Abstract
Docosahexaenoic acid (DHA) is a lipid peroxidation target in oxidative injury to retinal pigment epithelium (RPE) and retina. Photoreceptor and synaptic membranes share the highest content of DHA of all cell membranes. This fatty acid is required for RPE functional integrity; however, it is not known whether specific mediators generated from DHA contribute to its biological significance. We used human ARPE-19 cells and demonstrated the synthesis of 10,17S-docosatriene [neuroprotectin D1 (NPD1)]. This synthesis was enhanced by the calcium ionophore A-23187, by IL-1β, or by supplying DHA. Under these conditions, there is a time-dependent release of endogenous free DHA followed by NPD1 formation, suggesting that phospholipase A2 releases the mediator's precursor. Added NPD1 potently counteracted H2O2/tumor necrosis factor α oxidative-stress-triggered apoptotic RPE DNA damage. NPD1 also up-regulated the antiapoptotic proteins Bcl-2 and Bcl-xL and decreased proapoptotic Bax and Bad expression. Moreover, NPD1 (50 nM) inhibited oxidative-stress-induced caspase-3 activation. NPD1 also inhibited IL-1β-stimulated expression of cyclooxygenase 2 promoter transfected into ARPE-19 cells. Overall, NPD1 protected RPE cells from oxidative-stress-induced apoptosis, and we predict that it will similarly protect neurons. This lipid mediator therefore may indirectly contribute to photoreceptor cell survival as well. Because both RPE and photoreceptor cells die in retinal degenerations, our findings contribute to the understanding of retinal cell survival signaling and potentially to the development of new therapeutic strategies.
Keywords: age-related macular degeneration, docosanoids, neurodegeneration, neuroprotection, Bcl-2 proteins
Dietary omega-3 fatty acids are required to maintain cellular functional integrity, and overall they are necessary to human health (1). Docosahexaenoic acid (22:6, n-3, DHA), a major component of fish oil and marine algae, is most highly concentrated in photoreceptors, brain, and retinal synapses (2). Diet-supplied DHA or its precursor, 18:3, n-3, are initially taken up by the liver and then distributed through blood lipoproteins to meet the needs of organs, notably during photoreceptor cell biogenesis and synaptogenesis (3). Therefore, DHA is required for brain and retina development (4) and has been implicated in excitable membrane function (5, 6), memory, photoreceptor biogenesis and function (8–13), and neuroprotection (14).
Photoreceptor outer segments contain rhodopsin and the highest content of DHA of any cell type (2, 13). In contact with the photoreceptor tips is a monolayer of cells derived from the neuroectoderm, the retinal pigment epithelium (RPE). These cells are the most active phagocytes of the body: in a daily cycle they engulf and phagocytize the distal tips of photoreceptor outer segments, thereby participating in rod outer segment renewal (15) in a process that is balanced by addition of new membrane to the base of the outer segments. The conservation of DHA in photoreceptors is supported by retrieval through the interphotoreceptor matrix, which supplies the fatty acid for the biogenesis of outer segments (9, 16–19). The continuous renewal of photoreceptors is tightly regulated so that their length and chemical composition, including that of their phospholipids, are maintained. Photoreceptor phospholipids contain most of their DHA mainly in carbon 2 of the glycerol backbone, but they also display molecular species of phospholipids containing DHA in both C1 and C2 positions of the glycerol backbone (4, 20). Retina, as well as brain, displays an unusual DHA-retention ability, requiring very prolonged dietary deprivation of omega-3 fatty acids to reduce the tissue content (21). Under these conditions impairments of retinal function occur (9, 22). Moreover, DHA-supplemented infant formula enhances maturation of retinal function (23), visual acuity (24, 25), and mental performance (24, 25) in preterm and term infants.
RPE cells perform several other functions, including transport and reisomerization of bleached visual pigments, and contribute to the maintenance of the integrity of the blood outer retinal barrier. Retinal detachment or trauma triggers dysfunctions in the RPE cells that lead to the onset and development of proliferative vitreoretinopathy.
Because RPE cells are critical for photoreceptor survival, oxidative-stress-mediated injury and cell death in RPE in turn impair vision. The pathophysiology of many retinal degenerations (e.g., age-related macular degenerations, Stargardt disease) involves oxidative stress leading to apoptosis of RPE cells (26, 27).
Retinal DHA is a target of oxidative-stress-mediated lipid peroxidation (10). Oxidative stress in the brain generates neuroprostanes from DHA through an enzyme-independent reaction (28). In contrast, there are studies that demonstrate DHA-mediated neuroprotection in photoreceptors (29, 30) and in the brain (14). Is this prosurvival action the result of replenishing DHA into membranes? Or is it because of a more selective signaling by a DHA-derived mediator? To date, no specific DHA mediators have been identified that elicit neuroprotection in RPE, except certain docosanoids identified in retina, the formation of which is inhibited by lipoxygenase inhibitors (31). Although the bioactivity of those docosanoids has not been studied, they were suggested to be neuroprotective (2).
Here we report the isolation and structural characterization of 10,17S-docosatriene from DHA using tandem liquid chromatography (LC)–photodiode array–electrospray ionization–tandem MS (MS/MS)-based lipidomic analysis and ARPE-19 cells. These cells are spontaneously transformed human RPE cells that conserve many cell biological and functional properties (32). Moreover, we propose to term the newly isolated dihydroxy-containing DHA derivative from ARPE-19 cells neuroprotectin D1 (NPD1) (i) because of its neuroprotective properties in brain ischemia–reperfusion (33) and in oxidative-stress-challenged RPE cells (this work); (ii) because of its potent ability to inactivate proapoptotic signaling (this work); and (iii) because it is the first identified neuroprotective mediator of DHA.
