Abstract
N-methyl-d-aspartate receptor (NMDAR) activation requires both the binding of glutamate to its recognition site and occupancy of the strychnine insensitive glycine modulatory site (GMS). Pharmacological studies suggest that the glycine transporter, GlyT1, maintains subsaturating concentrations of glycine at synaptic NMDARs. To characterize further the role of GlyT1, we generated mice in which the gene encoding GlyT1 was inactivated by homologous recombination through insertion of a PGK-Neo cassette in place of exons 2 and 3. Real-time quantitative PCR revealed no transcripts in newborn homozygous [GlyT1(-/-)] mice and a 50% reduction in heterozygous (HZ) [GlyT1(+/-)] mice as compared with WT littermates. The activity of Na+-dependent glycine transport in forebrain homogenates was similarly affected. Homozygous mice died within 12 h of birth. In acute hippocampal slices, exogenous glycine or d-serine (10 μM) enhanced NMDAR currents with Schaffer collateral stimulation in WT mice but not HZ mice, suggesting that the GMS was more occupied in the latter. The NMDAR/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor ratio of the excitatory postsynaptic currents was significantly increased in the HZ mice. In the water maze, the HZ mice exhibited better spatial retention. Furthermore, HZ mice were less sensitive to an amphetamine disruption of prepulse inhibition than WT mice but were more sensitive to the effects of MK-801. Thus, reduced expression of GlyT1 enhances hippocampal NMDAR function and memory retention and protects against an amphetamine disruption of sensory gating, suggesting that drugs which inhibit GlyT1 might have both cognitive enhancing and antipsychotic effects.
Keywords: glutamate, glial cell, memory, prepulse inhibition
Glycine (1) and/or d-serine (2) are obligatory coagonists at the N-methyl-d-aspartate receptor (NMDAR) by binding to the strychnine-insensitive site on the NR1 subunit of the NMDAR permitting the subsequent binding of glutamate (3). The affinity of glycine for this glycine modulatory site (GMS) ranges from 0.1 to 3 μM depending on the NR2 subtype of the NMDAR (4). Because the concentration of glycine in the cerebrospinal fluid is 5–10 μM, it has been assumed that the GMS is saturated. However, Smith et al. (5) cloned a glycine high-affinity Na+-dependent transporter, GlyT1, which is expressed in brain with a distribution that mirrors NMDAR localization. With the development of potent and specific antagonists of GlyT1, the role of GlyT1 in maintaining subsaturating levels of glycine at the glutamatergic synapse was established. Thus, in the acute hippocampal slice preparation, Bergeron et al. (6) demonstrated a robust enhancement of NMDAR responses of CA1 pyramidal cell to Schaffer collateral stimulation in the presence of the potent GlyT1 antagonist N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)] propylsarosine (NFPS). These findings have been replicated in a rat prefrontal cortex acute slice preparation (7). Together, these findings suggest that GlyT1 maintains subsaturating concentrations of glycine at the GMS on synaptic NMDARs. Kinney et al. (8) have reported that systemic administration of NFPS increases longterm potentiation in the dentate gyrus and enhances prepulse inhibition of acoustic startle (PPI), suggesting that inhibition of GlyT1 affects NMDAR function in a behaviorally relevant fashion.
Although these findings with the relatively selective NFPS provide evidence of a functional role for GlyT1 in NMDAR function, we decided to examine further the effects of persistent hypofunction of GlyT1 by creating mice in which the gene encoding GlyT1 was inactivated by homologous recombination to ensure that these effects were indeed mediated by GlyT1.
Methods
Mice. All animal studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the appropriate institutional animal care and use committee. All mice used for functional studies were back-crossed at least nine generations to the 129/SvEvTac background.
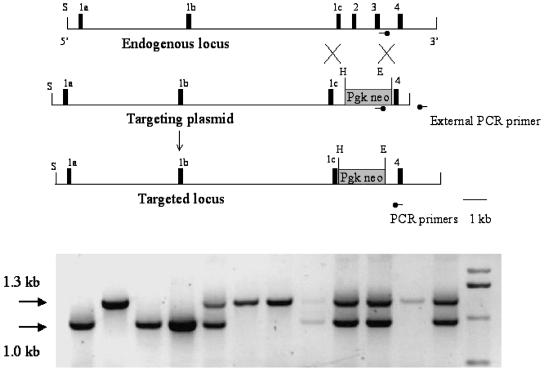
Targeting Vector and Generation of GlyT1 Mutant Mice. By PCR screening of the lambda FIXII 129/SvJ mouse genomic library (Stratagene) with mouse GlyT1-specific primers, we identified a positive ≈18.5-kb genomic clone that contained exons 1a–4. The phage clone containing GlyT1 exons 1a–4 was subcloned, mapped, and sequence-confirmed with mouse GlyT1-specific primers. A targeting plasmid was constructed by replacing the Eagl-Hpal 1.25-kb fragment, covering exons 2 and 3, with the 1.8-kb PGK-neomycin gene cassette in the opposite orientation of the GlyT1 gene. We targeted exons 2 and 3 because they are common to all of the isoforms of GlyT1A-D. The targeting vector contained 14.4 kb of homologous DNA upstream to the PGK-neomycin cassette and 1.05 kb downstream.
