Abstract
Objectives:
With the aging of HIV populations, vascular contributions to neuropathogenesis are increasingly important. Indirect analyses of cerebral small vessel disease have been performed, but there have been no direct studies of human brain to elucidate risk factors for arteriolar sclerosis.
Methods:
Mean arteriolar wall thickness (sclerotic index, SI) was measured in the deep cerebral white matter of 126 brains (96 HIV+, 30 HIV−). Correlations with SI were performed for age, sex, race, hypertension, hyperlipidemia, diabetes, obesity, cirrhosis, hepatitis C virus (HCV) infection, herpes infection, HIV infection, HIV risk, cocaine use, CD4 count, plasma HIV load, and combination antiretroviral therapy (cART) at the time of death.
Results:
Age, hypertension, race, HCV, and cirrhosis were associated with SI; of the HIV variables, only cART at death was associated with SI. To address colinearity, partial correlations were run with HCV and cirrhosis, hypertension and race, and hypertension and age. With HCV controlled, cirrhosis lost significance; with hypertension controlled, age lost significance. For the entire sample, HCV, African American race, and hypertension accounted for 15% of SI variance in multivariate analysis. Each was independently associated with SI, and HCV had the largest effect. For the HIV sample, inclusion of cART in the model increased R2 to 0.205, with only HCV, hypertension, and cART remaining significant or trend level.
Conclusions:
This tissue-based analysis of cerebral arteriolar disease demonstrates that HCV constitutes an independent risk, in addition to African American race, hypertension, and cART. Further study is needed to understand what aspects of HCV and cART contribute to cerebrovascular neuropathogenesis.
Cerebral small vessel disease (SVD) is associated with stroke, cerebral hemorrhage, and white matter rarefaction. By virtue of its role in white matter damage, cerebral SVD is postulated to contribute to cognitive impairment. Risk factors for SVD in general populations include increasing age, hypertension, and diabetes.1–4 With combination antiretroviral therapy (cART) and the aging of individuals with HIV, there is interest in a spectrum of CNS vascular disorders, their relationship to HIV-associated and traditional risk factors, and their neurologic and cognitive outcomes.5–7 Despite this interest, CNS SVD has been studied in only a few HIV populations.
In the Hawaii Aging with HIV Cohort, presence and severity of small vessel ischemic lesions, as determined by neuroimaging, was associated with age and higher mean systolic blood pressure but not HIV-associated immunovirologic indices.8 Funduscopic measurements of retinal arterioles, thought to reflect variations in cerebral small vessels, were performed in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA) cohort.9 In contrast to the Hawaiian study, LSOCA showed that retinal arteriolar caliber was associated with age, hypertension, and cART and CD4 count. In the Hawaiian cohort, as well as other HIV populations, vascular/metabolic risk factors were associated with cognitive impairments.5–7 In LSOCA, retinal vascular indices correlated with visual function.10
While studies of extracranial and large caliber intracranial vascular pathology have been performed in HIV,11 no neuropathologic studies have examined cerebral SVD and its relationship to general and HIV-associated risk factors. Herein, we report analysis of 126 individuals autopsied in the Manhattan HIV Brain Bank (MHBB) and describe risk factors for arteriolar thickening in the deep cerebral white matter of their brains.
METHODS
Patient population.
Patients were a subset of 292 individuals autopsied by MHBB under a Mount Sinai institutional review board–approved protocol. MHBB is a longitudinal study of HIV-associated nervous system complications; it enrolls HIV-positive and HIV-negative individuals who undergo prospective neuromedical evaluations, and its entry criteria were previously described.e1 Some individuals come to autopsy without premortem assessment; in these cases, extensive medical record reviews are performed. The 126 brains in this study were from 96 HIV+ and 30 HIV− individuals; 87 were from the longitudinal study and 39 were assessed at autopsy. Brains were chosen on the basis of having pertinent medical annotation and lack of focal neuropathologies in the deep cerebral white matter.
Clinical information, collected through a combination of patient or caregiver interview, laboratory testing, and/or medical record review, included the following: sex, race/ethnicity, age at death, HIV and hepatitis C virus (HCV) status, presence or absence of hypertension, diabetes, obesity, hyperlipidemia, historical herpes infections (herpes simplex virus, varicella-zoster virus, cytomegalovirus), and, for HIV-infected individuals, last CD4 count and plasma viral load prior to death, history of cocaine misuse, and use of cART at the time of death. Variables obtained from autopsy included presence of cirrhosis (used instead of clinical diagnosis because of greater accuracy) and heart weight.
Tissue analysis.
The MHBB neuropathology protocol has been published previously.e2 In brief, the brain is hemisectioned; half is formalin-fixed and used to generate 57 paraffin-embedded, hematoxylin and eosin (H&E)–stained, 4-μm sections. Standard H&E sections of deep frontal white matter, encompassing the periventricular watershed, were used for analysis and scanned at 200X with a high-speed, high-resolution Olympus VS110 virtual slide scanning system using VS-ASW software (Olympus America Inc, Center Valley, PA). For each patient, between 15 and 20 arterioles were manually identified and marked for analysis and calculation of the arteriolar sclerotic index (SI); the widest axes of these vessels ranged between 50 and 300 μm.
