Abstract
Inhibitors of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, namely statins, exert pleiotropic actions beyond lipid-lowering effects. Their pharmacological activity on atherosclerotic plaque stability and vascular inflammation appears to be mediated, at least in part, by nitric oxide (NO). With the aim of enhancing the nonlipid-lowering properties of selected statins, we introduced a NO-releasing moiety into the structure of pravastatin (NCX 6550) and fluvastatin (NCX 6553). NO release was evaluated as nitrosylhemoglobin adduct formation by using EPR spectroscopy in rat blood. Both compounds produced a linear time-dependent increase in nitrosylhemoglobin formation, which is consistent with slow NO release kinetics. In PC12 cells, unlike their native statins, both compounds stimulated cGMP formation (NCX 6550, EC50 = 2.3 ± 0.2 μM; NCX 6553, EC50 = 2.7 ± 0.2 μM). Moreover, NCX 6550 potently inhibited cell proliferation in rat aortic smooth muscle cells (IC50 = 2.2 ± 0.3 μM) with a mechanism that involved both the polyamine and HMG-CoA reductase signaling pathways. Hence, mevalonate or putrescine partially reverted the effects of NCX 6550 and their combination was fully effective. In RAW 264.7 murine macrophage cells stimulated with lipopolysaccharide (1 μg/ml), NCX 6550, but not pravastatin, significantly decreased inducible NO synthase and cyclooxygenase-2 protein expression as well as nitrite accumulation. All together, the data show that the previously undescribed NO-releasing statins retain HMG-CoA reductase inhibitory activity and release bioactive NO slowly. Among the additional properties, compared with native statins, the NO-releasing statins show enhanced antiinflammatory effects. Thus, NO-releasing statins represent an interesting class of drugs having potential in the therapy of disorders associated with endothelial dysfunction and vascular inflammation.
Inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase, commonly referred to as statins, are the most used class of drugs for the treatment of hypercholesterolemia. This pathological condition is strongly associated with the development of atherosclerosis, the underlying disorder in the majority of patients with cardiovascular diseases (1). Several clinical trials have established the efficacy of statins in reducing low-density lipoprotein–cholesterol levels and, as a secondary end point, the incidence of coronary heart disease (2). It is assumed that cholesterol reduction is the main achievement at the base of statin beneficial properties. However, the results of many studies have pointed out the therapeutic impact of the so-called “pleiotropic effects” of statins and have raised the possibility that mechanisms of action beyond lipid-lowering activity might be responsible for their beneficial effects in atherosclerotic patients (3, 4).
Among statin properties, independent of cholesterol biosynthesis inhibition, both nitric oxide (NO)-mediated antiinflammatory action at the level of the endothelium and the capacity of NO to inhibit vascular smooth muscle cell (SMC) proliferation appear to be critical (5). Indeed, statin-elicited increases in endothelial NO production have been shown to play a key role in vascular reactivity and modulation of SMC proliferation as well as cellular inflammation (6, 7).
Since its discovery, NO has been shown to be involved in modulating numerous vital functions, and it is recognized as a key regulator of cardiovascular homeostasis as well as inflammation, host–defense responses (8), and SMC proliferation (9). A key aspect of the regulatory activity of NO is the fine tuning of transcription of inducible genes, including those of inducible NO synthase (iNOS) or cyclooxygenase type 2 (COX-2). Thus, NO has been shown to have inhibitory (10, 11) or facilitatory (12, 13) activity on iNOS expression. Likewise, some authors reported that NO might exert stimulatory effects on COX-2 expression (14, 15), whereas others documented that slow NO-releasing drugs such as the nitro-aspirin derivative, 2-(acetyl)benzoic acid 3-(nitrooxy-methyl)phenyl ester (NCX 4016), show inhibitory effects on gene expression of either iNOS or COX-2 (16). This dual action of NO depends largely on the concentration that is released in specific tissues, which ultimately results in mimicking either the effects of the constitutive endothelial NO synthase or those of iNOS (17).
In view of this background, new pharmacological agents combining the properties of statins with those of a slow NO release compound might provide added therapeutic value, especially in those pathologies such as diabetes and atherosclerosis, where an impairment of endothelial function causes the inability to produce endogenous NO and plays a crucial role in disease progression. In an attempt to develop statin derivatives endowed with a broader action, we synthesized previously undescribed chemical entities by linking a NO-releasing moiety to the backbone structure of certain statins commonly used in therapy. Here we describe that nitric ester derivatives of pravastatin and fluvastatin not only retain the activity of the parent compounds but also possess the capacity to release functional NO, which in turn produces additional pharmacological effects including enhanced antiinflammatory activity in vitro.
Materials and Methods
Drugs and Reagents. 3-Isobutyl-1-methylxanthine (IBMX), 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1), S-nitroso-N-acetylpenicillamine (SNAP), isosorbide-5-mononitrate, putrescine, FBS, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, (3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, lipopolysaccharide (LPS), sulfanilamide, N-(1-naphthyl)ethylenediamine, glycerol, bromophenol blue, and 2-mercaptoethanol were purchased from Sigma. The undifferentiated pheochromocytoma (PC12) cell line was a gift from Emilio Clementi (San Raffaele Scientific Institute, Milan). Gentamicin, PBS, HBSS, and DMEM were from GIBCO (Invitrogen). The cGMP enzyme immunoassay kit was from Cayman Chemical (Ann Arbor, MI). The DC protein assay was from Bio-Rad.
