Abstract
A series of cyclometalated Z-selective ruthenium olefin metathesis catalysts with alterations to the N-heterocyclic carbene (NHC) ligand were prepared. X-Ray crystal structures of several new catalysts were obtained, elucidating the structural features of this class of cyclometalated complexes. The metathesis activity of each stable complex was evaluated, and one catalyst, bearing geminal dimethyl backbone substitution, was found to be comparable to our best Z-selective metathesis catalyst to date.
Introduction
The olefin metathesis reaction is arguably one of the most well-studied and useful tools in organic synthesis.1 The utility of this powerful methodology is demonstrated by its prevalence in numerous applications, including natural product synthesis,2 materials science,3 and biochemistry.4 Recent metathesis endeavors have addressed the challenge of stereoselectivity, as productive metathesis reactions may construct carbon–carbon double bonds with either Z- or E-stereochemistry. As a result of the reversible nature of the olefin metathesis reaction, the thermodynamically favored E-olefin is generally formed.5 In contrast, selective generation of the Z-olefin, a motif found in many natural products and pharmaceutical targets,2b via olefin metathesis has only recently been enabled by the development of novel metathesis catalysts. The Schrock and Hoveyda groups have achieved highly Z-selective metathesis utilizing monoaryloxide pyrrolide (MAP) molybdenum and tungsten catalysts, and have applied their systems to perform the Z-selective homodimerization and cross-metathesis of terminal olefins,6 ring-opening metathesis polymerization,7 as well as macrocyclic ring-closing metathesis.8
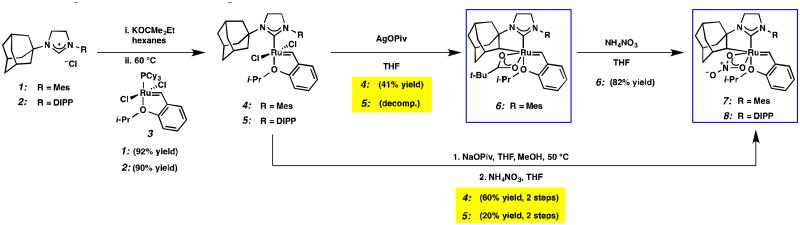
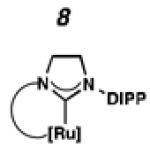
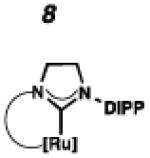
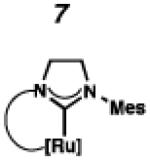
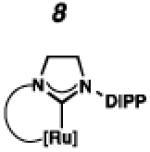
Recently, our group has developed Z-selective ruthenium-based catalysts with cyclometalated N-heterocyclic carbene (NHC) ligands.9,10 The general synthetic strategy used to access these complexes involves in situ generation of a carbene and subsequent metalation (e.g., 1 □ 4 and 2 □ 5, Scheme 1).11 The resulting NHC-ligated complex then undergoes carboxylate-driven C–H activation enabled by metal-bound carboxylates.12 In our early reports, it was discovered that while pivalate-ligated catalyst 6 was highly Z-selective,9a,b nitrato catalyst 7 had increased stability and selectivity with turnover numbers (TONs) approaching 1000 and Z-selectivity of approximately 90%.9c An analogous C–H activation strategy could not be used on complexes containing more sterically demanding NHCs; for example, treatment of 5, containing a N-2,6-diisopropylphenyl (DIPP) group, with silver pivalate (AgOPiv) led to complex mixtures.11 By employing the milder sodium pivalate (NaOPiv) in place of AgOPiv, it was discovered that C–H activation of 5, as well as other challenging complexes, could occur cleanly to generate catalyst 8.12a Gratifyingly, catalyst 8 was found to perform a number of homodimerization and cross-metathesis reactions with near perfect Z-selectivity (>95%) and unmatched TONs (≥ 7400). The power and industrial relevance of this class of cyclometalated ruthenium compounds has since been illustrated via applications in the synthesis of insect pheromone natural products13 and macrocyclic musks,14 in addition to more complicated natural products.15
Scheme 1.
Synthetic Route to Previously Reported C–H Activated Metathesis Catalysts.
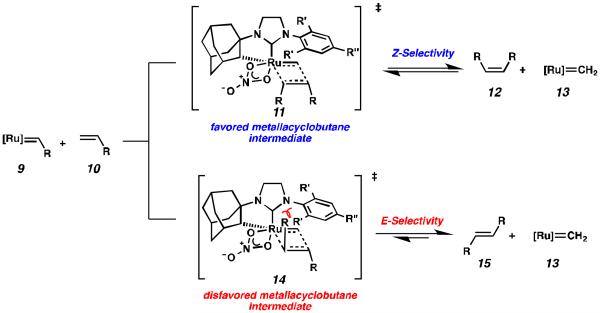
Computational studies provide a model for the Z-selectivity exhibited by cyclometalated metathesis catalysts 7 and 8, and also provide guidance for further catalyst development. The Houk group has previously shown that steric crowding over the ruthenium center forces the forming side-bound metallacyle into all-cis geometry (11, Figure 1), resulting in selective formation of the Z-isomer (12) via kinetic control.16 In the case of cyclometalated catalysts 7 and 8, the 5-membered imidazoline NHC holds the N-aryl group in close proximity to the intermediate metallacycle, thus explaining why the sterically more hindered N-DIPP group imparts greater selectivity than the N-Mes analogue.