The targeting vector was linearized and electroporated into embryonic stem (ES) cells derived from 129SvJ, which were maintained on subconfluent embryonic fibroblasts (9). Neomycin-resistant cell colonies were isolated and expanded. The ES cells harboring the homologous recombination were determined by PCR with primers external to the targeting construct as depicted in Fig. 1. Approximately 15% of the ES cell colonies had the correct recombination.
Fig. 1.
Targeting construct of GlyT1-null mutation (Upper), and genotype of postnatal day (P) 1 mice (Lower). (Upper)1c–4, Exons 1c–4; E, EagI; H, HpaI; S, SalI; Pgk neo, neomycin cassette with PGK promoter. (Lower) PCR genotyping of a litter of GlyT1 mutant mice. The first, third, and fourth lanes represent GlyT1(-/-) mice, the fifth, eighth, ninth, tenth, and twelfth lanes represent GlyT1(+/-) mice, and the rest represent WT mice.
The targeted ES cells were injected into C57BL/6 blastocysts. These blastocytes were transferred into pseudopregnant mothers. Chimeric mice were bred with 129S6/SvEvTac mice (Taconic Farms). The F1 mice with germ line transmission of the GlyT1 mutation were bred to F2. The colony was maintained on a 129S6/SvEvTac background. Genotypes of the mice were determined by PCR analyses of the mouse tail DNAs as in the ES cells. PCR primers for genotyping were as follows: 5′-GCCTTGGGAAAAGCGCCTCC-3′ from PGK-neomycin, 5′-CCCCTACTTCATCATGCTGATC-3′ for the WT allele, and 5′-CACCTACCAGTAGTTGCCTT-3′ as the primer common to both WT and mutant alleles (Fig. 1). The thermocycle of the PCR was 36 cycles at 94°C (30 s), 57°C (30 s), and 72°C (1 min 30 s). The amplicon from the WT allele is 1.3 kb, and the mutant allele is 1.0 kb (Fig. 1).
Real-Time Quantitative (RTQ)-PCR. Total RNA from brain regions was prepared by using TRIzol solution (GIBCO). The samples were treated for DNA contamination by using DNase I (Ambion, Austin, TX). For RTQ-PCR, primer pairs of exon 2 and 3, and 4 and 6, were applied. Each primer set was tested by conventional PCR before the RTQ-PCR to ensure that the amplicon yields a distinct single band. The RTQ-PCR was performed on an Opticon Continuous Florescence Detector (MJ Research, Waltham, MA). A standard curve of 109 to 102 molecules was also run simultaneously by using GlyT1-specific primers. Data were analyzed by using the Opticon software.
Glycine Uptake. Forebrains were homogenized in 20 vol of icecold 0.32 M sucrose, and the P2 fraction was isolated by differential centrifugation. The pellets were resuspended in 1 ml of Hepes-buffered saline and were incubated for 10 min at 37°C with [3H]glycine (10 nM) with transport stopped with ice-cold buffer, followed by centrifugation at 30,000 × g. KT and Vmax were determined by diluting [3H]glycine with unlabeled glycine. The resulting pellets were dissolved in 0.25 ml of 0.2 M NaOH and subjected to scintillation counting. Replacement of Na+ or Cl- with choline or acetate, respectively, completely abolished the [3H]glycine uptake.
Electrophysiologic Studies. The brains from heterozygous (HZ) and WT mice (12–13 weeks old) were removed and placed in an oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) at 4°C. Coronal sections (300 μm) containing the hippocampus were obtained with a vibrating microtome (VT 1000S, Leica, Bannockburn, IL) and stored for 1 h in an oxygenated chamber at room temperature before they were used for the experiments. As described in ref. 10, voltage–clamp recordings of CA1 pyramidal cells were obtained with a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA) under visual control by using differential interference contrast and infrared video microscopy (IR-DIC) (DMLFSA, Leica). Excitatory postsynaptic currents (EPSC) were evoked by electrical stimulation of the Schaffer collaterals with a bipolar microelectrode positioned in the stratum radiatum with 100-μs current pulses (0.1–1 mA, 0.3–0.01 Hz), which were adjusted to evoke a current amplitude in the range of 60–120 pA at Vm = -70 mV. The recordings were first obtained in normal ACSF, and then the NMDAR component was isolated pharmacologically.