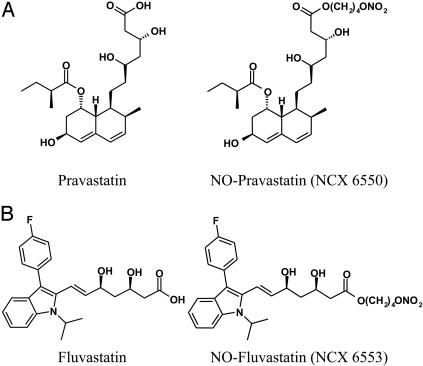
The NO-releasing statin derivatives were synthesized starting from the respective parent compound according to a synthetic procedure used for other NO-releasing agents (18). 1S-[1α(βS*, δS*),2α,6α,8β-(R*),8aα]]-1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-1-naphthaleneheptanoic acid 4-(nitrooxy)butyl ester (NCX 6550) is a derivative of pravastatin (Fig. 1). Its molecular weight is 541.64 (compared with 446.52 for pravastatin). R*,S*-(E)]-7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptanoic acid 4-(nitrooxy)butyl ester (NCX 6553) is a derivative of fluvastatin (Fig. 1). Its molecular weight is 528.58 (compared with 433.47 for fluvastatin).
Fig. 1.
Chemical structure of pravastatin and its NO-releasing derivative, NCX 6550 (A), and fluvastatin and its NO-releasing derivative, NCX 6553 (B). Other information is given in Materials and Methods.
The different statins are characterized by a different degree of lipophilicity (19). By changing their chemical structure, we modified the physicochemical properties. Taking the theoretical partition coefficients (LogP) as a measure of lipophilicity, we estimated the following changes: pravastatin and NCX 6550 from 2.5 to 4.1 and fluvastatin and NCX 6553 from 4.8 to 6.2.
NO Release as Measured by EPR Spectroscopy. Venous rat blood was deoxygenated for 30 min with a gas mixture containing 95% N2 and 5% CO2 in a sealed glass apparatus equipped with inlet and outlet purge valves and a water-warming jacket to maintain controlled temperatures. Blood samples were incubated at 37°C under anaerobic conditions. Test drugs were added to blood to a final concentration of 100 μM. At fixed times, 500-μl aliquots were taken by a degassed syringe and immediately transferred to EPR quartz tubes (4-mm i.d.). The plasma-erythrocytes were directly separated by tube centrifugation and stored in liquid nitrogen until the EPR spectroscopy assay.
EPR spectra were recorded at 100 K with an EMX (X band) Bruker spectrometer (Billerica, MA) equipped with a high-sensitivity cylindrical cavity and the concentration of the nitrosylhemoglobin complex HbFe(II)NO, expressed as micromolar, was determined by double integration of the signal by using CuSO4-ethylenediamine tetraacetic acid as reference standard (20).
Measurements of cGMP in the Rat PC12 Cell Line. The rat pheochromocytoma cell line was used. Cells were cultured according to previous reports (21). At the time of the experiments, the cells were washed once with HBSS supplemented with 0.05% ascorbic acid and then exposed for 45 min to either control conditions or increasing concentrations of test compounds in the presence of the phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (100 μM), and the NO-independent activator of soluble guanylyl cyclase, YC-1 (20 μM). The reaction was terminated by removal of the incubating buffer and consecutive addition of 100 μl of absolute ethanol. The organic extracts were then evaporated to dryness, and the residues were dissolved in aqueous buffer for quantitative determination of intracellular cGMP levels by using the cGMP enzyme immunoassay kit.
Effects on Proliferation of Rat Aortic SMC (RASMC). Cells were plated, grown, subcultured, and cultured as described (22). Briefly, RASMC (passages 15–25) were trypsinized and resuspended in fresh DMEM-Hepes medium supplemented with 10% FBS/2mM glutamine/100 units/ml penicillin/100 μg/ml streptomycin. The cells were seeded in 12-ml plates at a density of 3 × 103 cells per cm2 and incubated at 37°C in a humidified atmosphere of 5% CO2/95% air. After 24 h, the growth medium was replaced with 1 ml of arginine-free DMEM-Hepes supplemented with 5% FBS and 50 μM l-arginine and cell proliferation assessed by [3H]thymidine (0.5 μCi, 6.7 Ci/mmol; New England Nuclear) incorporation into the DNA during the following 24 h (23). Test agents were added to the cells at the time of [3H]thymidine addition and left throughout the experimental period. Where tested, putrescine (30 μM) or ornithine (100 μM) was added to the cells at the same time as the test agents. Cell proliferation data are expressed as percent of control values.
