Abstract
Objectives:
To describe the clinical course and neuropathology at autopsy of a patient with neuromyelitis optica (NMO) treated with alemtuzumab.
Methods:
Case report.
Results:
A 61-year-old woman with aquaporin-4 immunoglobulin G antibody seropositive NMO had 10 clinical relapses in 4 years despite treatment with multiple immunosuppressive therapies. Alemtuzumab was administered and was redosed 15 months later. For the first 19 months after the initial alemtuzumab infusion, the patient did not experience discrete clinical relapses or have evidence of abnormally enhancing lesions on brain or spinal cord MRI. However, she experienced insidiously progressive nausea, vomiting, and vision loss, and her brain MRI revealed marked extension of cortical, subcortical, and brainstem T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities. She died 20 months after the initial alemtuzumab infusion. Acute, subacute, and chronic demyelinating lesions were found at autopsy. Many of the lesions showed marked macrophage infiltration with a paucity of lymphocytes.
Conclusions:
Following alemtuzumab treatment, there appeared to be ongoing innate immune activation associated with tissue destruction that correlated with nonenhancing T2/FLAIR hyperintensities on MRI. We interpret the cessation of clinical relapses, absence of contrast-enhancing lesions, and scarcity of lymphocytes at autopsy to be indicative of suppression of adaptive immunity by alemtuzumab. This case illustrates that progressive worsening in NMO can occur as a consequence of tissue injury associated with monocytic infiltration. This observation may be relevant to multiple sclerosis (MS) as well as NMO and might explain why in previous studies of secondary progressive MS alemtuzumab did not seem to inhibit disability progression despite a dramatic decline in contrast-enhancing lesions.
Unlike multiple sclerosis (MS), a secondary progressive clinical course in neuromyelitis optica (NMO) is rare, and acute relapses in NMO account for most of the neurologic morbidity of the disease.1,2 Alemtuzumab is a humanized monoclonal antibody that targets CD52 and depletes lymphocytes and monocytes.3,4 Alemtuzumab reduces relapse activity in MS.5,6 Here we describe the clinical course and resultant neuropathology at autopsy of a patient with NMO following treatment with alemtuzumab.
CASE REPORT
A 61-year-old white woman with aquaporin-4 immunoglobulin (Ig) G antibody seropositive NMO had 10 clinical relapses in the first 4 years of her disease despite treatment with IV pulse cyclophosphamide, chronic glucocorticoids (up to 60 mg/day prednisone), mycophenolate mofetil (up to 3,000 mg/day), rituximab (2,000 mg IV, divided doses), and multiple courses of pulsed high-dose glucocorticoids and plasma exchange for acute flares. Relapses included 8 episodes of myelitis, one episode of intractable hiccups, and one episode of postchiasmal visual pathway injury. Functional recovery was poor, and her Expanded Disability Status Scale score ranged from 7.5 to 8.0 (severely paraparetic, essentially restricted to a wheelchair much of the day, preserved cognition). Important comorbidities included hypothyroidism for 10 years prior to diagnosis.
Alemtuzumab 12 mg IV × 5 days (total dose 60 mg) was administered. Prednisone was tapered from 20 mg/day to 10 mg/day over the next year. Fifteen months after her initial infusion, she was retreated with alemtuzumab 12 mg IV × 3 days (total dose 36 mg) and oral prednisone was discontinued the following month.
For the first 19 months after the initial alemtuzumab infusion, she did not experience any discrete clinical relapses or have evidence of abnormally enhancing lesions on brain or spinal cord MRI. However, she did experience insidiously progressive nausea and vomiting that became intractable, resulting in 27 kg of weight loss in the final year of life. Endoscopy did not reveal an alternate etiology for her symptoms. She also developed insidiously progressive bilateral vision loss: Jaeger 1 OD and J2 OS (20/25 and 20/30 Snellen equivalent respectively) to J2 OD and J3 OS (20/30 and 20/40 Snellen equivalent respectively) at 15 months post-alemtuzumab and J4 (20/50 Snellen equivalent) by 17 months. She remained seropositive for aquaporin-4 IgG.
Prior to receiving alemtuzumab, while on other immunosuppressive therapies, she had multiple urinary tract infections, one episode of urosepsis requiring hospitalization, herpes zoster ophthalmicus, a deep venous thrombosis in the leg, hematomas following plasma exchange, and a broken femur after falling from her wheelchair. Following alemtuzumab treatment, her course was complicated by Clostridium difficile colitis (2 months after the initial alemtuzumab infusion) and recurrent urosepsis (16 and 20 months after the first cycle of alemtuzumab).
Enteral feeding was initiated 20 months after the initial alemtuzumab infusion for refractory nausea and appetite loss. The patient and her family requested transition to comfort care, and she died at home.
