC, Bartsch R, Grunt TW. Drugs for HER-2-positive breast cancer. 1st ed. UK: Springer Basel AG; 2011. pp. vi–vi. [Google Scholar]
- 52.Kute T, Lack CM, Willingham M, Bishwokama B, Williams H, Barrett K, et al. Development of Herceptin resistance in breast cancer cells. Cytometry A. 2004;57(2):86–93. doi: 10.1002/cyto.a.10095. [DOI] [PubMed] [Google Scholar]