Materials and Methods
ARPE-19 Cell Culture. ARPE-19 cells were grown and maintained in DMEM-F12 medium supplemented with 10% FBS and incubated at 37°C with a constant supply of 5% CO2 (32).
LC-MS/MS Analysis and Synthesis of 10,17S-Docosatriene (NPD1). ARPE-19 lipid extracts were labeled with deuterated internal standards, purified by solid-phase extraction, and loaded onto a Biobasic-AX column (Thermo-Hypersil-Keystone, Bellefonte, PA) (100 mm × 2.1 mm; 5-μm particle size) run with a 45-min gradient protocol, starting with solvent A (40:60:0.01 methanol:water:acetic acid, pH 4.5; 300 μl/min); the gradient reached 100% solvent B (99.99:0.01 methanol:acetic acid) in 30 min and was run isocratically for 5 min. We used a TSQ Quantum (Thermo-Finnigan, San Jose, CA) triple quadrupole mass spectrometer; electrospray ionization spray voltage was 3 kV, sheath gas N2 (35 cm3/min, 275°C). Parent ions were detected on full-scan mode on Q1 quadrupole. Quantitative analysis was performed by selected reaction monitoring. Q2 collision gas was argon at 1.5 mTorr, and daughter ions were detected on Q3. Selected parent/daughter ion pairs for NPD1 and free DHA were 359/205 m/z and 327/283 m/z, respectively. Calibration curves were obtained running in parallel synthetic NPD1 and DHA (Cayman Chemical, Ann Arbor, MI). NPD1 for bioactivity was generated by biogenic synthesis using soybean lipoxygenase and DHA and characterized by LC-photodiode array-MS/MS according to reported physical criteria (33–35).
Human Cyclooxygenase 2 (COX-2) Promoter Induction. ARPE-19 cells growing in 12-well plates were transfected with 5 μg of a human COX-2–luciferase construct (830 bp) by FuGENE 6. Four hours later, fresh medium was added, and the cells were further incubated for 8 h at 37°C. Transfected cells were serum-starved for 4 h and challenged with IL-1β (10 ng/ml) for 8 h with or without added NPD1. Cell homogenates were made, protein concentrations were adjusted, and luciferase assays were performed (36).
Apoptosis by Mono- and Oligonucleosome Analysis. Cells were homogenized in a lysis buffer, and 20-μl aliquots were applied on streptavidin-coated 96-well plates. Cells were incubated for 2 h with 80 μl of incubation buffer containing the monoclonal antibodies directed against DNA and histones for detection of mono- and oligonucleosomes (catalog no. 1774425, Roche Diagnostics). After the unreacted antibodies were washed away, the immune complexes of DNA–histone antibodies remain bound to the streptavidin-coated plates, where detection is attained by horseradish peroxidase reaction.
DNA Fragmentation Assay. Cells (80% confluent) growing in DMEM (10% FBS) in six-well plates were labeled with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine per well for 24 h at 37°C. Then unincorporated [3H]thymidine was removed. Cells were then serum-starved for 1 h, treated with tumor necrosis factor α (TNF-α)/H2O2 for 6–8 h, harvested, and centrifuged at 200 × g for 10 min at 4°C. Supernatant was precipitated with 25% trichloroacetic acid to measure [3H]thymidine released during the induced oxidative stress. The cell pellet was solubilized in lysis buffer (0.2% Triton X-100 in 10 mM Tris/EDTA), and intact DNA and fragmented DNA were then separated by centrifugation at 13,000 × g for 10 min at 4°C. Fragmented DNA was precipitated from the supernatant with 25% trichloroacetic acid. The pellets were resuspended in 1% SDS, and radioactivity was measured (intact DNA in cells). The amount of DNA fragmentation is expressed as the percentage of fragmented DNA (14).
Analysis of Pro- and Antiapoptotic Proteins. Bcl-2 family proteins were analyzed by Western blot analysis. In brief, 20-μg equivalents of each cell extract were subjected to electrophoresis on an 8–16% gel (Promega) at 125 V for 2 h. The proteins were transferred to nitrocellulose membrane at 30 V for 1 h at 4°C. The membranes were subjected to treatment with primary antibodies of Bcl-2, Bcl-xL, Bax, and Bad for 1 h at room temperature and probed for 30 min with secondary antibody, goat anti-rabbit Ig:horseradish peroxidase, and horseradish peroxidase-conjugated anti-biotin antibody, and then proteins were evaluated by using an ECL kit.
Hoechst Staining. ARPE-19 cells were loaded with 2 μM Hoechst dissolved in a Locke's solution (Promega) and incubated for 1 h at 37°C before imaging. Cells were then washed once with PBS and viewed by using a Nikon DIAPHOT 200 microscope under UV fluorescence. Images were recorded by a Hamamatsu Color Chilled 3CCD camera and photoshop 5.0 software (Adobe Systems, Mountain View, CA) (37).
Results
10,17S-Docosatriene (NPD1) Generation by Human RPE Cells. ARPE-19 cells in culture contain detectable levels of NPD1 (Fig. 1). The MS/MS pattern of this lipid indicates that it represents a dihydroxy-DHA with a base peak [M-H] ion m/z 359, and with fragment ions at m/z 341, 297, 289, 261, 243, 205, 181, and 153. Diagnostic ions were essentially identical to those recently described in other tissues (34, 35). IL-1β stimulated the release of DHA and the synthesis of NPD1 (Fig. 1B) in a time-dependent fashion (data not shown). Furthermore, ARPE-19 cells stimulated with the calcium ionophore A-23187 also displayed a time-dependent increase of free DHA and in the synthesis of NPD1 (Fig. 1C). This experiment was performed without adding exogenous DHA as in Fig. 1B and demonstrated a 3- to 4-fold higher free DHA pool size available as compared to the amount of NPD1 formed. Thus, activation of a phospholipase A2 that releases free DHA is an event that precedes NPD1 synthesis. Fig. 1D depicts a possible NPD1 biosynthetic pathway. After phospholipase A2 releases DHA, a lipoxygenase-like enzyme catalyzes the synthesis of 17S-H(p)DHA, which in turn is converted to a 16(17)-epoxide that is enzymatically converted to NPD1. This finding is supported by early evidence of both the enzymatic synthesis of docosanoids in the retina (31) as well as the formation of a 17S-H(p)DHA preceding the synthesis of 10,17S-docosatriene in brain ischemia–reperfusion (33).