Gross neuropathologic evaluation of the spinal cord revealed severe destruction at the thoracic and upper lumbosacral levels such that the normal gray–white junction and the delineation of dorsal, lateral, and anterior columns were completely effaced. The optic nerves showed gray-tan discoloration on cross sections, with the left more prominent than the right. In addition, there were numerous areas of demyelination where gray, gelatinous lesions appeared to involve the periventricular white matter, middle cerebellar peduncles, cerebellar white matter, and brainstem. Foci suspicious for demyelination were noted in the area postrema of the medulla oblongata and the pontine tegmentum. Coronal sections of the cerebrum showed a 0.5 cm diameter brown-tan cavitary lesion in the periventricular white matter at the anterior horn of the left lateral ventricle extending into the genu of the corpus callosum. The edge of this lesion was firm, suggestive of gliosis. Another soft cavitary lesion was found in the periventricular white matter of the posterior horn of the right lateral ventricle.
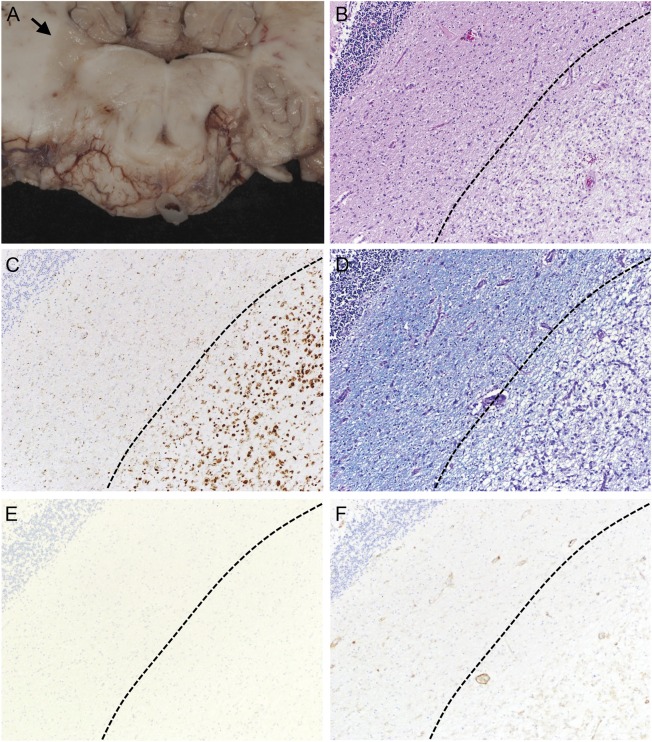
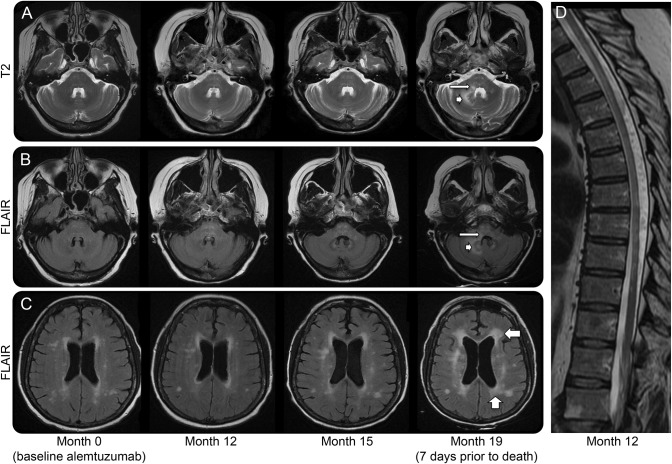
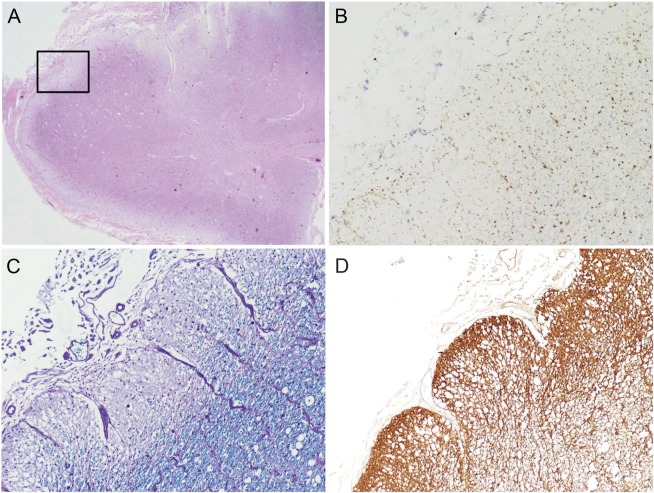
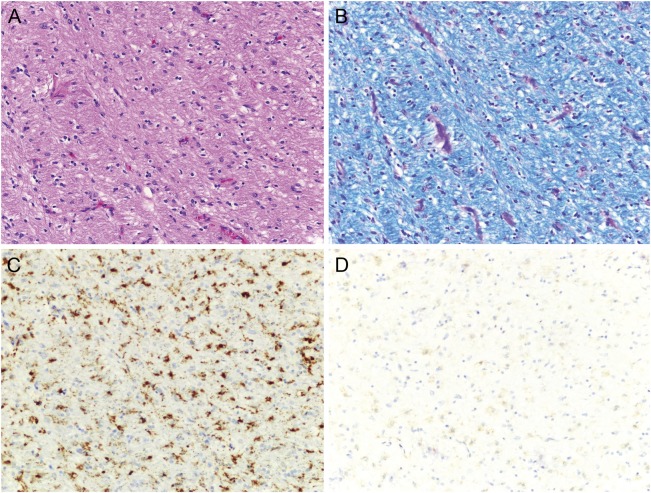
Microscopic examination showed a range of acute, subacute, and chronic demyelinating lesions in the aforementioned areas in the gross examination. One of the most extensively involved areas (figure 1) extended from the pons into the right middle cerebral peduncle and cerebellar white matter and correlated clinically with the MRI T2/fluid-attenuated inversion recovery abnormality shown in figure 2. All foci of demyelination demonstrated marked loss of myelin on luxol fast blue–periodic acid-Schiff stain and infiltration by CD68-positive macrophages. The actively demyelinating lesions showed dense macrophage infiltrates, whereas the subacute to chronic lesions showed cavity formation and relatively fewer macrophages. In all of the lesions there was a loss of the normal volume of oligodendroglia and a relative paucity of mature lymphocytes and neutrophils. Perivenular lymphocytic infiltrates typical of acute MS plaques were not seen. The spinal cord showed extensive demyelination in the thoracic segment and peripheral demyelination at the cervical level (figure 3). Both optic nerves showed infiltration by macrophages and variable astrogliosis (figure 4). There was no evidence of progressive multifocal leukoencephalopathy on autopsy.
Figure 1. Neuropathology of an inflammatory demyelinating lesion in the right cerebellar peduncle.