SI was calculated as described, using tools in the VS-ASW program.2,12 To eliminate variations in wall thickness due to vascular obliquity in the plane of section, measurements were done perpendicular to the widest axis of each arteriole, with SI = [1−(inner diameter/external diameter)] (figure). Thus, an SI of 0.99 implies near-total occlusion. A mean SI for each brain was calculated from measurements of 15–20 arterioles.
Figure. Sclerotic index (SI) calculation.
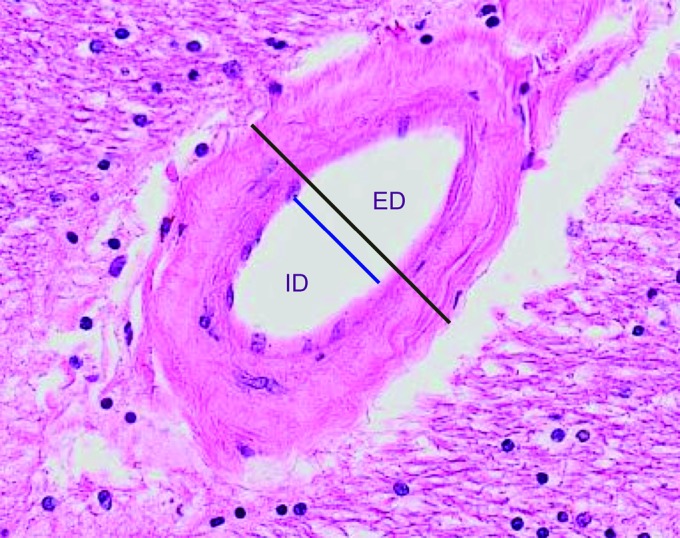
Measurements of the inner diameter (ID) and external diameter (ED) are taken perpendicular to the widest axis of the arteriole (lines separated here for visualization); these are used in calculating the SI = [1−(inner diameter/external diameter)].
Statistical analysis.
Statistics were performed using JMP version 9.0 (SAS, Cary, NC) and SPSS version 19 (IBM, Armonk, NY). Bivariate Pearson or Spearman correlations were conducted between clinical variables and mean SI; those significant at p ≤ 0.1 were considered for inclusion in multivariate (linear multiple regression) analyses after assessment for colinearity. Variables demonstrating colinearity were assessed for independent relationships with mean SI by way of partial correlations. Variables whose independent relationships with mean SI met significance at p ≤ 0.05 were included in multivariate analyses.
RESULTS
Cohort characteristics.
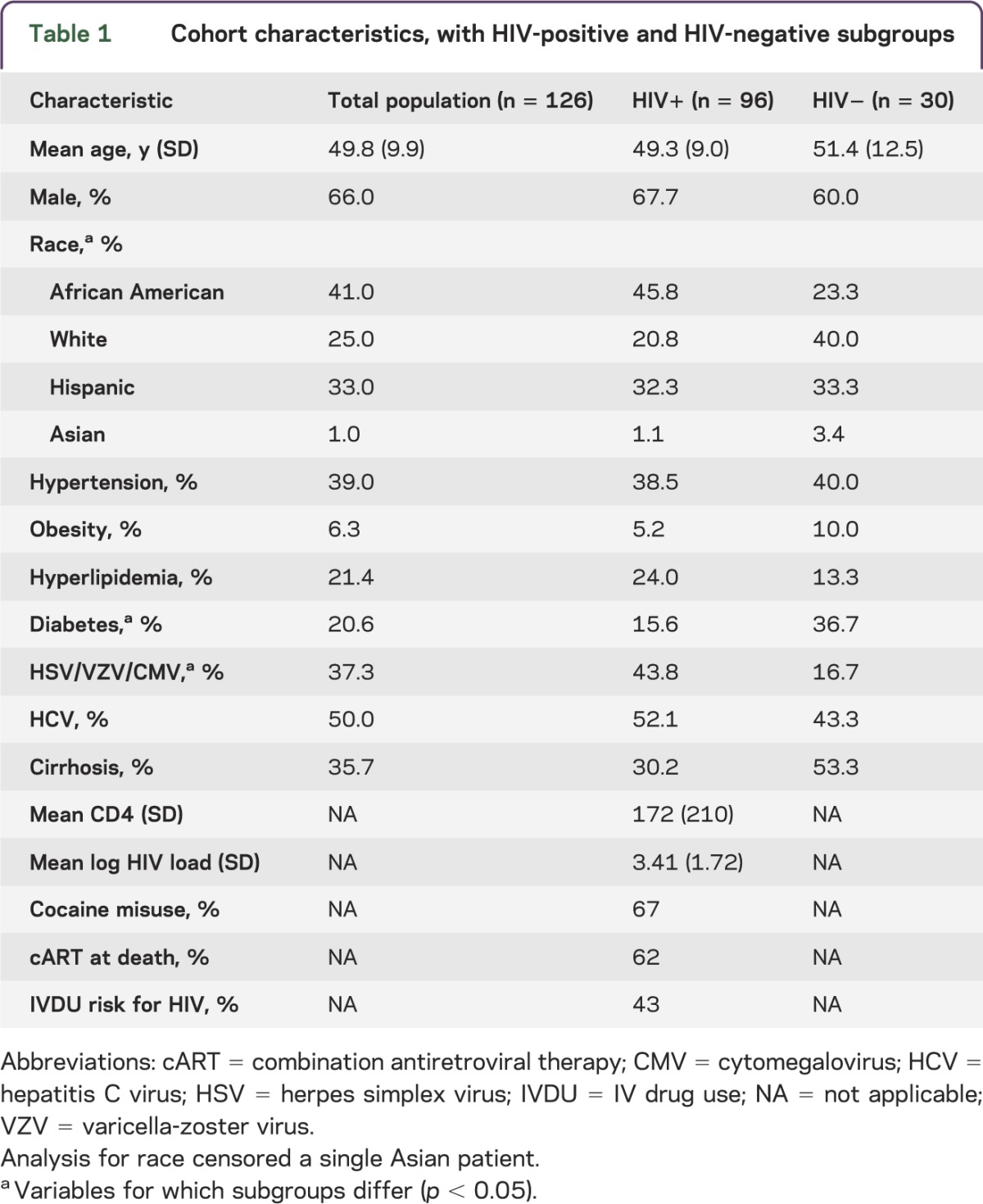
The overall population had a mean age of 50 years, was 66% male, and was 75% racial/ethnic minority, with significant representation of liver disease, hypertension, diabetes, hyperlipidemia, and herpes infections (table 1). HIV-positive and HIV-negative subgroups differed: the HIV-positive subgroup had more African Americans and individuals with herpes infections, whereas