Figure 1.
Steric crowding of metallacycle enforces Z-stereochemistry.
Despite the recent advances in catalyst development, there remains a need for more Z-selective ruthenium metathesis catalysts with expanded substrate scopes. In previous generations of ruthenium metathesis catalysts, modification of the NHC ligand has been well explored and has led to insights on the relation of structure, activity, and stability;19 however, modifications to the NHC ligand17 of cyclometalated catalysts have been largely unexplored, although proposed transition states suggest that this group is critical in imparting stereochemistry to the forming metallacycle.16 We hypothesized that modification to the identity of the NHC ligand could potentially lead to more active and more Z-selective metathesis catalysts.
Results and discussion
Selection and Synthesis of Catalysts
Herein, we report the preparation, characterization, and metathesis activity of seven new cyclometalated ruthenium metathesis catalysts with varying carbene ligands. While modifying the identity of the cyclometalated carbene, other features of the catalyst were left unaltered for the purpose of consistency. Although alternative X-type ligands have been explored, the nitrate ligand was selected for our catalyst series because it has been shown to impart exceptional stability and selectivity.9c In the past, we have seen that the N-1-adamantyl chelate is most stable,12a and for the purposes of this study this feature was left unchanged. Finally, our previous most promising Z-selective metathesis catalysts have possessed either N-Mes or N-DIPP substituents.
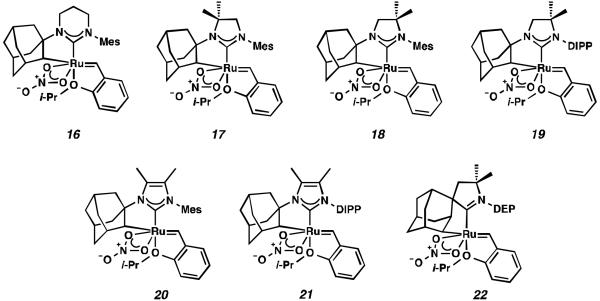
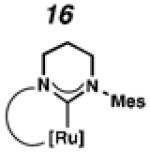
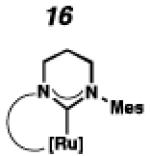
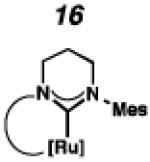
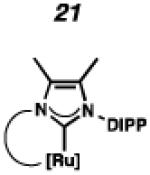
Cyclometalated complexes 16–22, bearing a number of unique carbene motifs, were selected as catalyst targets for our studies (Figure 2). Complex 16, possessing a 6-membered NHC, was of interest because crystal structures of similar non-cyclometalated ruthenium complexes have revealed that the N-aryl component exerts a larger steric influence on the benzylidene than with the 5-membered NHC analogue,18 thus implying that 16 may display improved Z-selectivity. Furthermore, increasing the ring size of the NHC could result in additional degrees of freedom, relieving ring strain of the NHC and leading to a more stable catalyst.
Figure 2.
New ruthenium complexes with modified cyclometalated carbene ligands.
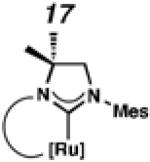
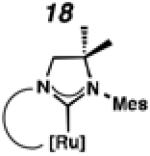
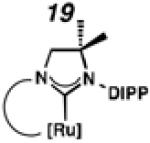
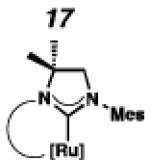
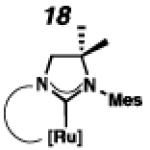
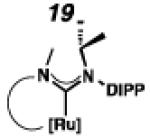
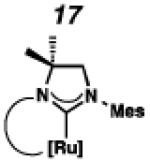
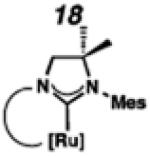
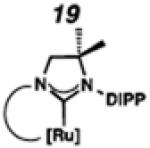
Backbone-substituted ruthenium complexes 17–19 were expected to display extended catalyst lifetimes and increased Z-selectivity.19 It was hypothesized that the geminal dimethyl substitution on compound 17 could push the N-adamantyl group closer to the metal center, resulting in a more stable chelate, thereby disfavoring common decomposition pathways.11 Alternatively, it was expected that the same substitution above the N-aryl group (e.g., 18 and 19) could prevent N-aryl rotation from occurring, and also position the aryl substituent closer to the forming metallacyclobutane; both effects could result in improved Z-selectivity.