The gray discoloration and softening noted in the right cerebellar peduncle (arrow, A) corresponds to an area of pallor on neuropathologic examination (B, hematoxylin & eosin, 100×). The pale area is infiltrated by numerous macrophages (C, CD68, 100×) and shows marked myelin loss (D, luxol fast blue–periodic acid-Schiff, 100×). Immunohistochemistry for lymphocyte markers CD4 and CD20 shows no evidence of an adaptive immune response (E and F, CD20/CD4, 100×).
Figure 2. Interval progression of nonenhancing T2/FLAIR lesions on brain MRI.
Brain MRI (A–C) shows interval progression of white matter lesions involving the pontine tegmentum (thin arrow in A and B), right cerebellum (arrowhead in A and B), and periventricular (wide arrows in C). There was no abnormal contrast enhancement in the first 3 studies shown; no contrast was administered for the last MRI. MRI thoracic spine (D) at month 12 after alemtuzumab administration shows multiple T2 parenchymal hyperintensities and prominent myelomalacia. There was no abnormal contrast enhancement (not shown). FLAIR = fluid-attenuated inversion recovery.
Figure 3. Neuropathology of the cervical spinal cord.
A cross-section of the cervical spinal cord shows similar areas of pallor on microscopic examination (A, hematoxylin & eosin, 20×). High-magnification examination of the boxed area from panel A shows macrophage infiltration (B, CD68, 100×) and loss of myelin staining (C, luxol fast blue–periodic acid-Schiff, 100×). There is also a marked increase in staining for glial fibrillary acidic protein (GFAP), corresponding to reactive astrogliosis (D, GFAP, 100×).
Figure 4. Neuropathology of the optic nerve.
A portion of the optic nerve (A, hematoxylin & eosin, 200×) shows some myelin loss (B, luxol fast blue–periodic acid-Schiff, 200×) and striking macrophage infiltration (C, CD68, 200×). Immunohistochemistry for mature T lymphocytes is negative (D, CD4, 200×).
DISCUSSION
We recognize that NMO can be a difficult disease to treat and that some patients do not respond to immune suppression. We believe that this case is noteworthy because following alemtuzumab treatment there appeared to be ongoing innate immune activation associated with tissue destruction. We interpret the cessation of clinical relapses, absence of contrast-enhancing lesions on MRI, and scarcity of lymphocytes at autopsy to be indicative of suppression of adaptive immunity as a consequence of alemtuzumab treatment. In contrast, extensive mononuclear cell infiltrates on neuropathology correlated with new, enlarging, nonenhancing lesions on T2-weighted MRI. The neuroanatomical areas of CNS involvement on imaging and neuropathology corresponded to the patient's progressive neurologic impairments.