the HIV-negative subgroup had more diabetes. Both subgroups had high rates of cirrhosis and HCV. In this cART-era population, 98% of HIV-positive patients had antiretroviral (ARV) exposures, but only 62% were on therapy at death; reasons for this were multifactorial and included noncompliance as well as regimens being withheld in late stages of other medical disorders.
Table 1.
Cohort characteristics, with HIV-positive and HIV-negative subgroups
Mean sclerotic indices.
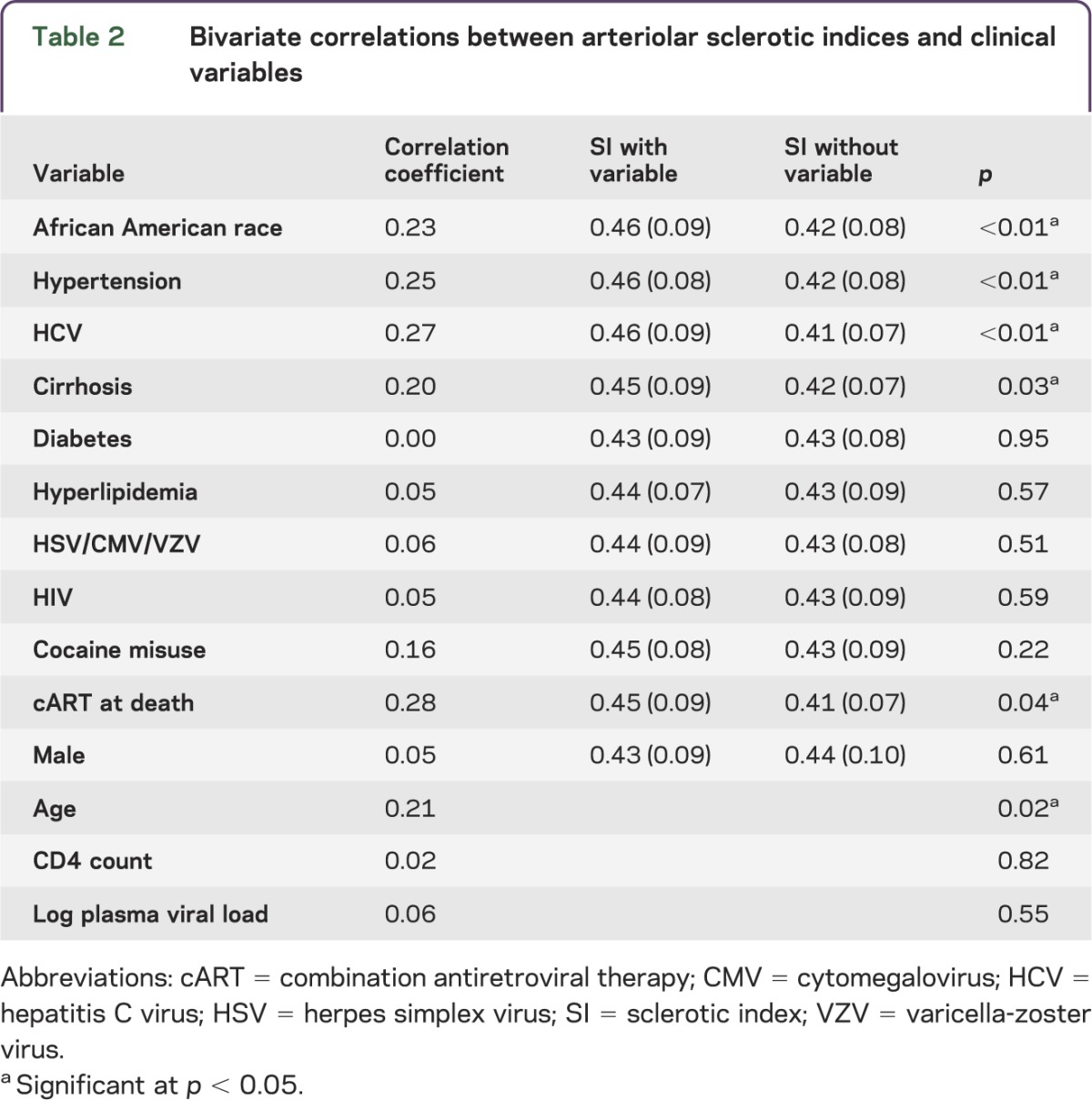
The mean SI for all categories assessed was >0.4, in the range of moderate SVD (table 2).2 Bivariate correlations were run between mean arteriolar SI and clinical/systemic autopsy variables. Significant correlations with SI were seen for African American race, age, hypertension, HCV, cirrhosis, and cART at death for HIV-positive patients. In the overall population, HIV status did not correlate with SI, nor did diabetes, hyperlipidemia, gender, or herpes infections. Within the HIV population, cocaine misuse, CD4 count, and plasma HIV load did not correlate with SI.
Table 2.
Bivariate correlations between arteriolar sclerotic indices and clinical variables
To address colinearity of significant factors, partial correlations were performed. Eighty-two percent of patients with cirrhosis had HCV; when HCV status was held constant, the cirrhosis effect on mean SI became nonsignificant (r = 0.08, p = 0.38). However, when cirrhosis was controlled, the HCV effect remained significant (r = 0.19, p = 0.04). Only HCV was entered into multivariate regression. Fifty-three percent of those with hypertension were African American; when hypertension was controlled, significant independent effects of African American race remained (r = 0.20, p = 0.03), and when African American race was