NMDAR/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) ratios were computed by taking the average of EPSC at +40 mV in ACSF containing picrotoxin (50 μM), CGP 52432 (10 μM), and strychnine (0.5 μM) in the absence and presence of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) (20 μM). The average response in the presence of NBQX (NMDAR only) was subtracted from that measured in its absence, and an average AMPAR-mediated current was then calculated. Data were collected by using pclamp 9 software (Axon Instruments). Analyses were performed off-line with igor software (WaveMetrics, Lake Oswego, OR) run on a PC.
Behavioral Tests. Mice were housed, up to five per cage, in a room with a 12-h light/dark cycle (lights on at 0600 hours), and behavioral testing was performed during the light phase of the cycle. Food (standard rodent diet) and water were provided ad libitum. At 12 weeks of age, mice were subjected to a neurological battery to assess reaching and righting reflexes and grip strength (11). Spatial learning and memory were assessed by using the 1-day water maze paradigm (12) in which mice were pretrained to escape from a pool of water by climbing onto a visible platform. The mice then learned to locate a hidden platform by using extra-maze visual cues. Spatial memory trials were arranged in three blocks of four trials. Thirty minutes after the third block, mice received one probe trial in which the platform initially was removed. Thirty seconds were allowed for mice to attempt to locate the platform. The location of a visible platform was changed to a different quadrant for each of four cued trials with the extra-maze cues removed. Data were recorded for subsequent analysis (HVS Images, Hampton, England). Parameters measured for spatial trials were swim time (time to platform), path length, corridor (a measure of the amount of time the mouse spends in a path, one platform wide, between the place of entry into the pool and the platform), and swim speed. For the probe trial, quadrant time (time spent in the former target quadrant) and platform crossings were measured during the first 30 s.
For behavioral pharmacologic studies, MK-801 and d-amphetamine were obtained from Sigma/RBI and dissolved in sterile water. Doses for each drug were chosen based on previous reports in the literature (13, 14). In PPI experiments, the first dose of a drug was assigned by using a Latin-square design, and animals received all doses to complete a within-subjects design. In locomotor experiments, each mouse was assigned one dose of a drug and also tested with vehicle. All drug experiments were separated by at least 1 week.
PPI was determined as described in ref. 14. Briefly, startle reactivity was measured by using four startle chambers (SR-LAB, San Diego Instruments, San Diego). Sixty-five readings were taken at 1-ms intervals, starting at stimulus onset, and the average amplitude was used to determine the acoustic startle response. The startle trials consisted of a 40-ms 120-dB pulse of broadband noise. PPI was measured by trials that consisted of a 20-ms noise prepulse, a 100-ms delay, then a 40-ms 120-dB startle pulse (120-ms onset-to-onset interval). The acoustic prepulse intensities were 3, 6, and 12 dB above the 65-dB background noise (i.e., 68, 71, and 77 dB). There was an average of 15 s (range 12–30 s) between trials. The mice were placed into the startle chambers immediately after each drug was injected, where background noise was presented for a 10-min acclimation period. The amount of PPI was calculated as a percentage score for each acoustic prepulse trial type: % PPI = 100 - {[(startle response for prepulse plus pulse)/(startle response for pulse alone)] × 100}. Acoustic startle magnitude was calculated as the average response to all of the pulse alone trials, excluding the first and last blocks of five pulse-alone trials presented.
Locomotor activity was measured by using the Photobeam Activity System (San Diego Instruments). The test sessions were 4 h in duration, and data were collected in 10-min time bins, where horizontal beam breaks, rearing, and repetitive movements comprised the total activity measure. On each drug test day, mice were allowed to acclimate to the locomotor chambers for 1 h and then removed, injected with vehicle, MK-801, or amphetamine, and returned to the locomotor chamber for the remaining 3 h of the session. The data from the 3-h posthabituation testing were used for data analyses.
Statistical Analyses. ANOVA was used to compare group values and pairwise comparisons were used to explore significant main effects or interactions. Drug effects were analyzed by using drug dose as a within-subject factor and genotype as a between-subjects factor.