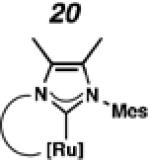
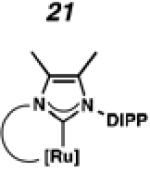
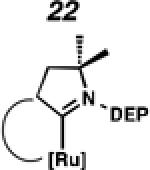
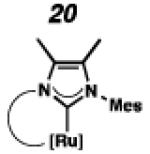
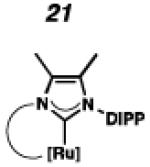
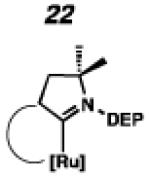
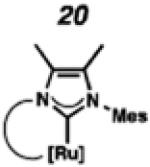
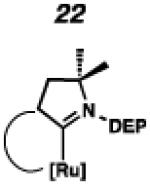
In the past, metathesis catalysts with unsaturated NHC ligands have been found to be less active than their saturated analogues.20 While it was expected that they would be less active, backbone unsaturated ruthenium complexes 20 and 21 were identified as synthetic targets. Finally, we decided to pursue ruthenium cyclic (alkyl)(amino)carbene (CAAC) complex 22, bearing a 2,6-diethylphenyl (DEP) substituent. In addition to being structurally interesting with a unique spiro-binding mode, CAAC ligands have been shown to impart a unique reactivity to catalysts of previous generations.21 In the case of each target shown in Figure 2, the carbene was synthesized using modifications of previously reported procedures.22 Elaboration to the cyclometalated complex was accomplished using strategies analogous to those shown in Scheme 1, involving metal-carboxylate mediated C–H activation, followed by ligand exchange with the addition of ammonium nitrate.23
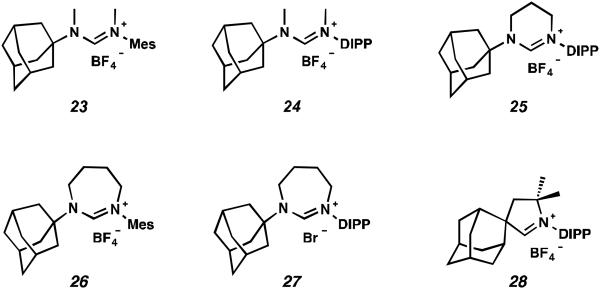
A number of formamidinium salts were synthesized that could not be elaborated to the desired cyclometalated ruthenium complexes (Figure 3). Previously, non-cyclometalated metathesis catalysts with acyclic diaminocarbenes have provided metathesis products with lower E:Z ratios than analogous catalysts containing NHC ligands.24 Based on these observations, it was anticipated that C–H activation of these acyclic diaminocarbene ruthenium complexes might lead to improved Z-selective metathesis catalysts. However, acyclic formamidinium salts 23 and 24 underwent metalation to produce an unstable dichloride that could not be isolated without substantial decomposition. We also attempted to synthesize a DIPP analogue to catalyst 16 with a 6-membered NHC, but found that the corresponding formamidinium salt (25) did not undergo successful metalation. Cyclometalated ruthenium complexes with 7-membered NHCs were also targeted because, like the 6-membered NHC complex, the N-aryl component was expected to have an increased steric effect on the forming metallacyclobutane. However, attempts to metalate 7-membered formamidinium salts 26 and 27 were unsuccessful, as was the metalation of previously reported CAAC salt 28.25
Figure 3.
Formamidinium salts that could not be elaborated to cyclometalated ruthenium metathesis catalysts.
Structural Analyses
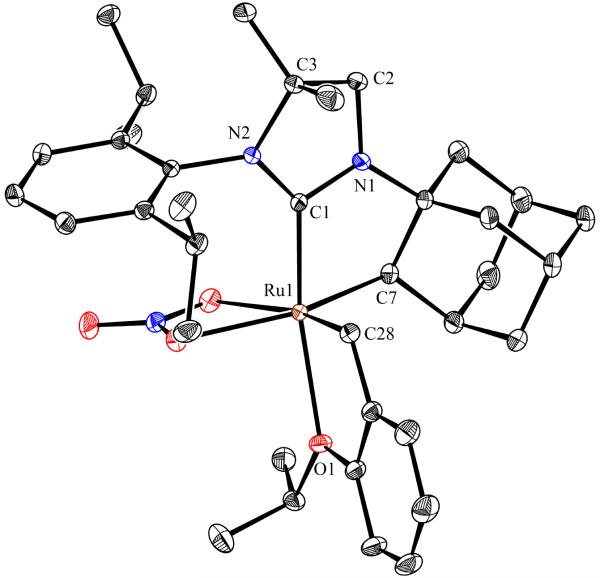
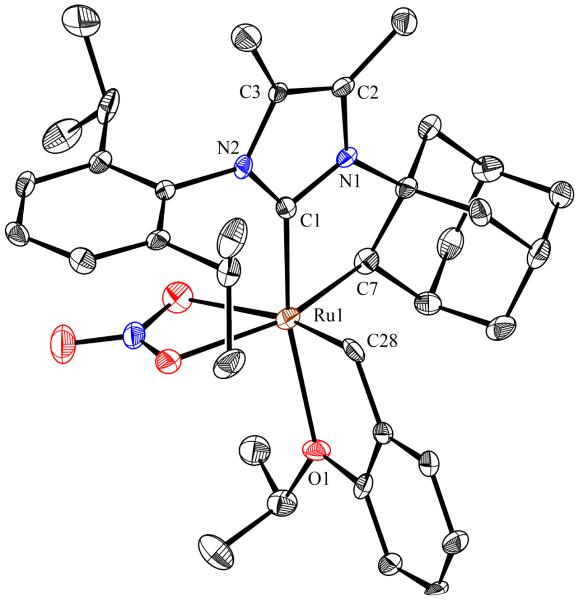
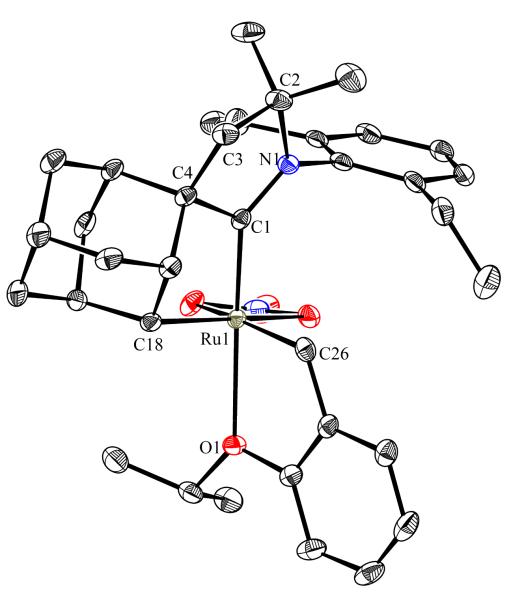
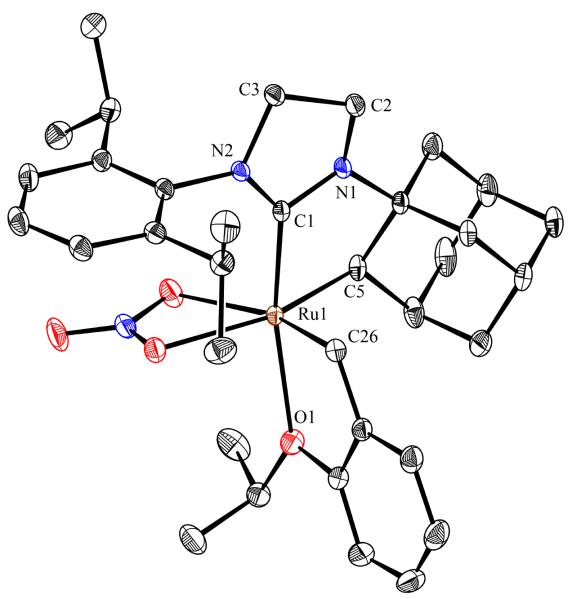
To probe the steric and electronic effects of cyclometalated carbene modification, crystals of 19, 21, and 22 were grown and their molecular structures were confirmed by single-crystal X-ray crystallographic analysis (Figures 5, 6, and 7, respectively). For comparison purposes, a crystal structure of 8, which previously had not been reported, was also obtained (Figure 4). The crystal structure of 8 did not vary significantly from the previously disclosed structure of 7.9c A comparison between structures reveals that the distance between Ru and the chelating adamantyl-carbon does not change significantly, with the exception of CAAC-ligated 22, which possesses a slightly longer bond length. Other key structural features are