Fig. 1.
10,17S-docosatriene (NPD1) is synthesized in ARPE-19 cells. (A) Elucidation of the structure of NPD1 by lipidomic analysis by LC-MS/MS (33–35). MS/MS spectrum for NPD1 shows a full scan of negative ion products for selected parent ion (m/z 359). (Inset) The UV spectrum. (B) Production of NPD1 by IL-1β (6 h). (C) Calcium ionophore A-23187 (10 μM) promoted the release of free DHA (blue bars) and the formation of NPD1 (red bars) as a function of incubation time. No exogenous DHA was added. As described in Materials and Methods, cells were treated with IL-1β or A-23187, then lipids were extracted and analyzed by LC-MS/MS.
Oxidative-Stress-Triggered Apoptosis Is Inhibited by NPD1. The combination of 10 ng/ml TNF-α and 400–800 μMH2O2 led to marked increase in Hoechst-positive cells (Fig. 2). Neither TNF-α nor H2O2 alone triggers a significant effect under these conditions. Serum starvation made ARPE-19 cells more sensitive to oxidative-stress-triggered apoptosis induced by TNF-α/H2O2. Serum starvation triggers the apoptotic cascade involving the activation of the caspase-3 pathway as demonstrated in bovine RPE in culture (38). Thus, we conducted studies whereby cells were grown in culture for 72 h in the presence of BSA with or without DHA. We found that endogenous NPD1 formation was enhanced by BSA–DHA (Fig. 2A). When these cells were serum-starved for 1 h and then exposed for 14 h to TNF-α/H2O2 during serum starvation, we observed a greatly reduced number of Hoechst-positive cells as a result of the preincubation with BSA–DHA (Fig. 2A). This experiment suggests that NPD1 synthesized in the ARPE-19 cells exerted its action at an early event of the apoptotic cascade. Thus, we decided to add NPD1 (50 nM) at the time of adding TNF-α/H2O2, and as a consequence we found inhibition of oxidative stress induction of Hoechst-positive cells (Fig. 2B). When the TNF-α concentration (10 ng/ml) was maintained and H2O2 was decreased from 800 to 400 μM, fewer Hoechst-positive cells were seen. NPD1 (50 nM) almost completely inhibited the oxidative stress caused by the lower H2O2 concentration. Moreover, this effect seems to be rather specific, because prostaglandin E2, leukotriene B4 (LTB4), and 20-OH-LTB4 were unable to elicit the degree of inhibition due to NPD1, although LTB4 was partially inhibitory (Fig. 2C). Fig. 2D illustrates further experiments that compared the effect of added arachidonic acid (50 nM) or DHA (50 nM) on oxidative-stress-induced apoptosis. Arachidonic acid was partially effective, but DHA proved to be a very potent inhibitor. Therefore, we then asked whether the inhibitory activity of added DHA might be correlated with NPD1 formation. In Fig. 2E, we show the time course of formation of NPD1 in cultures treated with DHA under conditions similar to those depicted in Fig. 2D. We observed synthesis of NPD1 as a function of incubation time. The free DHA content rose as a function of incubation time up to 2 h, then decreased. These changes in free DHA pool size during serum starvation (Fig. 2E) contrasted with those seen in Fig. 1 B and C, without serum starvation. In Fig. 1 B and C, stimulation by IL-1β or calcium ionophore promoted a sustained release of endogenous DHA from membrane phospholipids.
Fig. 2.
NPD1 attenuates oxidative-stress-induced apoptosis. (A) BSA–DHA enhanced NPD1 synthesis and led to decreased apoptosis. After plating, cells incubated for 72 h in the presence of either BSA (3.35 μM) or BSA plus DHA (6.7 μM). Then the cells were serum-starved for 1 h and TNF-α/H2O2 was added (14 h). Cells pretreated with BSA plus DHA yielded marked attenuation of Hoechst-positive cells. (B) NPD1 (50 nM) attenuated oxidative-stress-induced Hoechst-positive staining. After plating (72 h), cells were serum-starved for 8 h, TNF-α/H2O2 was added, and the cells were further incubated for 14 h and then stained with Hoechst reagent. The 800 and 400 μM refer to H2O2 concentrations. (C) Lack of inhibition by 50 nM prostaglandin E2, LTB4, or 20-OH-LTB4 of oxidative-stress-induced or Hoechst-positive staining. (D) DHA (50 nM) added as a free acid inhibited oxidative-stress-induced Hoechst-positive staining. Arachidonic acid (50 nM) exhibited far less protection. In C and D, 800 μM H2O2 was used; other conditions were as in B. (E) Synthesis of NPD1 in cultures exposed to DHA (50 nM) as in D. The pool size of free DHA is shown by blue bars.