Results
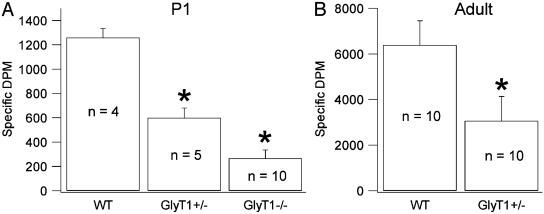
Expression of GlyT1 Is Reduced in Mutants. GlyT1(-/-) died within 12 h of birth because of respiratory distress. However, the body weights and total brain weights at birth did not differ significantly among GlyT1(-/-), HZ, and WT mice. Body weights of the adult male mice at 4 months were virtually identical between the HZ (25.4 ± 0.3 g, n = 10) and WT (24.3 ± 0.3 g, n = 10) mice. At birth, the uptake of [3H]glycine into brain homogenates was significantly reduced in HZ, and the GlyT1(-/-) mice had negligible glycine uptake (Fig. 2). At 12 weeks of age, the uptake of [3H]glycine was reduced by half in HZ compared with WT mice. Saturation isotherms indicated a Vmax of 0.36 ± 0.04 nmol per mg of protein per min for WT but 0.17 ± 0.03 nmol per mg of protein per min (P < 0.01) for the HZ with no significant difference in KT. The mRNA expression of GlyT1 paralleled the findings for glycine uptake. Total GlyT1 mRNA was quantified by applying primers common to both WT and mutant alleles; total RNA was similar across the three GlyT1 genotypes (+/+, +/-, and -/-). However, primers specific to the genetargeting region confirmed that the GlyT1(-/-) pups were missing the WT mRNA, whereas HZ pups had a significantly lower number of mRNA than WT pups (cortex: WT, 713 ± 59; HZ, 252 ± 41; cerebellum: WT, 846 ± 56; HZ, 294 ± 136; kidney: WT, 850 ± 72; HZ, 378 ± 91; P < 0.01 for all).
Fig. 2.
Na+-dependent [3H]glycine uptake in the forebrain homogenates of P1 pups and adult mice. Na+-dependent [3H]glycine uptake is determined in the P2 fraction from the forebrain of the P1 pups of WT, GlyT1(+/-), and GlyT1(-/-) mice and adults of WT and GlyT1(+/-) mice. (A) In P1 pups, [3H]glycine uptake is lower in GlyT1(+/-) mice compared with WT mice (*, P < 0.001), and [3H]glycine uptake in GlyT1(-/-) mice is lower than that in WT (*, P < 0.001) and GlyT1(+/-)(*, P = 0.005) mice. (B) In adult mice, [3H]glycine uptake is lower in GlyT1(+/-) mice than in WT mice (P < 0.001).
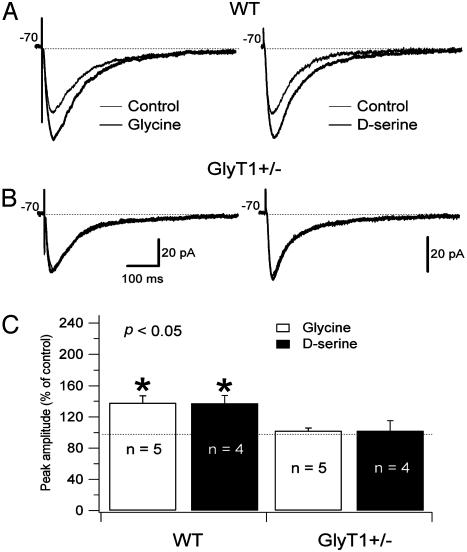
NMDAR Function Is Enhanced in GlyT1(+/-) CA1 Pyramidal Cells. To determine whether reduced GlyT1 expression in the HZ mice affects synaptic glycine levels, we tested the effect of glycine application on NMDAR currents. To evoke postsynaptic glutamatergic currents, the Schaffer collaterals were stimulated with a bipolar electrode, and postsynaptic CA1 pyramidal cells were held at Vm = -70 mV. The NMDAR-mediated component of the EPSC was isolated pharmacologically in a low Mg2+ ACSF (see Methods). Perfusion with glycine (10 μM) produced a significant increase in the amplitude of NMDAR currents of CA1 pyramidal cells in WT mice (37 ± 9%, n = 5; P < 0.005) while having no significant effect in HZ mice (2.2 ± 3.8%, n = 5; P > 0.05). Similarly, perfusion with d-serine, a full GMS agonist not transported by GlyT1, produced a significant increase in amplitude of NMDAR currents in WT mice (37 ± 10%) while having no significant effect in the HZ mice (Fig. 3). These data suggest that in HZ mice the number of GlyT1 expressed is insufficient to maintain a subsaturating concentration of glycine at the synapse. However, increased occupancy of the strychnine-insensitive GMS might increase NMDA function. To test this hypothesis, we examined the NMDAR/AMPAR ratio in HZ and WT mice. We found that the NMDAR/AMPAR ratio was significantly larger in HZ mice (4.76 ± 0.22, n = 12; P < 0.05) compared with WT mice (2.77 ± 0.88, n = 11). In a study of the spontaneous NMDAR EPSC, we observed that the NMDAR quantal size was the same in WT and HZ pyramidal cells, suggesting no difference in the number of NMDARs present at the synapses.