controlled, significant effects of hypertension remained (r = 0.21, p = 0.02). Both factors were entered into multivariate regressions. Mean age of patients with hypertension (54.8 ± 1.3 years) was significantly higher than those who were normotensive (46.6 ± 1.0 years, p < 0.01); when age was held constant, effects of hypertension on SI remained (r = 0.18, p = 0.05), but when controlling for hypertension, age became nonsignificant (r = 0.13, p = 0.15). Only hypertension was entered into multivariate regression.
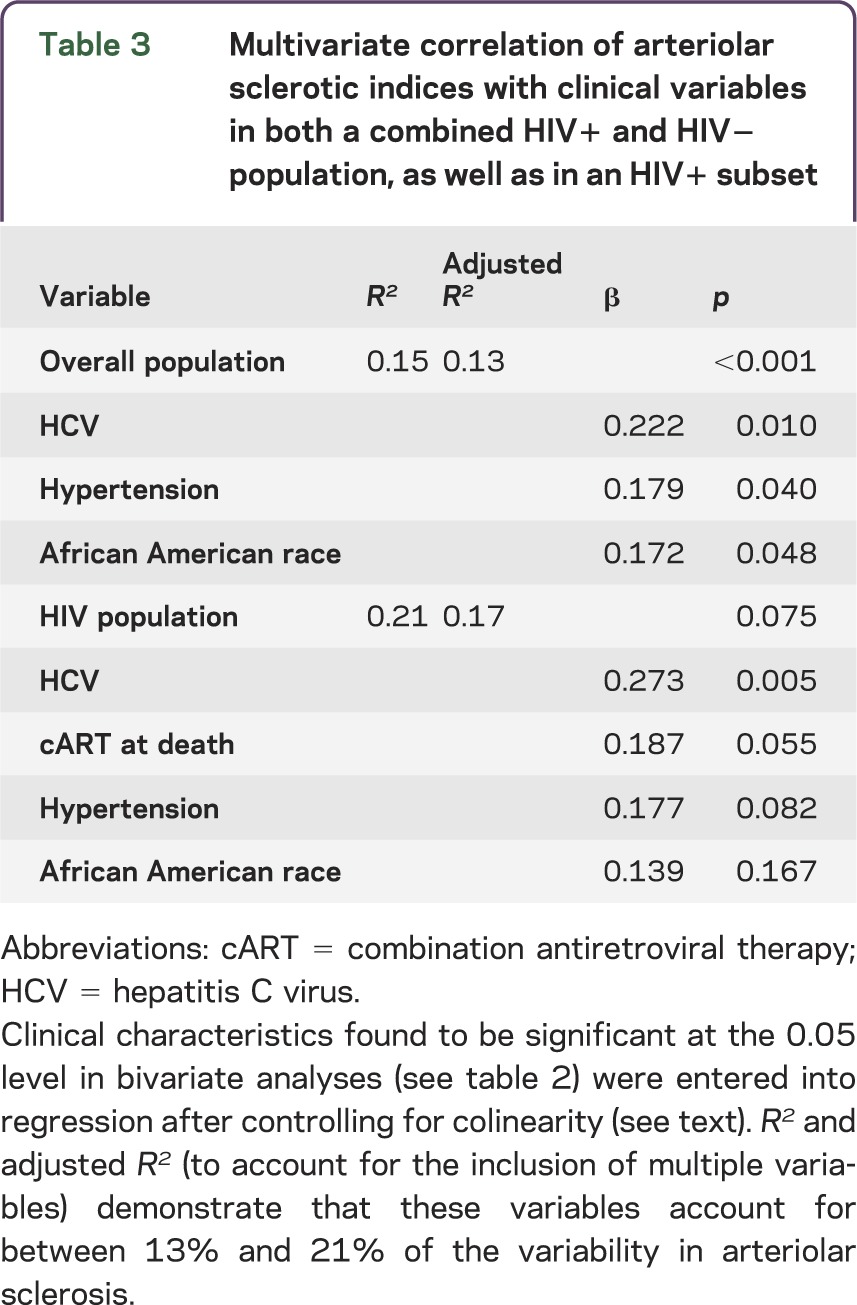
For the overall population, HCV, African American race, and hypertension accounted for 15% of the variance in SI in multiple regression (table 3). Each factor was independently associated with SI, and HCV had the largest effect. For the HIV-positive group, the inclusion of terminal cART in the model increased r2 to 0.21, with only HCV, hypertension, and cART remaining significant or at trend level; HCV again had the largest effect.
Table 3.
Multivariate correlation of arteriolar sclerotic indices with clinical variables in both a combined HIV+ and HIV− population, as well as in an HIV+ subset
Finally, by reviewing histories and on-study cART regimens, we estimated lifetime “ARV-month” exposures for 79 of the 96 HIV+ individuals. “ARV-month” exposures were calculated by multiplying the number of months a patient was on each ARV, summed over the lifetime. For this subsample, those on terminal cART had significantly greater “ARV-month” exposures than those not on terminal cART (on terminal cART: 204 ± 18 exposure months; off ARV at death: 98 ± 24 exposure months, p < 0.01), as well as a longer mean duration of HIV infection (on terminal cART 13.8 ± 0.7 years; off cART 10.8 ± 0.9 years, p < 0.01) and higher CD4 cell counts (on terminal cART: 212 ± 30 cells/mm3; off cART: 99 ± 38 cells/mm3, p = 0.02). Within the group on terminal cART, individuals on protease inhibitor (PI)–based regimens (n = 22) had higher SI than individuals on non-nucleoside reverse transcriptase inhibitor (NNRTI)–based regimens (n = 15) (SI 0.482 ± 0.016 for PI-based regimens; 0.425 ± 0.019 for NNRTI-based regimens, p = 0.03). Four individuals died on nucleoside reverse transcriptase inhibitors (NRTI) only, and 8 had other combinations that included other classes of agents.
DISCUSSION
Cerebral SVD is common in aging and has traditional risk associations with hypertension and diabetes, components of the metabolic syndrome now encountered with greater frequency in HIV populations.2,13 Concomitant with increased prevalence of metabolic abnormalities in HIV, increasing incidence of stroke and cerebral ischemia has been documented.14–16 While large artery atherosclerosis and cardiac embolism account for 30% of HIV-associated cerebrovascular events, small vessel occlusion is causative in approximately 20%.17 Authors have commented on the diversity of mechanisms underlying stroke in HIV, the prevalence of cryptogenic events, and the possibility that unique HIV-associated factors are important.11,14,15,17,18 Even with stable cART, arterial inflammation is increased in HIV, and a unique HIV-associated vasculopathy has been described.11,19 Alternatively, metabolic risk factors have been linked to cryptogenic stroke and HIV-associated cognitive dysfunction.5–7,20 Given the possibility of traditional and nontraditional risks for cerebrovascular events and the potential involvement of varying blood vessel calibers, direct neuropathologic study of the cerebral circulation in the context of HIV is necessary to better understand processes related to stroke and dementia.