These observations imply that oxidative stress triggers DNA fragmentation and RPE apoptosis and that NPD1 inhibits these events. Therefore, we decided to directly assess these mechanisms using two additional approaches: DNA fragmentation by differential sedimentation after [3H]thymidine labeling and ELISA detection of mono- and oligonucleosomes. TNF-α/H2O2 produced a marked DNA degradation as assessed by both of these methods (Fig. 3). Moreover, NPD1 inhibited DNA fragmentation, as indicated by the inhibition of DNA degradation by direct assessment using [3H]thymidine (Fig. 3A). In addition, the TNF-α/H2O2-induced accumulation of mono- and oligonucleosomes was inhibited by NPD1, further indicating that oxidative-stress-triggered DNA breakdown is attenuated by the DHA-derived mediator (Fig. 3B).
Fig. 3.
Protection against oxidative-stress-induced DNA fragmentation by NPD1. (A) DNA fragmentation assay using [3H]thymidine prelabeling. (B) DNA fragmentation detection by ELISA detection of mono- and oligonucleosomes. NPD1 (50 nM) was added at the same time as TNF-α/H2O2.
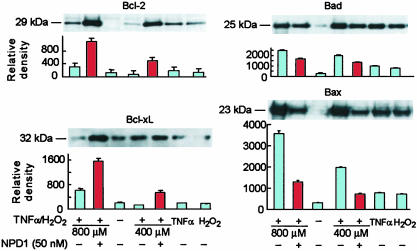
NPD1 Stimulated Antiapoptotic Bcl-2 Protein Expression and Decreased Proapoptotic Protein Expression During Oxidative Stress. Because Bcl-2 family proteins participate in the initiation and amplification of premitochondrial events in the apoptosis cascade, we investigated their participation in TNF-α/H2O2-induced ARPE-19 cell death as well as the possibility that they are a target for NPD1 action (Fig. 4). We studied two different concentrations of H2O2 (400 and 800 μM) plus TNF-α (10 ng/ml), which resulted in proportionally different numbers of Hoechst-positive cells (Fig. 2). In both instances, added NPD1 inhibited apoptosis (Fig. 2B). The antiapoptotic protein Bcl-xL was enhanced by TNF-α/H2O2 (800 μM), whereas Bcl-2 was not changed. NPD1 greatly activated expression of Bcl-xL and Bcl-2 (Fig. 4 A and B). The proapoptotic proteins Bax and Bad were up-regulated by TNF-α/H2O2 (800 μM; Fig. 4 C and D). A much lower Bax up-regulation was observed with 400 μMH2O2 plus TNF-α. However, Bad showed similar responses to both concentrations of H2O2 in combination with TNF-α. In addition, H2O2 or TNF-α alone somewhat increased Bax and Bad basal levels. NPD1 decreased Bax by 65% using 800 μMH2O2 along with TNF-α (Fig. 4D). However, the effect of the lipid mediator on oxidative-stress-increased Bad was smaller (52%) under similar conditions.
Fig. 4.
Expression of selected Bcl-2 family proteins in ARPE-19 cells: NPD1 up-regulated antiapoptotic proteins and down-regulated proapoptotic protein expression. Cells were grow for 72 h after plating, placed in serum-free medium for 1 h, then incubated with TNF-α/H2O2 for 6 h. Data represent four independent experiments with triplicate samples in each case.
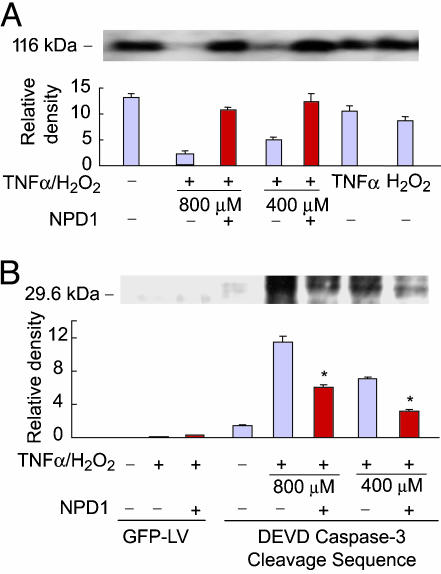
Oxidative-Stress-Mediated Caspase-3 Activation Is Attenuated by NPD1. Effector caspase-3, downstream of pro- and antiapoptotic proteins, is activated after mitochondrial cytochrome c release into the cytoplasm and activation of the apoptosome. In ARPE-19 cells, caspase-3-induced cleavage using endogenous substrate occurred when TNF-α/H2O2-triggered oxidative stress was present, as evidenced by cleavage of poly(ADP-ribose) polymerase (80% compared with the control; Fig. 5A). In addition, NPD1 was able to protect such cleavage when added with TNF-α/H2O2 (a 16% or 29% cleavage using 400 or 800 μM H2O2 plus TNF-α compared with controls). TNF-α or H2O2 alone elicits little caspase-3 activation. To further ascertain the involvement of caspase-3, we transduced ARPE-19 cells with a construct encoding a peptide containing the caspase-3 cleavage product sequence Asp-Glu-Val using a lentivirus. Then stably transfected cells (Fig. 5B) showed the cleavage product of caspase-3 during oxidative stress. NPD1 inhibited the cleavage of caspase-3 product by 50% and 60% in the presence of 400 or 800 μM H2O2 plus TNF-α, respectively.
Fig. 5.
NPD1 protects against caspase cleavage induced by TNF-α/H2O2. ARPE-19 cells were serum-starved for 1 h before a 6- to 8-h treatment with TNF-α (10 ng/ml)/H2O2 (either 400 or 800 μM as indicated) in the presence or absence of NPD1 (50 nM). (A) Caspase-3 cleavage was detected by Western blot analysis by using poly(ADP-ribose) polymerase (Santa Cruz Biotechnology) as antibody. (B) Cleavage of caspase-3 using cells stably transfected with a lentivirus construct containing the Asp-Glu-Val caspase-3 cleavage sequence. Red bars represent coincubations with 50 nM NPD1. The values are the average of triplicate samples in three independent experiments.