Fig. 3.
Administration of 10 μM glycine and 10 μM d-serine increase the amplitude of the evoked NMDAR currents of pyramidal cells in WT mice but not in GlyT1(+/-) mice. Pyramidal cells were recorded in a low Mg2+ ACSF (0.1 mM) in the presence of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) (20 μM), picrotoxin (50 μM), CGP 52432 (10 μM), and strychnine (0.5 μM). Responses were evoked by bipolar electrical stimuli at Vm = -70 mV in pyramidal cells of WT (A) and GlyT1(+/-) (B) mice. The NMDAR currents recorded in low Mg2+ ACSF (thin line) and during application of glycine (Left; thick line) or d-serine (Right; thick line) are superimposed. The application of glycine and d-serine did not change the amplitude of the NMDAR currents in GlyT1(+/-) pyramidal cells (B). (C) Histogram showing the effects of glycine (10 μM) and d-serine (10 μM) on NMDAR currents as percentage increase of the control. *, Difference was significant after to the application of glycine (open bar) and d-serine (filled bar) on WT mouse pyramidal cells (P < 0.005).
Spatial Retention Is Better in GlyT1(+/-) Mice. Both HZ and WT adult mice appeared healthy and had normal grooming behaviors. Reaching, righting, and grip reflexes were normal in both groups of mice. HZ and WT mice learned the 1-day swim task at the same rate with significant improvement in performance across trials (P < 0.05). Moreover, there were no significant differences between the two groups in swim time (P = 0.67) or path length (P = 0.88) measures during the spatial learning trials. However, on the probe trials, when the platform was removed from the maze, the HZ outperformed WT mice. HZ mice spent 1.9-fold more time in the former platform quadrant than WT mice [t(18) = 6.8, P = 0.018] and made 4-fold more platform crossings than WT mice [t(18) = 10.8, P = 0.004], suggesting better retention of the position of the platform for HZ mice. In the cued (visible platform) portion of the task, the HZ and WT mice performed similarly, with no significant differences in swim time, swim speed, or path length measures between the groups, indicating that their vision and motivation were not impaired.
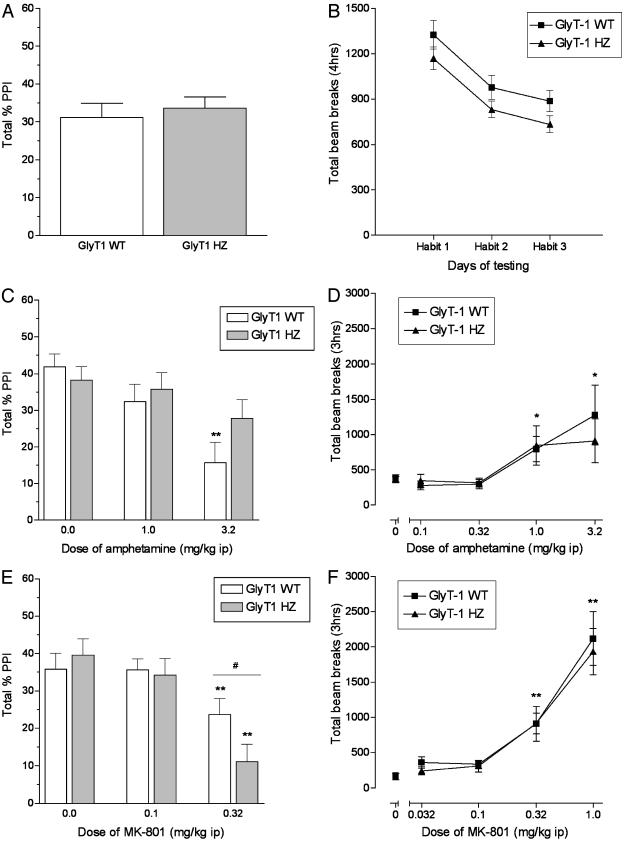
Prepulse Inhibition. HZ and WT mice had comparable levels of PPI in their initial characterization (Fig. 4A). In addition, HZ mice did not differ from WT mice in the 4-h assessments of spontaneous activity, and both groups showed habituation across test sessions (Fig. 4B). In tests with amphetamine, there was a significant main effect of drug on PPI [F(2,96) = 15.6, P < 0.001], and post hoc analyses revealed that only WT mice had disrupted PPI after administration of 3.2 mg/kg (P < 0.01) (Fig. 4C). There was also a significant main effect of MK-801 on PPI [F(2,96) = 24.5, P < 0.001]. In contrast to the effects of amphetamine, 0.32 mg/kg MK-801 disrupted PPI in both WT and HZ mice, and the effect of MK-801 was more profound in HZ than WT mice (P < 0.05) (Fig. 4E). Higher doses of amphetamine and MK-801 (10 mg/kg and 1.0 mg/kg, respectively) decreased PPI in a comparable manner in all mice regardless of genotype (data not shown). In tests of motor activity, both WT and HZ showed increased activity after 1.0 and 3.2 mg/kg amphetamine treatment [F(1,8) = 7.3 and P < 0.05, and F(1,7) = 7.0 and P < 0.05, respectively]. Both genotypes also showed increased activity after administration of 0.32 and 1.0 mg/kg MK-801 [F(1,11) = 13.7 and P < 0.01, and F(1,16) = 34.8 and P < 0.01, respectively] (Fig. 4 D and F).