Antiinflammatory Effects in the RAW 264.7 Cell Line. RAW 264.7, a murine cell line with monocyte/macrophage phenotype, was obtained from Sigma and cultured as described (21). Before the experiments, cells were incubated for 24 h in the presence of DMEM containing 0.4% FBS and gentamicin. Cells were preincubated for 30 min in the presence of drugs or vehicle (0.1% DMSO) and then subjected to LPS (1–1,000 ng/ml) for 16 h. At the end of the incubation period, the supernatant was collected and frozen. Mitochondrial respiration, as an indicator of cell viability, was assessed by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (0.5 mg/ml) to formazan. The extent of reduction of MTT to formazan was quantitated by measurement of optical density at 550 nm.
Nitrite content was determined in 100 μl of cell culture medium. After the addition of the sample in 96 multiwell plates, 50 μlof1% sulfanilamide in 5% H3PO4 solution was added to each well, before the final addition of 0.15% N-(1-naphthyl)ethylenediamine). The formation of the deep purple azo compound, indicative of nitrite presence, was analyzed by optical density measurement (540 nm) by using a spectrophotometer.
In selected experiments, RAW 264.7 cells were harvested in lysis buffer (20 mM Tris·HCl/1% CHAPS/1 mM EDTA/1 mM DTT/1 μg/ml leupeptin/1 mM PMSF) and Western blot analysis was performed. Cell lysates were mixed with Laemmli reagent (final concentrations: 1% wt/vol, SDS; 10%, vol/vol, glycerol; 0.5%, wt/vol, bromophenol blue) under reducing conditions (2%, vol/vol, 2-mercaptoethanol) and heated for 5 min at 85°C. SDS/PAGE was performed by using 8–12% and 4% acrylamide for separating and stacking gels, respectively. Protein transfer onto polyvinylidene difluoride membranes was performed at 200 mA for 2 h. Membranes were saturated overnight in blocking buffer containing 5% nonfat dry milk and incubated for 2 h at room temperature with different polyclonal antibody solutions. After washing with buffer (Tris·HCl, 0.42%, pH 7.4, containing 0.1% Tween 20), membranes were further incubated with an anti-rabbit IgG conjugated with horseradish peroxidase at 1:10,000 dilution (Santa Cruz Biotechnology) for 45 min at room temperature. Immunoreactive proteins were detected by using chemiluminescence substrates according to the manufacturer's instructions and later visualized after exposure to Hyperfilm. Quantitative analysis of the bands was performed by direct comparison of the intensity of the bands with the respective signals from β-actin. Band intensity was determined by using a Chemi Doc system (BioRad) and quantity one software (version 4.3.1; BioRad). The linearity of the signal was verified by loading different amounts of samples. In selected experiments, the identity of bands was ascertained by loading recombinant iNOS and COX-2 standards.
Data Analysis. Unless otherwise stated, all values are expressed as mean ± SEM. Cell proliferation data were analyzed by using the Bonferroni t test for unpaired values. All other data were analyzed by using ANOVA coupled to Dunnett's test for unpaired values. P < 0.05 was considered statistically significant.
Results
NO Release in Whole Rat Blood. NO binds to deoxyhemoglobin, thereby leading to the formation of a high-affinity paramagnetic complex [HbFe(II)NO] that produces a distinctive electron spin resonance spectrum (20). Thus, the EPR technique represents a valuable analytical tool for understanding the kinetics of NO release. To assess whether the pravastatin and fluvastatin derivatives can release NO in biological systems, we monitored HbFe(II)NO formation after the exposure of whole rat blood to either statins or their NO-releasing derivatives. HbFe(II)NO is stable in deoxygenated conditions, and its accumulation was followed for up to 4 h. SNAP (100 μM) induced a rapid and sustained increase of HbFe(II)NO that reached a maximum within 15 min (the earliest time point analyzed) and remained stable thereafter (Fig. 2). Conversely, equimolar concentrations of NCX 6550 and NCX 6553 elicited a response that was detectable starting ≈1 h after drug addition and increased linearly thereafter in a time-dependent fashion for 4 h (the latest time point analyzed). During this period, the rate of HbFe(II)NO formation (V[HbFe(II)NO]), as estimated from a linear regression analysis (NCX 6550, r2 = 0.94; NCX 6553, r2 = 0.98) of the data were: V[HbFe(II)NO] = 0.148 μM/min and V[HbFe(II)NO] = 0.121 μM/min, for NCX 6553 and NCX 6550, respectively (Fig. 2). The effects could not be attributed to endogenous NO production, because the parent drugs were devoid of any effect under these experimental conditions.
Fig. 2.
Effects of the NO-releasing derivatives of pravastatin (NCX 6550) and fluvastatin (NCX 6553) on nitrosylhemoglobin formation in whole rat blood as measured by using EPR spectroscopy. Experiments were performed in vitro by incubating venous rat blood with 100 μM NCX 6550 (filled triangles), NCX 6553 (filled squares), and the reference NO donor SNAP (filled circles) for up to 4 h. Data are representative of three separate experiments and are reported as mean ± SEM.