To date, HIV-associated cerebral SVD has been indirectly measured through funduscopic analysis of retinal arterioles and MRI of “ischemic” white matter changes.8,9 While valid techniques, both rely on assumptions that are not rigorously tested: that changes in retinal arterioles will reflect those in brain and that white matter lesions on neuroimaging can be ascribed to SVD and not other HIV-associated pathologies. Perhaps as a consequence of the indirect methodologies, these studies have been somewhat contradictory regarding cerebrovascular risks. Assessment of cerebral small vessels poses challenges not encountered in the study of large- and medium-caliber arteries, which are amenable to neuroimaging and for which neuropathologic investigations have been published.11,18 Thus, human brain collections present a unique opportunity to assess risk factors for cerebral SVD with concrete visualization of endpoints, albeit with the biases that typically accompany autopsy populations. Individuals succumbing to medical disorders are likely to be enriched for a variety of vascular risk factors that may or may not be representative of individuals with less dire medical conditions.
The characteristics of our population demonstrate this potential selection bias: there were high rates of liver disease in both our HIV+ and HIV− decedents. While a bias, this allowed us to investigate hepatic risks for SVD, which has not been previously undertaken. There is a growing, controversial literature on whether hepatic disorders are protective or permissive for vascular sclerosis, predicated on the liver's central role in metabolism, the relationship between fatty liver disease and metabolic syndrome, and inflammatory perturbations of viral hepatitis.21 HCV is of particular interest due to its prevalence, infecting 3% of the worldwide population. In a Taiwanese study, chronic HCV was an independent risk factor for cerebrovascular death.22 Chronic HCV has been linked to carotid artery atherosclerosis and intima-media thickness in patients with HIV,23,24 and positive-strand HCV RNA and replication intermediates were detected in carotid artery plaques.25 Both HCV and HCV/HIV coinfection were linked to increased cardiovascular risk and incident coronary artery disease.26,27 HCV was associated with elevations in plasma cell adhesion markers, potential indicators of endothelial dysfunction and precursors to atherosclerosis.28 However, it is important to note that while evidence of HCV-conferred atherogenic risk mounts, some have described the opposite.29,30 Thus, a definitive role for HCV in large vessel atherosclerosis remains unclear.21 HCV is also implicated in inflammatory SVD, as cryoglobulinemia and immune complex deposition may lead to leukocytoclastic vasculitis.31 CNS involvement is postulated to occur, but direct demonstration of vasculitic HCV brain pathology is not yet reported. Brain microvascular endothelial cell lines support HCV replication, and receptors for HCV cell entry are present in human brain blood vessels, but direct evidence of in vivo endothelial infection is lacking.32
In this study, we examined risk factors for cerebral SVD by direct examination of brain tissues. Our finding that hypertension was a risk is consistent with prior literature.2,4,12 Chronic hypertension causes arteriolar remodeling and changes cerebral blood flow autoregulation, with increased wall thickness and reductions in lumenal diameter,4 elevating risk for stroke and cognitive dysfunction. Our observation that hypertension accounts for age effects on SI suggests that blood pressure, and not aging per se, constitutes the primary vascular risk. Indeed, a prior autopsy study of putatively normotensive patients with cerebral SVD documented evidence of hypertension in two-thirds of the population.2
In contrast, in our cohort, race made independent contributions to SVD even when the effects of hypertension were considered. Increased prevalence of hypertension in African Americans33 was therefore not a reason for their greater SI. Racial differences in retinal arteriolar caliber have been documented,34 and smaller lumens/thicker walls in renal arterioles have been demonstrated in African American children compared to white children.35 Thus, it is not surprising that African American race conveys risk for increased cerebral arteriolar wall thickness, although the basis of this disparity is unclear. We did not find an effect of diabetes, surprising in light of its documented role in microangiopathies.1,3 While diabetes is thought to be a risk for cerebral SVD, some have noted a paucity of direct evidence for this association.36 Diabetes has complex vascular impacts and is related to large vessel arteriosclerosis, where decreased compliance in and of itself can be associated with cerebral SVD.37 In one cohort, diabetes constituted a risk for large but not small brain blood vessel thickening, suggesting a dissociation of these pathologies.36 In our cohort, we postulate that diabetes is not severe enough to result in end-organ microangiopathy; in our MHBB autopsy study of renal disease, diabetic arteriolar lesions were present in only 3% of kidneys examined.e3 We also failed to find an effect of cocaine misuse; however, we did not ascertain the severity (quantity and duration) of the cocaine disorders and thus cannot comment on this lack of association.
The strongest independent risk for cerebral SVD in our cohort was HCV, in both overall and HIV-positive populations. This is intriguing in light of increasing evidence for HCV-related vascular disorders and a recent publication documenting greater white matter abnormalities in HIV/HCV coinfected vs HIV monoinfected individuals.38 While it is possible that HCV directly affects cerebral small blood vessels,32 it is also possible that HCV-related large vessel arteriosclerosis23–25 results in arteriolar damage through reduced large vessel compliance and transmission of high pulse pressures during systole.37 It is possible that HCV-induced metabolic disorders predispose to arteriolar damage via dysregulated lipids and glucose and endothelial toxicity.23 It is possible that HCV-related systemic immunologic activation is vasculopathic, as HCV viremia results in elevated inflammatory burden.39 It is possible that HCV incites local inflammation contributing to vasculopathy, as compartmentalized HCV brain replication is documented.40 All these hypotheses merit examination in future studies.