Fig. 4.
PPI (Left) and locomotor activity (Right) in GlyT1 WT (+/+) and HZ (+/-) mice. (A and B) At baseline, there were comparable levels of PPI and locomotor activity in the two genotypes. (C) Amphetamine decreased PPI in WT mice but not HZ mice. (D) Amphetamine (1.0–3.2 mg/kg) increased locomotor activity in both WT and HZ mice. (E) MK-801 (0.32 mg/kg) decreased PPI more profoundly in HZ mice than in WT mice. (F) MK-801 (0.32–1.0 mg/kg) increased locomotor activity in both WT and HZ mice.
Discussion
Glycine transporters belong to the superfamily of 12 transmembrane domain, Na+-dependent, neurotransmitter transporters (5). Two different genes, GlyT1 and GlyT2, encode the glycine transporters. GlyT1 mRNAs are present heterogeneously in the frontal cortex and hippocampus, as well as the lower brainstem and spinal cord (15). Expression of GlyT1 corresponds closely to the expression pattern of NMDARs (5). GlyT1 is expressed in glia cells in close proximity to both excitatory and inhibitory synapses (16). Application of exogenous agonists for the GMS (glycine or d-serine) or antagonists for GlyT1 enhances the amplitude of NDMAR currents in in vitro experiments (6, 7, 10, 17). Sufficient numbers of GlyT1 appear critical in maintaining the glycine concentration gradient, hence modulating NMDAR functions (4).
To clarify further the role of GlyT1 in modulating NMDAR function at the synapse, we developed mice in which the expression of GlyT1 was disrupted by homologous recombination at exons 2 and 3, a mutation affecting all isoforms. Unfortunately, complete GlyT1 inactivation proved fatal soon after birth for the GlyT1(-/-) mice, apparently because of suppression of the respiratory center because of excessive glycinergic neurotransmission (18). Nevertheless, GlyT1 HZ mice exhibited normal growth and physical characteristics. RTQ-PCR revealed no GlyT1 transcripts in the GlyT1(-/-) mice and a 50% or more reduction in the cortex, cerebellum, and kidney of the HZ mice as compared with WT mice. Reduced expression of GlyT1 persisted into adulthood in the HZ mice. Additionally, Na+-dependent glycine uptake in forebrain homogenates for GlyT1(-/-) mice was profoundly reduced, and there was a >50% loss of activity in HZ versus WT mice, resulting from a loss of transporters as demonstrated by the reduced Vmax in HZ mice.
To determine the physiologic impact of reduced expression of GlyT1, we examined the function of the NMDAR at the Schaffer collateral CA1 pyramidal cell synapse in acute hippocampal slices prepared from adult HZ and WT mice. As we reported previously, application of exogenous glycine produces significant increases in the amplitude of NMDAR currents in CA1 pyramidal cells of WT mice (6). However, exogenous glycine had no significant effect in CA1 pyramidal cells of HZ mice. Furthermore, perfusion with d-serine, which is not a substrate for GlyT1, increased NMDAR current amplitudes in WT mice but not HZ mice. These data suggest that the number of GlyT1 transporters in HZ mice is insufficient to keep the glycine concentration in the synaptic cleft at subsaturating levels. Consistent with enhanced NMDAR function in the HZ mice, the NMDAR/AMPAR ratio is increased at CA1–Schaffer collateral synapses in HZ as compared with those of WT mice.