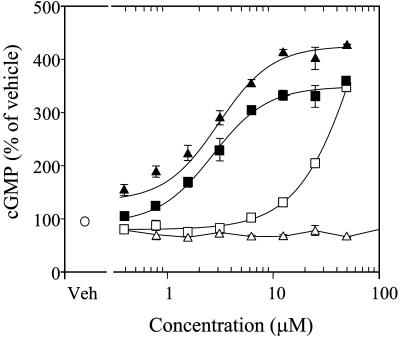
cGMP Accumulation in Rat PC12 Cells. Many biological actions of NO are mediated by cGMP (24). Therefore, we evaluated the extent of cGMP formation elicited by the drugs in PC12 cells. None of the statins examined induced appreciable cGMP accumulation (data not shown). Recently, the NO-independent activator of soluble guanylate cyclase, YC-1, has been shown to potentiate NO effects by stabilizing the binding of NO to the enzyme (25). We performed additional experiments in the presence of a maximally effective concentration of YC-1 (20 μM). The exposure of rat PC12 cells to statin derivatives resulted in a concentration-dependent potentiation of cGMP formation that was maximum at 50 μM. As illustrated in Fig. 2, the NCX 6550 profile is consistent with an estimated potency of 2.3 ± 0.2 μM (EC50), whereas pravastatin was completely inactive. However, pravastatin is known to be weakly effective in vitro because of its low lipophilicity (19). With the introduction of the NO-releasing moiety, the physicochemical characteristics of NCX 6550 are altered compared with pravastatin, making it more lipophilic and therefore increasing its penetration into the cells. To understand better the contribution of the NO moiety, we studied fluvastatin, which is a more liphophilic compound. Fluvastatin itself increased cGMP formation slightly, but only at concentrations above 25 μM, whereas its NO derivative, NCX 6553, was significantly more potent (EC50 = 2.7 ± 0.2 μM; Fig. 3).
Fig. 3.
Effects of pravastatin (open triangles) and its derivative, NCX 6550 (filled triangles), or fluvastatin (open squares) and its derivative, NCX 6553 (filled squares), on cGMP formation in PC12 cells. PC12 cells were incubated with YC-1 (20 μM) in the presence or absence of test compounds for 45 min. cGMP accumulation is indicated as percent of vehicle (open circle). Values are expressed as mean ± SEM; n = 6 for each treatment group.
Inhibition of RASMC Proliferation. It is well established that statins, as well as NO, can exert a cytostatic action on proliferating cells (9, 26). As expected, because of its low lipophilicity, pravastatin inhibited [3H]thymidine incorporation by RASMC only at high concentration, i.e., 100 μM (Fig. 4A). However, NCX 6550 caused a significant inhibition of [3H]thymidine incorporation with an estimated IC50 of 2.2 μM (Fig. 4A). Fluvastatin inhibited cell proliferation with an IC50 of 4.0 μM, whereas its NO-releasing derivative, NCX 6553, was more potent (IC50 of 0.5 μM).
Fig. 4.
Effects of pravastatin and its derivative, NCX 6550, on RASMC proliferation. (A) Concentration-dependent effects of pravastatin (open triangles) and NCX 6550 (filled triangles). The extent of inhibition is indicated as percent of vehicle (open circle). (B) RASMC were preincubated in the absence (white bars) or in the presence of 100 μM mevalonate (gray bars), 30 μM putrescine (hatched bars), and the combination of both (filled bars) before incubation with test compounds (SNAP, 30 μM; pravastatin, 100 μM; NCX 6550, 30 μM). Values are expressed as mean ± SEM of at least three independent experiments. *, P < 0.05 vs. control; #, P < 0.05 vs. respective indicated compound tested in absence of mevalonate and/or putrescine.
To gain more insight into the mechanism responsible for the antiproliferative effects of NO statins, we performed additional experiments with NCX 6550 either in the presence or absence of mevalonic acid (100 μM), the end product of 3-hydroxy-3-methylglutaryl CoA reductase enzyme, or putrescine (30 μM), which was previously shown to counteract the effects of NO donors by overriding the inhibitory action of NO on ornithine decarboxylase (9). As expected, the cytostatic action of pravastatin was completely reversed by mevalonate but not by putrescine. Conversely, consistent with previous results (9), the cytostatic action of the NO donor, SNAP, was inhibited in the presence of putrescine but not mevalonate (Fig. 4B). Interestingly, either putrescine or mevalonate partially prevented the cytostatic action of NCX 6550 (Fig. 4B). Furthermore, the combination of the two drugs nearly abolished the antiproliferative action of NCX 6550 (Fig. 4B). These observations support the concept that NO-releasing statins inhibit RASMC proliferation through two distinct mechanisms: one that depends on NO via the polyamine signaling pathway and the other that is linked to 3-hydroxy-3-methylglutaryl CoA reductase inhibitory activity.