Finally, of the HIV parameters, only cART at death conveyed independent risk for SVD, not CD4 cell count prior to death or plasma HIV load. As only 79 of our 96 HIV+ subjects had a lifetime ARV exposure history available at the time of autopsy, terminal cART was chosen as a variable for analysis because it could be accurately and reliably ascertained in the entire population. The significance of this observation is unclear. There is evidence that antiviral medications have direct endothelial toxicities, and drugs in the NRTI class used as common regimen backbones have been implicated in a variety of mitochondrial and oxidative stress endothelial pathways.41 Thus, it might be that the presence of a “vascular toxic” regimen at the time of death would result in enhanced vascular pathology; it is unclear whether this effect would be reversible upon therapy cessation. Other elements of contemporary cART regimens may also convey enhanced risk; there is a variable literature suggesting that PIs are of particular concern in the generation of vascular pathologies.41 Of interest, in our sample, individuals dying on PI-based regimens had significantly greater SI than those on NNRTI-based regimens. On the other hand, terminal cART may not have a direct effect on cerebral vasculature and may be a surrogate for some other confounding characteristic. One possibility is that the greater lifetime ARV exposures of those on terminal cART, in concert with the longer duration of their HIV disease, may have resulted in greater duration of proinflammatory and metabolic perturbations not captured in the syndromic diagnoses used for analyses. This hypothesis warrants further examination in prospective study.
Supplementary Material
GLOSSARY
- ARV
antiretroviral
- cART
combination antiretroviral therapy
- H&E
hematoxylin and eosin
- HCV
hepatitis C virus
- LSOCA
Longitudinal Study of the Ocular Complications of AIDS
- MHBB
Manhattan HIV Brain Bank
- NNRTI
non-nucleoside reverse-transcriptase inhibitor
- PI
protease inhibitor
- SI
sclerotic index
- SVD
small vessel disease
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Dr. Susan Morgello participated in study concept and design, analysis and interpretation, manuscript writing and revision, and study supervision. Jacinta Murray participated in study concept and design, acquisition of data, analysis and interpretation, and revision of the manuscript. Sarah Van Der Elst participated in acquisition of data, analysis and interpretation, and revision of the manuscript. Dr. Desiree Byrd participated in study concept and design, analysis and interpretation, and revision of the manuscript.
STUDY FUNDING
This study was supported by U24MH100931 from the NIH (to S.M.).
DISCLOSURE
S. Morgello is on the advisory board for Temple University Comprehensive NeuroAids Center, on the editorial board for Journal of Neurovirology, and receives research support from the NIH. J. Murray and S. Van Der Elst report no disclosures. D. Byrd receives research support from the NIH. Go to Neurology.org/nn for full disclosures.
REFERENCES
- 1.Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry 2007;78:702–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lammie GA, Brannan F, Slattery J, Warlow C. Non-hypertensive cerebral small-vessel disease: an autopsy study. Stroke 1997;28:2222–2229 [DOI] [PubMed] [Google Scholar]
- 3.Mayhan WG. Cerebral circulation during diabetes mellitus. Pharmacol Ther 1993;57:377–391 [DOI] [PubMed] [Google Scholar]
- 4.Nag S. Immunohistochemical localization of extracellular matrix proteins in cerebral vessels in chronic hypertension. J Neuropathol Exp Neurol 1996;55:381–388 [DOI] [PubMed] [Google Scholar]
- 5.Foley J, Ettenhofer M, Wright MJ, et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol 2010;24:265–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamoto BK, Valcour VG, Kallianpur K, et al. Impact of cerebrovascular disease on cognitive function in HIV-infected patients. J Acquir Immune Defic Syndr 2011;57:e66–e68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012;78:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: the Hawaii aging with HIV cohort study. Cerebrovasc Dis 2007;24:236–241 [DOI] [PubMed] [Google Scholar]
- 9.Gangaputra S, Kalyani PS, Fawzi AA, et al. Retinal vessel caliber among people with acquired immunodeficiency syndrome: relationships with disease-associated factors and mortality. Am J Ophthalmol 2012;153:434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyani PS, Fawzi AA, Gangaputra S, et al. Retinal vessel caliber among people with acquired immunodeficiency syndrome: relationships with visual function. Am J Opthalmol 2012;153:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez J, Glenn M, Isaacson RS, Marr AD, Mash D, Petito C. Thinning of the arterial media layer as a possible preclinical stage in HIV vasculopathy: a pilot study. Stroke 2012;43:1156–1158 [DOI] [PubMed] [Google Scholar]
- 12.Whitman GT, DiPatre PL, Lopez IA, et al. Neuropathology in older people with disequilibrium of unknown cause. Neurology 1999;53:375–382 [DOI] [PubMed] [Google Scholar]