HZ mice have better retention of the position of the platform during a spatial learning task, although they acquired the task at a similar rate to WT mice. Interestingly, during the probe trial the 129SvEv WT mice did not retain the platform quadrant as well after the intensive spatial training trials as did the C57BL/6 mice (12). The suboptimal performance of WT mice may have made it possible to discern the improved performance of the HZ mice. The improved performance on the probe trial of the HZ mice was not confounded by problems with general health or locomotor activity. The similarities in performances of the cued task between the groups suggest that both groups of mice had similar visual abilities and motivation to find the platform and climb onto it. Hence, the difference on the probe trial appears to reflect enhanced spatial memory in the HZ mice.
Spatial learning in the hidden-platform water maze requires the activation of NMDAR in the hippocampus (19, 20). Mice transgenic for and overexpressing NR2B exhibit increased NMDAR conductance, more robust hippocampal long-term potentiation, and enhanced performance on a number of memory tasks (21). Notably, NR2B transgenics exhibit significantly greater preference for the former platform quadrant during probe trials, similar to our findings in the GlyT1 HZ mice, consistent with enhanced NMDAR function in both mutants. In contrast, pharmacological blockade of the NR1/NR2B subunit does not impair acquisition or retention of a spatial navigation task in rats (22), suggesting that the different subunits may play different roles in spatial learning and memory.
Two different propsychotic classes of drugs (the stimulants, such as amphetamine, and NMDAR antagonists, such as MK-801) disrupt PPI in rodents (23, 24). Whereas amphetamine disrupts PPI through enhanced dopaminergic function, MK801 exerts its effects by blocking NMDAR. Although a dose–response curve with amphetamine did not reveal any significant differences in its effects on locomotor activity between HZ and WT, WT mice exhibited a significant impairment in PPI after 3.2 mg/kg, whereas the HZ mice did not. In addition, although MK-801 produced identical effects on locomotor activity, the HZ mice exhibited a more profound PPI disruption after 0.32 mg/kg of MK-801 than WT mice. This dissociation with regard to PPI effects in GlyT1 HZ mice between amphetamine and MK-801 may reflect the fact that MK-801 acts as an open-channel blocker (25). Consistent with this mechanism, the neurophysiologic studies indicate greater NMDAR activity without a change in NMDAR number in HZ as compared with WT, perhaps accounting for this greater sensitivity of the HZ mice to MK-801.
Considerable evidence has accrued to support the hypothesis that hypofunction of a subpopulation of NMDAR may account for the endophenotype of schizophrenia, especially the negative symptoms and cognitive impairments (26). These findings include the fact that the NMDAR-blocking dissociative anaesthetics can recapitulate core symptoms and physiologic deficits of schizophrenia and agents that enhance GMS occupancy such as d-serine, glycine, and sarcosine, a GlyT1 inhibitor, improve negative symptoms and cognition in schizophrenic subjects receiving typical antipsychotics. Given the present findings with a GlyT1 hypomorph, drugs that inhibit GlyT1 may prove effective in treating the cognitive deficits as well as the psychosis in schizophrenia.
Acknowledgments
This work was supported by National Institute of Mental Health Conte Center Grants PO50MH60450 and MH51290 (to J.T.C.), National Institute on Drug Abuse Grants DA07252 (to R.J.R.-W.) and DA12142 (to S.B.C.), the Canadian Institute of Health Research and National Alliance for Research on Schizophrenia and Depression (R.B.), and National Institute of Neurological Disorders and Stroke Grant R44NS3459 (to J.B.-S.).
Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ES, embryonic stem; GlyT, glycine transporter; HZ, heterozygous; NMDAR, N-methyl-d-aspartate receptor; P1, postnatal day 1; PPI, prepulse inhibition; RTQ, real-time quantitative.