IL-1β-Induced COX-2 Expression Is Inhibited by NPD1. Proinflammatory gene expression is enhanced during oxidative stress and represents an important signaling route in RPE cell injury. COX-2 is an inducible enzyme that catalyzes the synthesis of prostaglandins and is involved in oxidative stress and cell function. COX-2 is actively regulated in the RPE (39). Therefore, we transfected ARPE-19 cells with a 5′ deletion construct of the human COX-2 promoter containing 830 bp fused to a luciferase reporter gene. IL-1β induces a prominent increase in COX-2 promoter expression. In a dose-dependent fashion, NPD1 potently counteracted the cytokine induction of the COX-2 promoter. The results presented in Fig. 6 indicate that, under these conditions, NPD1 displays IC50 below 5 nM.
Fig. 6.
NPD1 inhibits IL-1β-induced COX-2 expression. ARPE-19 cells grown in six-well plates were transfected with human COX-2 (830 bp) promoter-luciferase construct as described in Materials and Methods.
Discussion
The results reported here demonstrate that human ARPE-19 cells synthesize 10,17S-docosatriene (NPD1), a stereospecific mediator derived through a DHA oxygenation pathway. The structure of NPD1, elucidated by lipidomic analysis using LC–photodiode array–electrospray ionization–MS/MS, agrees with that of the recently described docosatriene found in human blood, glial cells, and mouse brain (35) and during brain ischemia–reperfusion (33). In addition, we provide evidence for the biological activity of NPD1 as a potent inhibitor of oxidative-stress-induced apoptosis and of cytokine-triggered proinflammatory COX-2 gene promoter induction in human ARPE-19 cells.
NPD1 is formed through enzyme-mediated steps involving a phospholipase A2 followed by a 15-lipoxygenase-like activity. We previously reported that retina forms mono-, di-, and trihydroxy derivatives of DHA, and that certain lipoxygenase inhibitors block this synthesis, suggesting an enzymatic process of a lipoxygenase nature (31). Although we did not know the precise stereochemistry and bioactivity of these DHA-oxygenated derivatives, we suggested that these docosanoids might be neuroprotective (2). In the RPE cells, as in the brain, the availability of free (unesterified) DHA is tightly regulated, which is demonstrated here in ARPE-19 cells (Fig. 1 B and C) and by the fact that the retina and brain free DHA pool size is negligible under basal, unstimulated conditions (40–42). Therefore, the regulation of the phospholipase A2 that releases free DHA plays an important role in the pathway leading to the formation of NPD1. The importance of this step is highlighted by the action of ischemia or seizures that elicit rapid activation of free DHA release in brain (40, 42). Our present results demonstrate that calcium ionophore, or, to a lesser extent, IL-1β, activates the synthesis of NPD1 in ARPE-19 cells. Under these conditions, there is a time-dependent increase in endogenous free DHA that is ≈3- to 4-fold higher than the amount of NPD1 being synthesized. The RPE cell actively recycles DHA from phagocytized disk membranes back to the inner segment of the photoreceptor cell (16). In addition, the RPE cell takes up DHA from the bloodstream through the choriocapillaris and, in turn, supplies the fatty acid to photoreceptors through the interphotoreceptor matrix (9, 16–19). This uptake is very active during early postnatal development, when photoreceptor outer segment biogenesis occurs (3). In addition, active docosahexaenoyl CoA synthases in the RPE and the retina channel free DHA to acyltransferases that incorporate the fatty acid into membrane phospholipids (43). Thus, the RPE cell is very active in the uptake, conservation, and delivery of DHA to photoreceptors (2, 16). Here we demonstrate an additional function of the RPE cell, i.e., its capacity to synthesize NPD1. The biological activity of NPD1 may be elicited through a receptor and, in turn, modulate signaling including repression of NF-κB and other transcription factors, and, as a consequence, down-regulate proinflammatory genes (33). NPD1 may act in autocrine fashion and/or may diffuse through the interphotoreceptor matrix and act as a paracrine signal on photoreceptor cells.