summarized in Table 1. Of note, catalyst 8, bearing a 5-membered saturated NHC, has the ability to kink its backbone, as shown by the N(1)–C(2)–C(3)–N(2) torsional angle of 23.33°. This kinked geometry relieves ring strain and allows for a more stable catalyst. Interestingly, catalyst 19 possesses an approximately equal but opposite torsional angle (–23.66°). As expected, unsaturation of the NHC results in a nearly planar geometry, as indicated by 21’s torsional angle of 1.16°. Furthermore, from the crystal structure of CAAC-ligated 22, it is apparent that the N-aryl ring is situated further away from the benzylidene moiety. This structural feature may explain the diminished Z-selectivity observed in metathesis studies, along with the fact that 22 is a less stable complex.
Figure 5.
ORTEP drawing of 19. Displacement ellipsoids are drawn at 50% probability. For clarity, hydrogen atoms have been omitted.
Figure 6.
ORTEP drawing of 21. Displacement ellipsoids are drawn at 50% probability. For clarity, hydrogen atoms have been omitted.
Figure 7.
ORTEP drawing of 22. Displacement ellipsoids are drawn at 50% probability. For clarity, hydrogen atoms have been omitted.
Figure 4.
ORTEP drawing of 8. Displacement ellipsoids are drawn at 50% probability. For clarity, hydrogen atoms have been omitted.
Table 1.
Selected X-Ray Data for 8, 19, 21, and 22.
|
| ||||
|---|---|---|---|---|
| 8 | 19 | 21 | 22 | |
|
| ||||
| Bond Lengths (Å) | ||||
| Ruthenium-NHC Carbene Carbon Ru(1)–C(1) |
1.954 | 1.974 | 1.965 | 1.939 |
| Ruthenium-Chelating Isopropoxy Group Ru(1)–O(1) |
2.311 | 2.320 | 2.317 | 2.329 |
| Ruthenium-Chelating Adamantyl Carbon |
2.062 Ru(1)–C(5) |
2.064 Ru(1)–C(7) |
2.060 Ru(1)–C(7) |
2.096 Ru(1)–C(18) |
| Ruthenium Alkylidene Styrene Carbon |
1.849 Ru(1)–C(26) |
1.851 Ru(1)–C(28) |
1.841 Ru(1)–C(28) |
1.852 Ru(1)–C(26) |
| Torsional Angle (deg) | ||||
| N(1)–C(2)–C(3)–N(2) | 23.33 | −23.66 | 1.16 | n/a |
Homodimerization Metathesis Activity
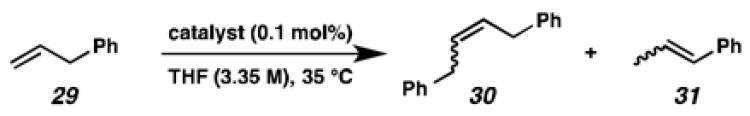
To probe the metathesis ability of each new cyclometalated complex, homodimerization studies were performed using three standard substrates. Allyl benzene (29) was selected for our initial studies because this substrate is prone to double bond migration and the extent of isomerization to form 31 is a good litmus test to assess the overall stability of new metathesis catalysts.26 All homodimerization reactions were performed in tetrahydrofuran (THF) at 35 °C with an olefin concentration of 3.35 M and 0.1 mol% catalyst loading. Aliquots were removed at 1-, 3-, 7-, and 12-hour time points, and conversion as well as Z-selectivity were calculated by analysis of the 1H NMR spectra of the unpurified reaction mixtures (Table 2). Complexes 7 and 8 were able to catalyze the homodimerization of allyl benzene with excellent conversions and high selectivities, and were in good agreement with previously published data.12a A number of catalysts were found to homodimerize allyl benzene with similar efficacy. While catalysts 17, 20, and 21 demonstrated erosion of Z-selectivity over time, catalyst 19, bearing geminal dimethyl substitution on the NHC backbone, displayed higher Z-selectivities than 8 (98% conversion, 92% Z-selectivity vs. >99% conversion, 83% Z-selectivity at 12 h of reaction time). The efficacy of 19 at preserving Z-selectivity highlights this new catalyst structure’s unique attributes. However, complex 16, with a 6-membered NHC, was not metathesis active, while CAAC-ligated 22 was found to be highly unstable under the reaction conditions.