References
- 1.Johnson, J. W. & Ascher, P. (1987) Nature 325, 529-531. [DOI] [PubMed] [Google Scholar]
- 2.Mothet, J. P., Parent, A. T., Wolosker, H., Brady, R. O., Jr., Linden, D. J., Ferris, C. D., Rogawski, M. A. & Snyder, S. H. (2000) Proc. Natl. Acad. Sci. USA 97, 4926-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laube, B., Hirai, H., Sturgess, M., Betz, H. & Kuhse, J. (1997) Neuron 18, 493-503. [DOI] [PubMed] [Google Scholar]
- 4.Danysz, W. & Parsons, A. C. (1998) Pharmacol. Rev. 50, 597-664. [PubMed] [Google Scholar]
- 5.Smith, K. E., Borden, L. A., Hartig, P. R., Branchek, T. & Weinshank, R. L. (1992) Neuron 8, 927-935. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron, R., Meyerm, T. M., Coyle, J. T. & Greene, R. W. (1998) Proc. Natl. Acad. Sci. USA 95, 15730-15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L., Muhlhauser, M. & Yang, C. R. (2003) J. Neurophysiol. 89, 691-703. [DOI] [PubMed] [Google Scholar]
- 8.Kinney, G. G., Sur, C., Burno, M., Mallorga, P. J., Williams, J. B., Figueroa, D. J., Wittmann, M., Lemaire, W. & Conn, P. J. (2003) J. Neurosci. 23, 7586-7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudnicki, M. A., Schnegelsberg, P. N., Stead, R. H., Braun, T., Arnold, H. H. & Jaenisch, R. (1993) Cell 75, 1351-1359. [DOI] [PubMed] [Google Scholar]
- 10.Martina, M., Krasteniakov, N. V. & Bergeron, R. (2003) J. Physiol. 548, 411-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul, C. A., Beltz, B. & Berger-Sweeney, J. (1997) In Discovering Neurons: The Experimental Basis of Neuroscience (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 324-337.
- 12.Frick, K. M., Stillner, E. T. & Berger-Sweeney, J. (2000) NeuroReport 11, 3461-3465. [DOI] [PubMed] [Google Scholar]
- 13.Ralph, R. J., Paulus, M. P. & Geyer, M. A. (2001) J. Pharmacol. Exp. Ther. 298, 148-155. [PubMed] [Google Scholar]
- 14.Ralph-Williams, R. J., Lehmann-Masten, V., Otero-Corchon, V., Low, M. J. & Geyer, M. A. (2002) J. Neurosci. 22, 9604-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borowsky, B., Mezeym, E. & Hoffman, B. J. (1993) Neuron 10, 851-863. [DOI] [PubMed] [Google Scholar]
- 16.Zafra, F., Aragon, C., Olivares, L., Danbolt, N. C., Gimenez, C. & Storm-Mathisen, J. (1995) J. Neurosci. 15, 3952-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox, C. S., Fitzsimonds, R. M., Johnson, B. & Dichter, M. A. (1996) J. Neurophysiol. 76, 3415-3424. [DOI] [PubMed] [Google Scholar]
- 18.Gomeza, J., Hulsmann, S., Ohno, K., Eulenburg, V., Szoke, K., Richter, D. & Betz, H. (2003) Neuron 40, 785-796. [DOI] [PubMed] [Google Scholar]
- 19.Morris, R. G., Garrud, P., Rawlins, J. N. & O'Keefe, J. (1982) Nature 297, 681-683. [DOI] [PubMed] [Google Scholar]
- 20.Tsien, J. Z., Huerta, P. T. & Tonegawa, S. (1996) Cell 87, 1327-1338. [DOI] [PubMed] [Google Scholar]
- 21.Tang, Y. P., Shimizu, E., Dube, G. R., Rampon, C., Kerchner, G. A., Zhuo, M., Liu, G. & Tsien, J. Z. (1999) Nature 401, 63-69. [DOI] [PubMed] [Google Scholar]
- 22.Guscott, M. R., Clarke, H. F., Murray, F., Grimwood, S., Bristow, L. J. & Hutson, P. (2003) Eur. J. Pharmacol. 476, 193-199. [DOI] [PubMed] [Google Scholar]
- 23.Geyer, M. A., Krebs-Thomson, K., Braff, D. L. & Swerdlow, N. R. (2001) Psychopharmacology 156, 117-154. [DOI] [PubMed] [Google Scholar]
- 24.Geyer, M. A., McIlwain, K. L. & Paylor, R. (2002) Mol. Psychiatry 7, 1039-1053. [DOI] [PubMed] [Google Scholar]
- 25.Huettner, J. E. & Bean, B. P. (1998) Proc. Natl. Acad. Sci. USA 85, 1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyle, J. T., Tsai, G. & Goff, D. (2003) Ann. N.Y. Acad. Sci. 1003, 318-327. [DOI] [PubMed] [Google Scholar]