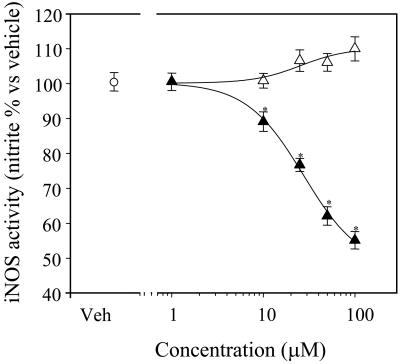
Effects on Inflammatory Pathways in RAW 264.7 Cells. Monocyte–macrophage cells are considered to play a central role in the development and progression of the inflammatory processes underlying atherogenesis (27). Moreover, overexpression of inducible enzymes such as iNOS and COX-2 in this cell type is considered the hallmark of an ongoing inflammatory response (28, 29). Thus, consistent with previous reports (30), 16-h exposure of RAW 264.7 cells to bacterial LPS (1 μg/ml) induced marked de novo synthesis of iNOS protein and consequent nitrite (one of the inactive oxidation products derived from NO) accumulation in the cell culture medium. NCX 6550 (1–100 μM) produced a concentration-dependent inhibition of nitrite accumulation in the cell culture medium (IC50 = 27.7 ± 6.3 μM), whereas no effect was observed in the presence of pravastatin alone (Fig. 5).
Fig. 5.
Effects of pravastatin and its derivative, NCX 6550, on nitrite accumulation in the RAW 264.7 cell line. Cells were pretreated for 30 min with either pravastatin (open triangles) or NCX 6550 (filled triangles) and then stimulated with bacterial LPS (1 μg/ml). After a 16-h incubation, iNOS activity was measured by the Griess method and was expressed as nitrite accumulation in the cell culture medium. Values are expressed as mean ± SEM (% vs. LPS); n = 20 determinations obtained from three separate experiments. *, P < 0.05 vs. vehicle or pravastatin alone.
Under the same experimental conditions, cell viability was not affected after incubation with NCX 6550. In fact, there was no correlation between inhibition of nitrite accumulation (pharmaco-logical effect) and decrease in cell viability (nonspecific metabolic effect), as shown by linear regression analysis (r2 = 0.405; not significant). Moreover, to rule out the possibility that the observed effect was related to an increased lipophilicity of NCX 6550 attributable to the modified chemical structure, experiments were performed with fluvastatin and its NO-releasing derivative, which are both lipophilic. NCX 6553 (10 μM), the fluvastatin derivative, was able to inhibit nitrite accumulation, an effect not shared by the parent compound (data not shown).
The decrease in nitrite accumulation in the cell culture medium could have been attributed to the blockade of iNOS enzymatic activity and/or inhibition of iNOS protein synthesis. To address this point, we studied the effects of NCX 6550 on iNOS protein expression. Consistent with the data obtained in the nitrite accumulation experiments, NCX 6550, but not pravastatin, reduced iNOS protein expression in a concentration-dependent fashion at 16 h after the exposure of cells to LPS (Fig. 6). These results link the action of NCX 6550 directly to iNOS protein synthesis rather than to iNOS enzymatic activity. Finally, we determined the effects of NCX 6550 on the expression of COX-2. Similarly to that observed for iNOS, the NO-releasing pravastatin derivative, but not the parent compound, showed an inhibitory action at the level of COX-2 protein expression (Fig. 6).
Fig. 6.
Effects of pravastatin and its derivative NCX 6550 on iNOS and COX-2 protein expression in RAW 264.7 cells. After preincubation (30 min) with vehicle (open bars), pravastatin (50 or 100 μM, gray bars) or NCX 6550 (50 or 100 μM, filled bars), cells were stimulated with LPS (1,000 ng/ml) for 16 h. At the end of the incubation period, the supernatants were collected and cells harvested with lysis buffer and Western blot analysis performed. β-Actin was used as the reference housekeeping gene. Values are expressed as mean ± SEM of relative arbitrary units (RAU) and are representative of six determinations from two separate experiments. *, P < 0.05 vs. vehicle or pravastatin alone.
Discussion
This paper describes chemical compounds that incorporate two biologically active moieties: statins and NO. The main finding is that two prototype compounds, the NO-releasing pravastatin, NCX 6550, and the NO-releasing fluvastatin, NCX 6553, show pharmacological properties that differ from those of the native statins. These compounds release NO and produce biological activity that appears to be mediated largely by NO.
Using PC12 cells, which are sensitive to NO-induced stimulation of cGMP production, both NO-releasing statins produced a concentration-dependent increase in cGMP, which was much greater than that observed with the parent statins. Specifically, although pravastatin produced no effect on cGMP levels, its NO-releasing derivative, NCX 6550, increased cGMP levels with an EC50 of 2.3 μM. However, being a hydrophilic statin (19), one might expect that pravastatin would show little or no activity in cell assay systems. Incorporation of the nitric ester moiety increases lipophilicity and therefore allows the compound to better penetrate into the cells. This change in physicochemical properties of the compounds may have accounted for the differences in pharmacology between pravastatin and NO-releasing pravastatin. To confirm this possibility, we examined fluvastatin and its NO-releasing derivative, NCX 6553. Fluvastatin is considered a lipophilic statin that readily penetrates into the cells to produce its effects (19). In PC12 cells, although fluvastatin increased cGMP at concentrations above 25 μM, its NO derivative was much more potent (EC50 of 2.7 μM).