Table 2.
Homodimerization of allyl benzene (29).
| ||||
|---|---|---|---|---|
| catalyst | time (h) | conversion (%)a | Z-selectivity (%)a | 30/31 a |
|
| ||||
|
1 | 93 | 92 | >50 |
| 3 | 98 | 84 | >50 | |
| 7 | >99 | 66 | >50 | |
| 12 | >99 | 45 | >50 | |
|
| ||||
|
1 | 92 | >95 | >50 |
| 3 | 98 | >95 | >50 | |
| 7 | >99 | 91 | >50 | |
| 12 | >99 | 83 | >50 | |
|
| ||||
|
1 | 0 | n/a | n/a |
| 3 | 0 | n/a | n/a | |
| 7 | 0 | n/a | n/a | |
| 12 | 0 | n/a | n/a | |
|
| ||||
|
1 | 72 | >95 | >50 |
| 3 | 92 | 95 | >50 | |
| 7 | 97 | 72 | >50 | |
| 12 | 99 | 53 | >50 | |
|
| ||||
|
1 | 82 | >95 | >50 |
| 3 | 94 | 95 | >50 | |
| 7 | 97 | 90 | >50 | |
| 12 | 99 | 79 | >50 | |
|
| ||||
|
1 | 75 | >95 | >50 |
| 3 | 91 | >95 | >50 | |
| 7 | 97 | >95 | >50 | |
| 12 | 98 | 92 | >50 | |
|
| ||||
|
1 | 84 | 97 | >50 |
| 3 | 95 | 90 | >50 | |
| 7 | >99 | 54 | >50 | |
| 12 | >99 | 25 | >50 | |
|
| ||||
|
1 | 13 | >95 | >50 |
| 3 | 75 | >95 | >50 | |
| 7 | 95 | >95 | >50 | |
| 12 | 98 | 93 | >50 | |
| 25 | >99 | 74 | >50 | |
|
| ||||
|
1 | 4 | 50 | 17 |
| 3 | 6 | 49 | 2 | |
| 7 | 10 | 49 | 1 | |
| 12 | 15 | 49 | 0.6 | |
Determined from analysis of the 1H NMR spectrum.
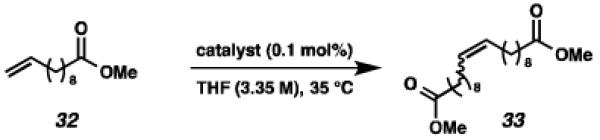
Next, the efficacy of our new complexes was further examined using two more challenging homodimerization substrates. 10-Methyl undecenoate (32, Table 3) was selected because the ester functionality is far removed from the double bond, while 4-pentenol (34, Table 4) has been linked to decomposition pathways of prior generations of Ru-metathesis catalysts.27 Furthermore, pronounced Z-content degradation has been observed in the homodimerization of 4-pentenol with previous Z-selective metathesis catalysts 7 and 8. Metathesis reactions were executed utilizing the standard conditions described above. Catalyst 19 effected the homodimerization of 10-methyl undecenoate with approximately the same conversions and near-perfect selectivities as catalyst 8 (96% vs. 97% conversion at 12 h for 19 and 8, respectively), and 21 showed a similar level of Z-selectivity and efficiency. As expected, 4-pentenol was a difficult substrate and erosion of Z-selectivity was observed for all catalysts. Of note, 20 and 21 have improved solubility compared to 7 and 8, and could perform neat, homogeneous homodimerizations of allylbenzene (29), 10-methyl undecenoate (32), and pentenol (34) without reduced activity.
Table 3.
Homodimerization of 10-methy1 undecenoate (32).