- 13.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005;352:48–62 [DOI] [PubMed] [Google Scholar]
- 14.Evers S, Nabavi D, Rahmann A, Heese C, Reichelt D, Husstedt IW. Ischaemic cerebrovascular events in HIV infection: a cohort study. Cerebrovasc Dis 2003;15:199–205 [DOI] [PubMed] [Google Scholar]
- 15.Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 2007;78:1320–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology 2011;76:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology 2007;68:1257–1261 [DOI] [PubMed] [Google Scholar]
- 18.Nagel MA, Mahalingam R, Cohrs RJ, Gilden D. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets 2010;10:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012;308:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ances BM, Bhatt A, Vaida F, et al. Role of metabolic syndrome components in human immunodeficiency virus-associated stroke. J Neurovirol 2009;15:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purnak T, Efe C, Beyazit Y, et al. Recent insights into the relationship between inflammatory liver diseases and atherosclerosis. J Investig Med 2011;59:904–911 [DOI] [PubMed] [Google Scholar]
- 22.Lee MH, Yang HI, Wang CH, et al. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke 2010;41:2894–2900 [DOI] [PubMed] [Google Scholar]
- 23.Adinolfi LE, Restivo L, Zampino R, et al. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis 2012;221:496–502 [DOI] [PubMed] [Google Scholar]
- 24.Sosner P, Wangermez M, Chagneau-Derrode C, leMoal G, Silvain C. Atherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infection. Atherosclerosis 2012;222:274–277 [DOI] [PubMed] [Google Scholar]
- 25.Boddi M, Abbate R, Chellini B, et al. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol 2010;47:72–75 [DOI] [PubMed] [Google Scholar]
- 26.Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging 2012;32:421–430 [DOI] [PubMed] [Google Scholar]
- 27.Kakinami L, Block RC, Adams RJ, Cohn SE, Maliakkal B, Fisher SG. Risk of cardiovascular disease in HIV, hepatitis C, or HIV/hepatitis C patients compared to the general population. Int J Clin Pract 2013;67:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masia M, Padilla S, Robledano C, Ramos JM, Gutierrez F. Evaluation of endothelial function and subclinical atherosclerosis in association with hepatitis C virus in HIV-infected patients: a cross-sectional study. BMC Infect Dis 2011;11:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arcari CM, Nelson KE, Netski DM, Nieto FJ, Gaydos CA. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis 2006;43:e53–e56 [DOI] [PubMed] [Google Scholar]
- 30.Bilora F, Campagnolo E, Rinaldi RRossato A, Arzenton M, Petrobelli F. Carotid and femoral atherosclerosis in chronic hepatitis C: a 5-year follow-up. Angiology 2008;59:717–720 [DOI] [PubMed] [Google Scholar]
- 31.Ko HM, Hernandez-Prera JC, Zhu H, et al. Morphologic features of extrahepatic manifestations of hepatitis C virus infection. Clin Dev Immunol 2012;2012:740138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects endothelial cells of the blood-brain barrier. Gastroenterology 2012;142:634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu W, Pratt JH. A consideration of genetic mechanisms behind the development of hypertension in blacks. Curr Hypertens Rep 2013;15:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TT, Wang JJ, Sharrett AR, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the multi-ethnic study of atherosclerosis (MESA). Diabetes Care 2008;31:544–549 [DOI] [PubMed] [Google Scholar]
- 35.Rostand SG, Cross SK, Kirk KA, Lee JY, Kuhlmann A, Amann K. Racial differences in renal arteriolar structure in children with minimal change nephropathy. Kidney Int 2005;68:1154–1160 [DOI] [PubMed] [Google Scholar]
- 36.Kim BJ, Lee SH, Kang BS, Yoon BW, Roh JK. Diabetes increases large artery diseases, but not small artery diseases in the brain. J Neurol 2008;255:1176–1181 [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Choi JH, Moon JS, Kim HJ, Cha JK. Association between the severity of cerebral small vessel disease, pulsatility of cerebral arteries, and brachial ankle pulse wave velocity in patients with lacunar infarction. Eur Neurol 2010;64:247–252 [DOI] [PubMed] [Google Scholar]
- 38.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol 2011;17:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armah KA, Quinn EK, Cheng DM, et al. Human immunodeficiency virus, hepatitis C, and inflammatory biomarkers in individuals with alcohol problems: a cross-sectional study. BMC Infect Dis 2013;13:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishman SL, Murray J, Eng FJ, Walewski JL, Morgello S, Branch AD. Molecular and bioinformatics evidence of hepatitis C virus evolution in brain. J Infect Dis 2008;197:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kline ER, Sutliff RL. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med 2008;56:752–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