Consistent with these data, another study conducted in the rabbit aorta showed that NCX 6550 (IC50 = 2.4 ± 0.6 μM) and NCX 6553 (IC50 = 8.7 ± 3.3 μM) counteracted norepinephrine-induced contraction, whereas pravastatin was not effective and fluvastatin produced vasorelaxation only at a very high concentration. Moreover, the vasorelaxant effect was prevented by the soluble guanylyl cyclase inhibitor 1-H-(1,2,4-)oxadiozolo(4,3-a)-quinoxalin-1-one (data not shown).
In rat whole blood, using a direct method to measure bioactive NO (nitrosylhemoglobin formation as measured with EPR spectroscopy), both NCX 6550 and NCX 6553 released NO slowly. This is consistent with the results of other studies on drugs incorporating a similar NO-releasing moiety, such as the NO-releasing derivative of aspirin, NCX 4016 (31). This property makes these drugs of potential clinical interest because they produce little or no change in systemic blood pressure and do not cause other effects typically associated with the nitrovasodilators that release NO rapidly (32).
An interesting model that allowed us to delineate the distinct contributions of the statin and the NO moiety is that of vascular SMC proliferation. The 3-hydroxy-3-methylglutaryl CoA reductase inhibitors are known to inhibit cell proliferation, an effect that is prevented by the addition of mevalonate (33). When tested in the RASMC, fluvastatin at concentrations ranging from 0.1 to 100 μM and pravastatin only at 100 μM or greater inhibited cell proliferation, and this was reversed by addition of mevalonate. The NO-releasing derivative of pravastatin, NCX 6550, allowed us to further investigate the signaling pathway involved in this effect. NCX 6550 was much more potent than pravastatin. When tested in the presence of the polyamine putrescine, which is required for cell proliferation, the antiproliferative effects of NCX 6550 were partially prevented. These data indicate that one key component of the NCX 6550 antiproliferative action is attributable to NO. Importantly, such effects have been demonstrated to occur through a mechanism that is cGMP independent (9). Moreover, as expected, mevalonate also partially reversed the cytostatic action of NCX 6550. The combined addition of mevalonate plus putrescine caused a nearly complete reversal of the cytostatic effect of NCX 6550.
These observations reveal clearly that NO-releasing statins inhibit cell proliferation through two distinct mechanisms: one relying on the biosynthetic pathway leading to cholesterol and isoprenoids and the other through a NO-mediated inhibition of the ornithine decarboxylase-polyamine pathway. Consistent with this, it has also been shown that another slow NO-release drug, NCX 4016, elicited similar effects (9). Putrescine, which is produced from ornithine via ornithine decarboxylase, can therefore override the antiproliferative action of NO.
Statins produce a variety of effects independent of their plasma cholesterol-lowering properties. A number of studies have shown that statins exert vascular protection through mechanisms involving NO production (34). Indeed, the so-called pleiotropic effects of statins appear to depend on the inhibition of the synthesis of isoprenoid intermediates of the mevalonate pathway that are critical for the posttranslational modification of several proteins, including the small GTP-binding Ras or Rho (35, 36). Direct inhibition of Rho has been shown to mediate the increase in endothelial NO synthase (eNOS) expression by statins (35). Increasing evidence suggests that the activation of the protein kinase Akt may also contribute to NO-mediated effects in endothelial cells (37). The net result of these actions is that statins increase NO production via eNOS activation in a variety of cells. Interestingly, exogenous NO provided by the NO-releasing statin markedly potentiates the NO-mediated component of the statin in different test systems. This property may well enhance the therapeutic actions of the statins. The first expected effect is a significant restoration of endothelial function beyond that induced by statins alone in atherosclerotic disease. Decreased synthesis and/or activity of NO underlies endothelial dysfunction, which is an important factor leading to atherosclerosis and acute coronary syndrome. Thus, reduced NO availability contributes to a variety of effects such as impairment of vascular relaxation, platelet aggregation, increased vascular smooth muscle proliferation, and enhanced leukocyte and monocyte adhesion to the endothelium. The data presented in this study show that the NO-releasing statins contribute to the reduction in both SMC proliferation and inflammatory events.
There is evidence that low physiological concentrations of NO are inhibitory on iNOS expression through a negative feedback mechanism (17). Consistent with this mechanism, the NO-releasing statins inhibited both expression and function of iNOS in LPS-stimulated macrophages, thereby suggesting that the quantity of NO released mimics that normally produced by constitutive NOS under physiological conditions. Recent findings have suggested that statins might exert their cardiovascular protective effects by arresting monocyte differentiation into macrophages and promoting monocyte apoptotic cell death (38). The possibility exists that the effects on iNOS may be consequent to an apoptotic event elicited by either the statin, NO, or a combination of both. Although this possibility cannot be ruled out, we did not observe any cell death at pharmacologically effective concentrations of the test agents. Therefore, under the experimental conditions used, there appears to be little or no contribution of apoptosis to iNOS inhibition caused by the NO-releasing statins.