| |||
|---|---|---|---|
| catalyst | time (h) | conversion (%)a | Z-selectivity (%)a |
|
| |||

|
1 | 85 | 92 |
| 3 | 94 | 89 | |
| 7 | 96 | 88 | |
| 12 | 97 | 83 | |
|
| |||
|
1 | 70 | >95 |
| 3 | 91 | >95 | |
| 7 | 96 | >95 | |
| 12 | 97 | >95 | |
|
| |||
|
1 | 0 | n/a |
| 3 | 0 | n/a | |
| 7 | 0 | n/a | |
| 12 | 0 | n/a | |
|
| |||
|
1 | 18 | >95 |
| 3 | 56 | >95 | |
| 7 | 79 | 94 | |
| 12 | 86 | 91 | |
|
| |||
|
1 | 35 | >95 |
| 3 | 65 | >95 | |
| 7 | 78 | >95 | |
| 12 | 82 | 94 | |
|
| |||
|
1 | 68 | >95 |
| 3 | 88 | >95 | |
| 7 | 94 | >95 | |
| 12 | 96 | >95 | |
|
| |||
|
1 | 28 | >95 |
| 3 | 56 | 92 | |
| 7 | 75 | 85 | |
| 12 | 82 | 78 | |
|
| |||
|
1 | 5 | >95 |
| 3 | 27 | >95 | |
| 7 | 65 | >95 | |
| 12 | 81 | >95 | |
| 25 | 85 | >95 | |
|
| |||
|
1 | 0 | n/a |
| 3 | 0 | n/a | |
| 7 | 0 | n/a | |
| 12 | 0 | n/a | |
Determined from analysis of the 1H NMR spectrum.
Table 4.
Homodimerization of 4-pentenol (34).

| |||
|---|---|---|---|
| catalyst | time (h) | conversion (%)a | Z-selectivity (%)a |
|
| |||
|
1 | 52 | >95 |
| 3 | 92 | 88 | |
| 7 | 96 | 58 | |
| 12 | 99 | 24 | |
|
| |||
|
1 | 37 | >95 |
| 3 | 87 | 80 | |
| 7 | 94 | 33 | |
| 12 | 97 | 21 | |
|
| |||
|
1 | 0 | n/a |
| 3 | 0 | n/a | |
| 7 | 0 | n/a | |
| 12 | 0 | n/a | |
|
| |||
|
1 | 6 | >95 |
| 3 | 55 | 88 | |
| 7 | 73 | 78 | |
| 12 | 85 | 76 | |
|
| |||
|
1 | 20 | >96 |
| 3 | 63 | 95 | |
| 7 | 71 | 82 | |
| 12 | 81 | 63 | |
|
| |||
|
1 | 14 | >95 |
| 3 | 63 | 82 | |
| 7 | 79 | 30 | |
| 12 | 86 | 29 | |
|
| |||
|
1 | 22 | n/a |
| 3 | 84 | 75 | |
| 7 | 95 | 35 | |
| 12 | significant isomerization | ||
|
| |||
|
1 | 0 | n/a |
| 3 | 10 | >95 | |
| 7 | 49 | 72 | |
| 12 | 65 | 47 | |
| 25 | 88 | 27 | |
|
| |||
|
1 | 0 | n/a |
| 3 | 0 | n/a | |
| 7 | 0 | n/a | |
| 12 | 0 | n/a | |
Determined from analysis of the 1H NMR spectrum.
Cross-Metathesis Activity
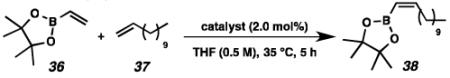
Based on the results of the homodimerization studies, our two most active and selective new catalysts 19 and 21 were selected to be evaluated in more complicated cross-metathesis reactions. Cross-metathesis with allylic-substituted olefins is a particularly difficult transformation because increased steric bulk further destabilizes the Z-geometry of the cross product.28 However, the utility of Z-selective cyclometalated metathesis catalyst 8 has previously been illustrated in the cross-metathesis of vinyl pinacol boronate (36) with 1-dodecene (37).29 In the case of catalyst 8, the reaction proceeds with 81% yield and 92% Z-selectivity. Catalyst 19 performed with comparable yields and selectivities, while unsaturated catalyst 21 displayed low activity under these conditions.
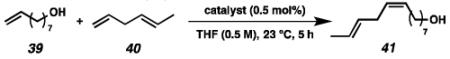
Chemoselective cross-metathesis of dienes is another underdeveloped reaction30 that can be accomplished by cyclometalated ruthenium metathesis catalysts. Previously, 8 was demonstrated to catalyze the Z-selective cross-metathesis of 39 and 40 with complete chemoselectivity.31 Under identical reaction conditions, the catalytic abilities of 8, 19, and 21 were compared (Table 6). Catalysts 8 and 19 afforded cross-product in similar yields (77% and 64%, respectively), with near perfect Z-selectivities, while 21 was again found to be an ineffective catalyst.
Table 6.
Chemoselective cross-metathesis of 8-nonenol (39) and trans-1,4-hexadiene (40).
Isolated yields.
Z-Selectivity determined by analysis of the 1H NMR spectrum of the purified product.
Conclusions
A variety of new cyclometalated ruthenium alkylidene complexes have been prepared that exhibit high Z-selectivity. The use of NaOPiv to perform C–H activation has allowed access to a number of unique structures. This approach was used to synthesize seven new cyclometalated species, which were evaluated in a number of metathesis reactions. As expected, it was found that N-DIPP-substituted catalysts were more Z-selective than the analogous N-Mes-substituted complexes. One catalyst in particular, 19, was found to be comparable to the best Z-selective metathesis catalyst to date in this series (8) in homodimerizations and more complicated cross-metathesis reactions. Future studies into catalyst development will focus on investigating modification of the X-type ligand, N-chelate, and the chelating isopropoxy styrene group.
Supplementary Material
Table 5.
Cross-metathesis of vinyl pinacol boronate (36) and 1-dodecene (37).
Isolated yields.