Oxidative stress triggers multiple signaling pathways, including some that are cytoprotective and others that contribute to cell damage and eventually cell death. Among these are the Bcl-2 family proteins. In fact, expression of pro- and antiapoptotic Bcl-2 family proteins is altered by oxidative stress, and these proteins represent a major factor, insofar as the outcome of the apoptotic signaling, because cell survival reflects the predominance of one set of proteins over the other. In the RPE and retina, oxidative stress, increased by several factors including light exposure or reactive oxygen species, shifts the balance of Bcl-2 family protein expression toward those that favor cell damage (44, 45). Because our results show oxidative-stress-induced changes in the expression of Bcl-2 proteins, they imply that the early RPE response to oxidative stress includes transcriptional, translational, and/or posttranslational events upstream of the mitochondrial apoptotic step. In this pathway we have observed that oxidative-stress-triggered ARPE-19 cell damage includes changes in the expression of Bcl-2, Bcl-xL, Bax, and Bad. It is conceivable that oxidative stress, cytokines, and other intercellular signals (e.g., growth factors) may activate NPD1 formation in an effort to counteract the injury/proinflammatory response and restore homeostasis. Exogenous NPD1 (50 nM) promotes a differential modification in the expression of Bcl-2 family proteins under these conditions, up-regulating the protective Bcl-2 proteins and attenuating the expression of the proteins that challenge cell survival, particularly Bax and Bad. These observations suggest a critical, coordinated regulation of the availability of Bcl-2 proteins for subsequent downstream signaling. NPD1 may act at the level of signaling that regulates promoters of the genes encoding death repressors and effectors of the Bcl-2 family of proteins. In contrast, translational or posttranslational events may also integrate a concerted response to counteract oxidative stress. The precise molecular mechanisms remain to be defined. The exploration of these events will provide an important insight into regulatory survival signaling, because Bcl-2 family proteins regulate apoptotic signaling at the level of the mitochondria and endoplasmic reticulum. Subsequently, cytochrome c is released from mitochondria and effector caspase-3 is activated. In agreement with this sequence, we show that oxidative stress activated caspase-3 in ARPE-19 cells. The lipid mediator NPD1 (50 nM) decreased oxidative-stress-activated caspase-3. Moreover, apoptosis was an outcome of TNF-α/H2O2-induced oxidative stress in ARPE-19 cells. Interestingly, NPD1 was very effective in counteracting oxidative-stress-induced apoptosis in ARPE-19 cells. This action was not mimicked by classic eicosanoids such as prostaglandin E2, LTB4, or even arachidonic acid. This finding supports the selectivity of the new class of mediator, NPD1. Perhaps one of the most interesting observations is that DHA itself inhibited oxidative-stress-induced apoptosis. Under those conditions, a remarkable, time-dependent formation of NPD1 occurred. Significantly, the potency of DHA for cytoprotection was much higher than that of added NPD1 (Fig. 2 B and D). This suggests that endogenously generated NPD1 may exert its action near the subcellular site of its synthesis. Alternatively, it may imply that other NPD1-like mediators may participate in promoting RPE cell survival. It is indeed possible that related NPD1 mediators are formed in an attempt to cope with the multiplicity of cellular signaling that has the potential of going awry in RPE or neurons when confronted with oxidative stress. In support of this possibility, the brain makes a series of other potentially bioactive DHA-oxygenated derivatives such as those generated in the presence of aspirin during ischemia–reperfusion (33).
To date, the high content of DHA in photoreceptors and RPE has been linked mainly to endowing photoreceptor membrane domains with physical properties that contribute to functional modulation. For example, in other cells DHA modulates G protein-coupled receptors and ion channels. Moreover, DHA has been suggested to regulate membrane function by maintaining its concentration in phosphatidylserine (46). DHA is also envisioned as a target of oxidative stress, mainly by reactive oxygen intermediates that generate DHA peroxidation products and in turn participate in RPE and photoreceptor cell damage.
Rats with rhodopsin mutations that are homologous to human retinitis pigmentosa have a decreased content of DHA in photoreceptors (13). This observation is interpreted as a possible retinal response to a metabolic stress, whereby decreasing the amount of the major target of lipid peroxidation, DHA, contributes to protection (13). Alternately, in view of our present results, the retinal DHA pool size available for synthesis of neuroprotective docosanoids may be compromised because of lipid peroxidation. Retinal degeneration induced by constant light promotes DHA loss from photoreceptors, and rats reared in bright cyclic light are protected (47). These studies suggest adaptation/plasticity that may involve endogenous molecules (47) that have not been characterized to date. Some of these may be lipid mediators such as NPD1. Moreover, photoreceptor degenerations (e.g., inherited retinal degenerations) may involve an impairment in NPD1-mediated cell-survival signaling in RPE and/or photoreceptors.
Are growth factors involved in the survival-promoting activity of NPD1? There is no evidence as yet; however, fibroblast growth factor 2 induces bovine RPE cell survival through a sustained adaptive phenomenon that involves extracellular signal-regulated kinase 2 (ERK2) activation by secreted fibroblast growth factor 1 and ERK2-dependent Bcl-xL (38). Bcl-xL may play a key role in integrating and transmitting exogenous fibroblast growth factor 2 signals for RPE cell survival. Issues such as this remain to be explored.
A consequence of RPE cell damage and apoptosis is photoreceptor cell death, a dominant factor in age-related macular degeneration (48). For example, in Stargardt disease (a juvenile form of macular degeneration), oxidative stress mediated by the lipofuscin fluorophore A2E produces RPE damage involving caspase-3, whereas Bcl-2 exerts cellular protection (49).
Collectively our findings demonstrate that NPD1, a DHA-derived mediator endogenously synthesized by RPE cells, is a modulator of signaling pathways that promote cell survival. The regulation of Bcl-2 family protein expression is a premitochondrial apoptotic target of NPD1 under conditions of oxidative stress. As a consequence, downstream signaling that includes effector caspase-3 activation and DNA degradation is attenuated. This lipid mediator also potently counteracted cytokine-triggered proinflammatory COX-2 gene induction, another major factor in cell damage. In ischemia–reperfusion-injured hippocampus and in neural progenitor cells stimulated by IL-1β, COX-2 expression seems to be related to NF-κB activation. NPD1 inhibits NF-κB and COX-2 induction under those conditions (33). Therefore, a similar regulatory mechanism may operate in RPE cells, i.e., NPD1 down-regulation of cytokine-mediated NF-κB activation. Proinflammatory injury of the RPE is involved in pathoangiogenesis and proliferative vitreoretinopathy, which occur in several diseases, including diabetic retinopathy. The neuroprotective bioactivity of NPD1 in brain ischemia–reperfusion includes decreased infarct size and inhibition of polymorphonuclear leukocyte infiltration (33). In the present work, the addition of DHA to the culture medium promoted strong cytoprotection when RPE cells were confronted with oxidative stress. In vivo the active DHA supply to brain and retina from the liver through the bloodstream (3) is necessary for cell development and function and may play a critical role in conditions where, because of enhanced oxidative stress, the polyunsaturated fatty acyl chains of membrane phospholipids are decreased as a consequence of lipid peroxidation, as occurs in aging, retinal degenerations, and neurodegeneration such as Alzheimer's disease (3, 50). In addition, greater understanding of the signals that modulate NPD1 synthesis may be of therapeutic value for neurodegenerative diseases. Overall, selective DHA-delivery systems to the retina and brain may be vital in combating these diseases. Moreover, NPD1 and its cellular target(s) may also allow the design of novel therapeutic approaches to manage RPE cytoprotection and in turn enhance photoreceptor survival in retinal degenerations.