There are additional properties that make the NO-releasing statins more effective than the native statins. For example, using the same NO-releasing derivative, NCX 6550, Gresele et al.¶ showed that the compound inhibited platelet aggregation in vitro and reduced mortality in the thromboembolism mouse model. Moreover, unlike pravastatin, NCX 6550, inhibited LPS and phorbol myristate acetate-induced tissue factor expression in isolated human mononuclear cells. These observations indicate that the NO-releasing pravastatin possesses antiplatelet and anticoagulant effects, and that such actions may afford NO-releasing pravastatin with antiatherothrombotic properties.¶
Despite numerous experimental studies showing nonlipid lowering effects of statins, there is no clear indication that such effects have any clinical value. However, it appears that the cardiovascular-protecting properties of statins are at least partially independent of their lipid-lowering effects. The results of the present study show that the incorporation of NO moiety into the statin structure significantly potentiates the nonlipid-lowering mechanism of action of this class of drugs. These findings strongly suggest that these NO-releasing statins may offer an opportunity to target atherosclerosis through a multipathway approach. The multiple mechanisms of action of the NO-releasing statins may provide significantly enhanced therapeutic benefits over the standard statin drugs. Moreover, the NO-releasing statins may provide short-term clinical efficacy not observed with statins.
Acknowledgments
We thank Dr. Emilio Clementi (San Raffaele Scientific Institute, Milan) for providing the pheochromocytoma (PC12) cell line. The editorial support of Dr. Barbara Gallone is greatly appreciated. This work was supported in part by National Institute of Health Grants HL 58433 and HL 66999.
Abbreviations: COX-2, cyclooxygenase type 2; YC-1, 3′-(5′-hydroxymethyl-2-Furyl)-1-benzylindazole; iNOS, inducible NO synthase; LPS, lipopolysaccharide; NCX 6550, [1S-[1α(βS*, δS*),2α,6α,8β-(R*),8aα]]-1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-1-naphthaleneheptanoic acid 4-(nitrooxy)butyl ester; NCX 6553, [R*,S*-(E)]-7-[3-(4-fluorophenyl)-1(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptanoic acid 4-(nitrooxy)butyl ester; SNAP, S-nitroso-N-acetyl-penicillamine; SMC, smooth muscle cell; RASMC, rat aortic SMC.
Footnotes
Gresele, P., Rossiello, M. R., Momi, S., Semeraro, N., Caracchini, R., Ongini, E., Del Soldato, P. & Colucci, M. (2003) Circulation 108, Suppl IV, 161 (Abstract).
References
- 1.Shepherd, J., Cobbe, S. M. & Ford, I. (1995) N. Engl. J. Med. 333, 1301-1307. [DOI] [PubMed] [Google Scholar]
- 2.Heart Protection Study Collaborative Group (2002) Lancet 360, 7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonetti, P. O., Lerman, L. O., Napoli, C. & Lerman, A. (2003) Eur. Heart J. 24, 225-248. [DOI] [PubMed] [Google Scholar]
- 4.Marz, W. & Koenig, W. (2003) J. Cardiovasc. Risk 3, 169-179. [DOI] [PubMed] [Google Scholar]
- 5.Weitz-Schmidt, G. (2002) Trends Pharmacol. Sci. 23, 482-486. [DOI] [PubMed] [Google Scholar]
- 6.Ni, W., Egashira, K., Kataoka, C., Kitamoto, S., Koyanagi, M., Inoue, S. & Takeshita, A. (2001) Circ. Res. 89, 415-421. [DOI] [PubMed] [Google Scholar]
- 7.Dichtl, W., Dulak, J., Frick, M., Alber, F. H., Schwarzacher, S. P., Ares, M. P. S., Nilsson, J., Pachinger, O. & Weidinger, F. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 58-63. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan, C. (2001) Nat. Immunol. 2, 907-916. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro, L. J., Buga, G. M., Wei, L. H., Bauer, P. M., Wu, G. & Del Soldato, P. (2001) Proc. Natl. Acad. Sci. USA 98, 4202-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colasanti, M., Persichini, T., Menegazzi, M., Mariotto, S., Giordano, E., Caldarera, C. M., Sogos, V., Lauro, G. M. & Suzuki, H. (1995) J. Biol. Chem. 270, 26731-26733. [DOI] [PubMed] [Google Scholar]