Z-Selectivity determined by analysis of the 1H NMR spectrum of the purified product.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers F32GM102984 (fellowship to S.M.B.) and NIH R01-GM031332, the NSF under award number CHE-1212767, and the NSERC of Canada (fellowship to V.M.M.). NMR spectra were obtained by instruments supported by the NIH (RR027690). The Bruker KAPPA APEXII X-ray diffractometer was purchased via an NSF:CRIF:MU award to the California Institution of Technology (CHE-0639094). Lawrence Henling and Dr. Michael Takase are acknowledged for X-ray crystallographic analysis. Naseem Torian and Dr. Mona Shahgholi are acknowledged for high-resolution mass spectrometry assistance. Dr. David VanderVelde is acknowledged for NMR assistance. Brendan Quigley, Dr. Jeff Cannon, and Dr. Daryl Allen are acknowledged for helpful discussions. David Weinberger (Bertrand Group) is gratefully acknowledged for providing CAACs. Materia, Inc. is thanked for its donation of 3 and metathesis catalysts 7 and 8.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
References
- 1.(a) Fürstner A. Angew. Chem. Int. Ed. 2000;39:3012–3043. [PubMed] [Google Scholar]; (b) Trnka TM, Grubbs RH. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]; (c) Schrock RR. Chem. Rev. 2002;102:145–180. doi: 10.1021/cr0103726. [DOI] [PubMed] [Google Scholar]; (d) Schrock RR, Hoveyda AH. Angew. Chem. Int. Ed. 2003;42:4592–4633. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]; (e) Vougioukalakis GC, Grubbs RH. Chem. Rev. 2010;110:1746–1787. doi: 10.1021/cr9002424. [DOI] [PubMed] [Google Scholar]; (f) Samojłowicz C, Bieniek M, Grela K. Chem. Rev. 2009;109:3708–3742. doi: 10.1021/cr800524f. [DOI] [PubMed] [Google Scholar]
- 2.(a) Fürstner A. Chem. Commun. 2011;47:6505–6511. doi: 10.1039/c1cc10464k. [DOI] [PubMed] [Google Scholar]; (b) Cossy J, Arseniyadis S, Meyer C. Metathesis in Natural Product Synthesis: Strategies, Substrates, and Catalysts. Wiley-VCH; Weinheim: 2010. [Google Scholar]
- 3.(a) Leitgeb A, Wappel J, Slugovc C. Polymer. 2010;51:2927–2946. [Google Scholar]; (b) Sutthasupa S, Shiotsuki M, Sanda F. Polym. J. 2010;42:905–915. [Google Scholar]; (c) Liu X, Basu A. J. Organomet. Chem. 2006;691:5148–5154. [Google Scholar]
- 4.Binder JB, Raines RT. Curr. Opin. Chem. Biol. 2008;12:767–773. doi: 10.1016/j.cbpa.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grubbs RH, editor. Handbook of Metathesis. Wiley-VCH; Weinheim: 2003. [Google Scholar]
- 6.(a) Meek SJ, O’Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Flook MM, Jiang AJ, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:7962–7963. doi: 10.1021/ja902738u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Marinescu SC, Levine DS, Zhao Y, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2011;133:11512–11514. doi: 10.1021/ja205002v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jiang AJ, Zhao Y, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:16630–16631. doi: 10.1021/ja908098t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yu M, Ibrahem I, Hasegawa M, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2012;134:2788–2799. doi: 10.1021/ja210946z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flook MM, Ng VWL, Schrock RR. J. Am. Chem. Soc. 2011;133:1784–1786. doi: 10.1021/ja110949f. [DOI] [PubMed] [Google Scholar]
- 8.(a) Wang C, Yu M, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Chem.–Eur. J. 2013;19:2726–2740. doi: 10.1002/chem.201204045. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu M, Wang C, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Nature. 2011;479:88–93. doi: 10.1038/nature10563. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang C, Haeffner F, Schrock RR, Hoveyda AH. Angew. Chem. Int. Ed. 2013;52:1939–1943. doi: 10.1002/anie.201209180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Endo K, Grubbs RH. J. Am. Chem. Soc. 2011;133:8525–8527. doi: 10.1021/ja202818v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keitz BK, Endo K, Herbert MB, Grubbs RH. J. Am. Chem. Soc. 2011;133:9686–9688. doi: 10.1021/ja203488e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Keitz BK, Endo K, Patel PR, Herbert MB, Grubbs RH. J. Am. Chem. Soc. 2012;134:693–699. doi: 10.1021/ja210225e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Khan RKM, O’Brien RV, Torker S, Li B, Hoveyda AH. J. Am. Chem. Soc. 2012;134:12774–12779. doi: 10.1021/ja304827a. For other Z-selective ruthenium metathesis catalysts, see: [DOI] [PubMed] [Google Scholar]; (b) Khan RKM, Torker S, Hoveyda AH. J. Am. Chem. Soc. 2013;135:10258–10261. doi: 10.1021/ja404208a. [DOI] [PubMed] [Google Scholar]; (c) Occhipinti G, Hansen FR, Törnroos KW, Jensen VR. J. Am. Chem. Soc. 2013;135:3331–3334. doi: 10.1021/ja311505v. [DOI] [PubMed] [Google Scholar]