Acknowledgments
We are grateful to Stephen Prescott and Dan A. Dixon (Huntsman Cancer Institute, Salt Lake City) for the COX-2 830-bp promoter construct, to Pierluigi Nicotera and Daniele Bano (University of Leicester, Leicester, U.K.) for the lentivirus construct, and to Katherine Gotlinger for expert technical assistance. This work was supported by National Institutes of Health Grants EY05121, P20RR16816 (to N.G.B.), and GM38765 (to C.N.S.), the Eye, Ear, Nose, and Throat Foundation (N.G.B.), and the Ernest C. and Yvette C. Villere Chair in Retinal Degenerations (N.G.B.).
Abbreviations: NPD1, neuroprotectin D1; COX-2, cyclooxygenase 2; TNF-α, tumor necrosis factor α; DHA, docosahexaenoic acid; MS/MS, tandem MS; LC, liquid chromatography; RPE, retinal pigment epithelium; LTB, leukotriene B4.
References
- 1.Simopoulos, A. P. (1999) Am. J. Clin. Nutr. 70, Suppl. 3, 560S-569S. [DOI] [PubMed] [Google Scholar]
- 2.Bazan, N. G. (1990) in Nutrition and the Brain, eds. Wurtman, R. J. & Wurtman, J. J. (Raven, New York), Vol. 8, pp. 1-24. [Google Scholar]
- 3.Scott, B. L. & Bazan, N. G. (1989) Proc. Natl. Acad. Sci. USA 86, 2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aveldaño di Caldironi, M. I. & Bazan, N. G. (1977) Adv. Exp. Med. Biol. 83, 249-256. [DOI] [PubMed] [Google Scholar]
- 5.Salem, N., Jr., Kim, H. Y. & Yergey, J. A. (1986) in The Health Effects of Polyunsaturated Fatty Acids in Seafoods, eds. Simopoulos, A. P., Kifer, R. R. & Martin, R. (Academic, New York), pp. 263-317.
- 6.Litman, B. J., Niu, S. L., Polozova, A. & Mitchell, D. C. (2001) J. Mol. Neurosci. 16, 237-242. [DOI] [PubMed] [Google Scholar]
- 7.Moriguchi, T. & Salem, N., Jr. (2003) J. Neurochem. 87, 297-309. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler, T. G., Benolken, R. M. & Anderson, R. E. (1975) Science 188, 1312-1314. [DOI] [PubMed] [Google Scholar]
- 9.Stinson, A. M., Wiegand, R. D. & Anderson, R. E. (1991) J. Lipid Res. 32, 2009-2017. [PubMed] [Google Scholar]
- 10.Organisciak, D. T., Darrow, R. M., Jiang, Y. L. & Blanks, J. C. (1996) Invest. Ophthalmol. Vis. Sci. 37, 2243-2257. [PubMed] [Google Scholar]
- 11.Anderson, R. E, Maude, M. B. & Bok, D. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1715-1720. [PubMed] [Google Scholar]
- 12.Bicknell, I. R., Darrow, R., Barsalou, L., Fliesler, S. J. & Organisciak, D. T. (2002) Mol. Vis. 8, 333-340. [PubMed] [Google Scholar]
- 13.Anderson, R. E, Maude, M. B., McClellan, M., Matthes, M. T., Yasumura, D. & LaVail, M. M. (2002) Mol. Vis. 8, 351-358. [PubMed] [Google Scholar]
- 14.Kim, H. Y., Akbar, M., Lau, A. & Edsall, L. (2000) J. Biol. Chem. 275, 35215-35223. [DOI] [PubMed] [Google Scholar]
- 15.Hu, J. & Bok, D. (2001) Mol. Vis. 7, 14-19. [PubMed] [Google Scholar]
- 16.Bazan, N. G., Birkle, D. L. & Reddy, T. S. (1985) in Retinal Degeneration: Experimental and Clinical Studies, eds. LaVail, M. M., Anderson, R. E. & Hollyfield, J. (Liss, New York), pp. 159-187.