- 11.Peng, H. B., Spiecker, M. & Liao, J. K. (1998) J. Immunol. 161, 1970-1976. [PubMed] [Google Scholar]
- 12.Pérez-Sala, D., Cernuda-Morollòn, E., Dìaz-Cazorla, M., Rodriguéz-Pascual, F. & Lamas, S. (2001) Am. J. Physiol. 280, 466-473. [DOI] [PubMed] [Google Scholar]
- 13.Connelly, L., Jacobs, A. T., Palacios-Callender, M., Moncada, S. & Hobbs, A. J. (2003) J. Biol. Chem. 278, 26480-26487. [DOI] [PubMed] [Google Scholar]
- 14.Tetsuka, T., Daphna-Iken, D., Miller, B. W., Guan, Z., Baier, L. D. & Morrison, A. R. (1996) J. Clin. Invest. 97, 2051-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Knethen, A., Callsen, D. & Brune, B. (1999) Mol. Biol. Cell. 10, 361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorucci, S., Mencarelli, A., Meneguzzi, A., Lechi, A., Morelli, A., Del Soldato, P. & Minuz, P. (2002) Circulation 106, 3120-3125. [DOI] [PubMed] [Google Scholar]
- 17.Colasanti, M. & Suzuki, H. (2000) Trends Pharmacol Sci. 21, 249-252. [DOI] [PubMed] [Google Scholar]
- 18.Burgaud, J. L., Benedini, F., Robinson, E. M. & Del Soldato, P. (1999) Drugs Future 24, 1-4. [Google Scholar]
- 19.Serajuddin, A. T., Ranadive, S. A. & Mahoney, E. M. (1991) J. Pharmacol. Sci. 80, 830-834. [DOI] [PubMed] [Google Scholar]
- 20.Kosaka, H., Saway, Y., Sakaguchi, H., Kumura, E., Harada, N., Watanabe, M. & Shiga, T. (1994) Am. J. Physiol. 266, 1400-1405. [DOI] [PubMed] [Google Scholar]
- 21.Bulotta, S., Barsacchi, R., Rotiroti, D., Borgese, N. & Clementi, E. (2001) J. Biol. Chem. 276, 6529-6536. [DOI] [PubMed] [Google Scholar]
- 22.Wei, L. H., Jacobs, A. T., Morris, Jr., S. M. & Ignarro, L. J. (2000) Am. J. Physiol. 279, 248-256. [DOI] [PubMed] [Google Scholar]
- 23.Buga, G. M., Singh, R., Pervin, S., Rogers, N. E., Schmitz, D. A., Jenkinson, C. P., Cederbaum, S. D. & Ignarro, L. J. (1996) Am. J. Physiol. 271, 1988-1998. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro, L. J. (1989) Circ. Res. 65, 1-21. [DOI] [PubMed] [Google Scholar]
- 25.Koglin, M. & Behrends, S. (2003) J. Biol. Chem. 278, 12590-12597. [DOI] [PubMed] [Google Scholar]
- 26.Bellosta, S., Bernini, F., Ferri, N., Quarato, P., Canavesi, M., Arnaboldi, L., Fumagalli, R., Paoletti, R. & Corsini, A. (1998) Atherosclerosis 137 Suppl, 101-109. [DOI] [PubMed] [Google Scholar]
- 27.Li, A. C. & Glass, C. K. (2002) Nat. Med. 8, 1235-1242. [DOI] [PubMed] [Google Scholar]
- 28.Kujubu, D. A. & Hershman, H. R. (1992) J. Biol. Chem. 267, 7991-7994. [PubMed] [Google Scholar]
- 29.Posadas, I., Terencio, M. C., Guillen, I., Ferrandiz, M. L., Coloma, J., Paya, M. & Alcaraz, M. J. (2000) Naunyn Schmiedeberg Arch. Pharmacol. 361, 98-106. [DOI] [PubMed] [Google Scholar]
- 30.Wu, W. P., Hao, J. X., Ongini, E., Impagnatiello, F., Presotto, C., Wiesenfeld-Hallin, Z. & Xu, X. J. (2004) Br. J. Pharmacol. 141, 65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carini, M., Aldini, G., Stefani, R., Orioli, M. & Facino, R. M. (2001) J. Pharmacol. Biomed. Anal. 26, 509-518. [DOI] [PubMed] [Google Scholar]
- 32.Wallace, J. L., Ignarro, L. J. & Fiorucci, S. (2002) Nat. Rev. Drug Discov. 1, 375-382. [DOI] [PubMed] [Google Scholar]
- 33.Negre-Aminou, P., Van Vliet, A. K., Van Erck, M., Van Thiel, G. C., Van Leeuwen, R. E. & Cohen, L. H. (1997) Biochim. Biophys. Acta 1345, 259-268. [DOI] [PubMed] [Google Scholar]
- 34.Wolfrum, S., Jensen, K. S. & Liao, J. K. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 729-736. [DOI] [PubMed] [Google Scholar]
- 35.Laufs, U. & Liao, J. K. (1998) J. Biol. Chem. 273, 24266-242671. [DOI] [PubMed] [Google Scholar]
- 36.Nubel, T., Dippold, W., Kleinert, H., Kaina, B. & Fritz, G. (2004) FASEB J. 18, 140-142. [DOI] [PubMed] [Google Scholar]
- 37.Kureishi, Y., Luo, Z., Shiojima, I., Bialik, A., Fulton, D., Lefer, D. J., Sessa, W. C. & Walsh, K. (2000) Nat. Med. 6, 1004-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vamvakopoulos, J. E. & Green, C., (2003) BMC Cardiovasc. Disord. 3, 6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]