- 11.Herbert MB, Lan Y, Keitz BK, Liu P, Endo K, Day MW. J. Am. Chem. Soc. 2012;134:7861–7866. doi: 10.1021/ja301108m. For metalation of 1 see ref 9a. For metalation of 2, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Rosebrugh LE, Herbert MB, Marx VM, Keitz BK, Grubbs RH. J. Am. Chem. Soc. 2013;135:1276–1279. doi: 10.1021/ja311916m. For the C–H activation of 4 see ref 9a. For the C–H activation of 8 see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cannon JS, Zou L, Liu P, O’Leary DJ, Houk KN, Grubbs RH. J. Am. Chem. Soc. 2014;136:6733–6743. doi: 10.1021/ja5021958. For more detailed studies on carboxylate-assisted C–H activation, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert MB, Marx VM, Pederson RL, Grubbs RH. Angew. Chem. Int. Ed. 2013;52:310–314. doi: 10.1002/anie.201206079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx VM, Herbert MB, Keitz BK, Grubbs RH. J. Am. Chem. Soc. 2013;135:94–97. doi: 10.1021/ja311241q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Chung W.-j., Carlson JS, Bedke DK, Vanderwal CD. Angew. Chem. Int. Ed. 2013;52:10052–10055. doi: 10.1002/anie.201304565. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chung W.-j., Carlson JS, Vanderwal CD. J. Org. Chem. 2014;79:2226–2241. doi: 10.1021/jo5000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu P, Xu X, Dong X, Keitz BK, Herbert MB, Grubbs RH, Houk KN. J. Am. Chem. Soc. 2012;134:1464–1467. doi: 10.1021/ja2108728. [DOI] [PubMed] [Google Scholar]
- 17.Martin D, Canac Y, Lavallo V, Bertrand G. J. Am. Chem. Soc. 2014;136:5023–5030. doi: 10.1021/ja412981x. For a discussion on the reactivity of various carbenes, see: [DOI] [PubMed] [Google Scholar]
- 18.(a) Yun J, Marinez ER, Grubbs RH. Organometallics. 2004;23:4172–4173. [Google Scholar]; (b) Yang L, Mayr M, Wurst K, Buchmeiser MR. Chem.–Eur. J. 2004;10:5761–5770. doi: 10.1002/chem.200400278. [DOI] [PubMed] [Google Scholar]
- 19.(a) Kuhn KM, Bourg J-B, Chung CK, Virgil SC, Grubbs RH. J. Am. Chem. Soc. 2009;131:5313–5320. doi: 10.1021/ja900067c. For previous reports of non-cyclometalated metathesis catalysts with back-bone substituted NHCs, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chung CK, Grubbs RH. Org. Lett. 2008;10:2693–2696. doi: 10.1021/ol800824h. [DOI] [PubMed] [Google Scholar]
- 20.Scholl M, Trnka TM, Morgan JP, Grubbs RH. Tetrahedron Lett. 1999;40:2247–2250. [Google Scholar]
- 21.(a) Anderson DR, Ung T, Mkrtumyan G, Bertrand G, Grubbs RH, Schrodi Y. Organometallics. 2008;27:563–566. doi: 10.1021/om7008028. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anderson DR, Lavallo V, O’Leary DJ, Bertrand G, Grubbs RH. Angew. Chem. Int. Ed. 2007;46:7262–7265. doi: 10.1002/anie.200702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Kuhn KM, Grubbs RH. Org. Lett. 2008;10:2075–2077. doi: 10.1021/ol800628a. For general procedures for the synthesis of imidazolium salts, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hirano K, Urban S, Wang C, Glorius F. Org. Lett. 2009;11:1019–1022. doi: 10.1021/ol8029609. [DOI] [PubMed] [Google Scholar]
- 23. For catalyst synthesis details, see Supporting Information.
- 24.Rosen EL, Sung DH, Chen Z, Lynch VM, Bielawski CW. Organometallics. 2010;29:250–256. [Google Scholar]
- 25.Lavallo V, Frey GD, Kousar S, Donnadieu B, Bertrand G. Proc. Nat. Acad. Sci. 2007;104:13569–13573. doi: 10.1073/pnas.0705809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter T, Heji A, Wenzel AG, Funk TW, Grubbs RH. Organometallics. 2006;25:5740–5745. [Google Scholar]
- 27.(a) Trnka TM, Morgan JP, Sanford MS, Wilhelm TE, Scholl M, Choi T-L, Ding S, Day MW, Grubbs RH. J. Am. Chem. Soc. 2003;125:2546–2558. doi: 10.1021/ja021146w. [DOI] [PubMed] [Google Scholar]; (b) Beach NJ, Lummiss JAM, Bates JM, Fogg DE. Organometallics. 2012;31:2349–2356. [Google Scholar]
- 28.Turner RB, Jarrett AD, Goebel P, Mallon BJ. J. Am. Chem. Soc. 1973;95:790–792. [Google Scholar]
- 29.Quigley BL, Grubbs RH. Chem. Sci. 2014;5:501–506. doi: 10.1039/c3sc52806e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Nolan SP, Clavier H. Chem. Soc. Rev. 2010;39:3305–3316. doi: 10.1039/b912410c. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y, Arpin CC, Cullen AJ, Mitton-Fry MJ, Sammakia T. J. Org. Chem. 2011;76:7641–7653. doi: 10.1021/jo2012658. [DOI] [PubMed] [Google Scholar]; (c) Carter KP, Moser DJ, Storvick JM, O’Neil GW. Tetrahedron Lett. 2011;52:4494–4496. [Google Scholar]; (d) Diver ST, Giessert AJ. Chem. Rev. 2004;104:1317–1382. doi: 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]
- 31.Cannon JS, Grubbs RH. Angew. Chem. Int. Ed. 2013;52:9001–9004. doi: 10.1002/anie.201302724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.