- 17.Gordon, W. C., Rodriguez de Turco, E. B. & Bazan, N. G. (1992) Curr. Eye Res. 11, 73-83. [DOI] [PubMed] [Google Scholar]
- 18.Bazan, N. G., Reddy, T. S., Redmond, T. M., Wiggert, B. & Chader, G. J. (1985) J. Biol. Chem. 260, 13677-13680. [PubMed] [Google Scholar]
- 19.Chen, Y., Houghton, L. A., Brenna, J. T. & Noy, N. (1996) J. Biol. Chem. 271, 20507-20515. [DOI] [PubMed] [Google Scholar]
- 20.Wiegand, R. D. & Anderson, R. E. (1983) Exp. Eye Res. 37, 159-173. [DOI] [PubMed] [Google Scholar]
- 21.Neuringer, M., Connor, W. E., Lin, D. S., Barstad, L. & Luck, S. (1986) Proc. Natl. Acad. Sci. USA 83, 4021-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuringer, M., Connor, W. E., Van Petten, C. & Barstad, L. (1984) J. Clin. Invest. 73, 272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uauy, R. D., Birch, D. G., Birch, E. E., Tyson, J. E. & Hoffman, D. R. (1990) Pediatr. Res. 28, 485-492. [DOI] [PubMed] [Google Scholar]
- 24.Birch, E. E., Hoffman, D. R., Uauy, R., Birch, D. G. & Prestidge, C. (1998) Pediatr. Res. 44, 201-209. [DOI] [PubMed] [Google Scholar]
- 25.Birch, E. E., Garfield, S., Hoffman, D. R., Uauy, R. & Birch, D. G. (2000) Dev. Med. Child Neurol. 42, 174-181. [DOI] [PubMed] [Google Scholar]
- 26.Sieving, P. A., Chaudhry, P., Kondo, M., Provenzano, M., Wu, D., Carlson, T. J., Bush, R. A. & Thompson, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparrow, J. R., Vollmer-Snarr, H. R., Zhou, J., Jang, Y. P., Jockusch, S., Itagaki, Y. & Nakanishi, K. (2003) J. Biol. Chem. 278, 18207-18213. [DOI] [PubMed] [Google Scholar]
- 28.Roberts, L. J., II, Montine, T. J., Markesbery, W. R., Tapper, A. R., Hardy, P., Chemtob, S., Dettbarn, W. D. & Morrow, J. D. (1998) J. Biol. Chem. 273, 13605-13612. [DOI] [PubMed] [Google Scholar]
- 29.Rotstein, N. P., Aveldano, M. I., Barrantes, F. J, Roccamo, A. M. & Politi, L. E. (1997) J. Neurochem. 69, 504-513. [DOI] [PubMed] [Google Scholar]
- 30.Rotstein, N. P., Politi, L. E., German, O. L. & Girotti, R. (2003) Invest. Ophthalmol. Vis. Sci. 44, 2252-2259. [DOI] [PubMed] [Google Scholar]
- 31.Bazan, N. G., Birkle, D. L. & Reddy, T. S. (1984) Biochem. Biophys. Res. Commun. 125, 741-747. [DOI] [PubMed] [Google Scholar]
- 32.Philp, N. J., Wang, D., Yoon, H. & Hjelmeland, L. M. (2003) Invest. Ophthalmol. Vis. Sci. 44, 1716-1721. [DOI] [PubMed] [Google Scholar]
- 33.Marcheselli, V. L., Hong, S., Lukiw, W. J., Tian, X. H., Gronert, K., Musto, A., Hardy, M., Gimenez, J. M., Chiang, N., Serhan, C. N. & Bazan, N. G. (2003) J. Biol. Chem. 278, 43807-43817, and erratum (2003) 278, 51974. [DOI] [PubMed] [Google Scholar]
- 34.Serhan, C. N., Clish, C. B., Brannon, J., Colgan, S. P., Chiang, N. & Gronert, K. (2000) J. Exp. Med. 192, 1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong, S., Gronert, K., Devchand, P. R., Moussignac, R. L. & Serhan, C. N. (2003) J. Biol. Chem. 278, 14677-14687. [DOI] [PubMed] [Google Scholar]
- 36.Bazan, N. G., Fletcher, B. S., Herschman, H. R. & Mukherjee, P. K. (1994) Proc. Natl. Acad. Sci. USA 91, 5252-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee, P. K., DeCoster, M. A., Campbell, F. Z., Davis, R. J. & Bazan, N. G. (1999) J. Biol. Chem. 274, 6493-6498. [DOI] [PubMed] [Google Scholar]
- 38.Bryckaert, M., Guillonneau, X., Hecquet, C., Courtois, Y. & Mascarelli, F. (1999) Oncogene 18, 7584-7593. [DOI] [PubMed] [Google Scholar]
- 39.Ershov, A. V. & Bazan, N. G. (1999) J. Neurosci. Res. 58, 254-261. [PubMed] [Google Scholar]
- 40.Bazan, N. G. (1970) Biochim. Biophys. Acta 218, 1-10. [DOI] [PubMed] [Google Scholar]
- 41.Aveldano, M. I. & Bazan, N. G. (1974) FEBS Lett. 40, 53-56. [DOI] [PubMed] [Google Scholar]
- 42.Aveldano, M. I. & Bazan, N. G. (1975) Brain Res. 100, 99-110. [DOI] [PubMed] [Google Scholar]
- 43.Reddy, T. S. & Bazan, N. G. (1984) Curr. Eye Res. 3, 1225-1232. [DOI] [PubMed] [Google Scholar]
- 44.Osborne, N. N., Cazevieillem C., Pergandem, G. & Wood, J. P. (1997) Invest. Ophthalmol. Vis. Sci. 38, 1390-1400. [PubMed] [Google Scholar]
- 45.Liang, Y. G., Jorgensen, A. G., Kaestel, C. G., Wiencke, A. K., Lui, G. M., la Cour, M. H., Ropke, C. H. & Nissen, M. H. (2000) Curr. Eye Res. 20, 25-34. [PubMed] [Google Scholar]
- 46.Salem, N., Jr., Litman, B., Kim, H. Y. & Gawrisch, K. (2001) Lipids 36, 945-959. [DOI] [PubMed] [Google Scholar]
- 47.Li, F., Cao, W. & Anderson, R. E. (2001) Exp. Eye Res. 73, 569-577. [DOI] [PubMed] [Google Scholar]
- 48.Hinton, D. R., He, S. & Lopez, P. F. (1998) Arch. Ophthalmol. 116, 203-209. [DOI] [PubMed] [Google Scholar]
- 49.Sparrow, J. R. & Cai, B. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1356-1362. [PubMed] [Google Scholar]
- 50.Nourooz-Zadeh, J., Liu, E. H. C., Yhlen, B., Δnggård, E. E. & Halliwell, B. (1999) J. Neurochem. 72, 734-740. [DOI] [PubMed] [Google Scholar]