We believe that there is a possible relationship between these observations and alemtuzumab's presumed mechanism that primarily targets adaptive immune function.7 We speculate that an underlying disease process involving the innate immune system causing massive monocytic infiltration and tissue destruction occurred. This observation may be relevant to MS as well as NMO and might explain why in secondary progressive MS alemtuzumab did not seem to inhibit disability progression despite a dramatic decline in contrast-enhancing lesions.8 Mononuclear inflammation is a prominent histologic feature of progressive MS, particularly foamy macrophages in periplaque white matter,9 subpial mononuclear inflammatory infiltrates associated with cortical demyelination,10 and widespread microglial activation.10 In progressive MS, T-cell infiltration typically is also present in normal-appearing white matter associated with diffuse axonal injury10 and in perivascular cuffs.10,11
It is possible that alemtuzumab treatment contributed to worsening in this patient, possibly through triggering monocyte activation; however, alemtuzumab treatment does not appear to precipitate worsening in most patients with relapsing or progressive MS.5,6,8 Therefore, if alemtuzumab somehow contributed to the decline in this patient, this effect may be patient- or disease-specific. It is also important to note that our patient was already severely disabled at the time of alemtuzumab treatment.
NMO can be an exceedingly difficult disease to treat, with some patients succumbing to their illness despite empiric use of immune suppressants. Currently there are no proven treatments for prevention of relapses in NMO. We believe that this case illustrates that progressive worsening in NMO can occur in the absence of clinical relapses or contrast-enhancing lesion activity on MRI as a consequence of tissue injury associated with monocytic infiltration.
GLOSSARY
- Ig
immunoglobulin
- MS
multiple sclerosis
- NMO
neuromyelitis optica
AUTHOR CONTRIBUTIONS
Dr. Gelfand: drafting the manuscript, study concept, analysis/interpretation of data, acquisition of data. Dr. Cotter: revising the manuscript for important intellectual content, analysis/interpretation of data, acquisition of data. Dr. Klingman: revising the manuscript for important intellectual content, acquisition of data. Dr. Huang: revising the manuscript for important intellectual content, analysis/interpretation of data, acquisition of data. Dr. Cree: revising the manuscript for important intellectual content, study concept/design, analysis/interpretation of data, acquisition of data, study supervision.
STUDY FUNDING
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR000143 and American Academy of Neurology Clinical Research Training Fellowship (J.M.G.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURE
J.M. Gelfand has received research support from the National Center for Advancing Translational Sciences of the NIH and he and his spouse have received compensation for expert witness medical-legal consulting. J. Cotter and J. Klingman report no disclosures. E.J. Huang is an associate editor for The Journal of Neuroscience and is on the editorial board for Brain Pathology. B.A.C. Cree is an editor for Annals of Neurology; is a consultant for Abbvie, Biogen Idec, EMD Serono, Genzyme, Novartis, Sanofi Aventis, and Teva Neurosciences; has received research support from Acorda, Avanir, Biogen Idec, EMD Serono, Hoffman La Roche, and Novartis; and has been an expert consultant for Biogen Idec. Go to Neurology.org/nn for full disclosures.
REFERENCES
- 1.Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 2007;68:603–605 [DOI] [PubMed] [Google Scholar]
- 2.Cree B. Neuromyelitis optica: diagnosis, pathogenesis, and treatment. Curr Neurol Neurosci Rep 2008;8:427–433 [DOI] [PubMed] [Google Scholar]
- 3.Lowenstein H, Shah A, Chant A, Khan A. Different mechanisms of Campath-1H-mediated depletion for CD4 and CD8 T cells in peripheral blood. Transpl Int 2006;19:927–936 [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009;128:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–1839 [DOI] [PubMed] [Google Scholar]
- 6.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–1828 [DOI] [PubMed] [Google Scholar]
- 7.Freedman MS, Kaplan JM, Markovic-Plese S. Insights into the mechanisms of the therapeutic efficacy of alemtuzumab in multiple sclerosis. J Clin Cell Immunol 2013;4:1000152. [PMC free article] [PubMed] [Google Scholar]
- 8.Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 2006;253:98–108 [DOI] [PubMed] [Google Scholar]
- 9.Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 2001;50:646–657 [DOI] [PubMed] [Google Scholar]
- 10.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128:2705–2712 [DOI] [PubMed] [Google Scholar]
- 11.Revesz T, Kidd D, Thompson AJ, Barnard RO, McDonald WI. A comparison of the pathology of primary and secondary progressive multiple sclerosis. Brain 1994;117:759–765 [DOI] [PubMed] [Google Scholar]