Abstract
Background:
Nowadays, owing to medicinal plants as a candidate to obtain promising new medicinal agents, there is a renewed interest in the use of these natural sources for drug development.
Objective:
In the present study, we aimed to assess the anticholinesterase, antioxidant, and neuropotective effects of Tripleurospermum disciforme and Dracocephalum multicaule extracts.
Materials and Methods:
Methanolic extract of the plants was prepared by maceration method. Anticholinesterase effect of different concentrations of the plants was studied by colorimetric method and antioxidant activity was evaluated using diphenypicrylhydrazil (DPPH) assay. Protective effect of the extracts against amyloid β (Aβ)-induced toxicity in PC12 cells was determined by MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method.
Results:
Both T. disciforme and D. multicaule extracts could inhibit acetylcholinesterase (AChE) in a dose-dependent manner. The highest inhibition occurred at 5 μg/ml (71.18 ± 4.9 and 79.06 ± 3.1% inhibition respectively by T. disciforme and D. multicaule) in comparison to tacrine (86.37 ± 3.24%). The greatest DPPH inhibition of T. disciforme and D. multicaule was shown at 800 μg/ml (89.04 ± 3.9 and 78.5 ± 3.7%, respectively). None of tested extracts induced protection against βA toxicity in PC12 cell.
Conclusion:
Although the results indicated anticholinesterase and antioxidant of the T. disciforme and D. multicaule, further specific studies and scientific validity are needed.
Keywords: Anticholinesterase, antioxidant, Dracocephalum multicaule, Tripleurospermum disciforme
INTRODUCTION
Alzheimer's disease (AD) is the most common form of dementia in elders worldwide, which can affect memory and the other cognitive functions.[1] The loss of cholinergic synapses has been a consistent finding in AD, so increasing the brain's level of acetylcholine (ACh) through inhibition of acetylcholinesterase (AChE) has been one of the primary strategies for management of AD.[2] In addition, the role of oxidative stress has been proven in the pathogenesis of AD.[3,4] Neuronal systems appear to be especially sensitive to oxidation. Free radicals formation leads to inflammatory reactions which causes AD development. One of the other pathological features identified in AD is the presence of neurofibrillary tangles, amyloid plaques, and inflammations. Accumulation of amyloid β (Aβ) peptide acts as an inhibitor of certain enzyme functions. The presence of this peptide is the hallmark of the AD pathology.[5,6,7] There are obstacles in successful treatment of AD such as lack of full effectiveness of the current drugs in treatment of all aspects of AD, high costs, and adverse effects. In earlier studies, we have reported antioxidant and anticholinesterase effect of some medicinal plants.[8,9,10] There has been growing interest on traditional herbal medicines. Presently, in continuing to focus on the future-promising herbs against AD, anticholinesterase, antioxidant, and protective effect of Tripleurospermum disciforme and Dracocephalum multicaule against toxicity of βA peptide have been studied. These two plants have been used in folk medicine for memory enhancing.[11] T. disciforme (C.A. Mey) Schultz Bip. known as “Babooneh dashti” belongs to Asteraceae family and has similar uses to Matricaria chamomilla. This plant has been used as a popular treatment for sleep disorders, inflammations, carminative, and as a hair color.[12] Anti-ulcer effect of this plant has been reported in mice.[13] D. multicaule Montbr. and Auch. known in Persian as “Palang moshk” has been widely distributed in northwestern of Iran and is from Lamiaceae family.[14] Antioxidant effect of D. moldavica has been reported previously.[15,16] Up to now, it is for the first time that the extracts of T. disciforme and D. multicaule have been studied for anticholinesterase, antioxidant, and protective effect against Aβ-induced toxicity. Primary phytochemical studies were performed for detection of classes of active constituents in tested plants which might be responsible for biological activities of the plants.
MATERIALS AND METHODS
Plant materials
Flowering tops of T. disciforme and D. multicaule were collected from Bidkhoon, Kerman province and Rasht in Gilan province, respectively in June 2011. The plants were authenticated by a botanist and a voucher specimen was deposited in Herbarium Center, Faculty of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran (KF1225, KF1238).
Chemicals
Acetylthiocholine iodide (ATCI), AChE (EC 3.1.1.7, type VI-S from Electric Eel), and 5,5’- dithio-bis (2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich, Switzerland. Tacrine was purchased from Fluka Chemie (Buchs, Switzerland). Other chemicals were from analytical grade.
Preparation of plant extract and phytochemical screening
About 200 g of each plant was extracted by maceration method with methanol 80% for 72 h. The extracts were concentrated under vacuum. Dried extracts were stored at −20°C until test. Phytochemical screening of the plants was performed to screen the presence of alkaloids, terpenoids, steroids, saponins, and flavonoids.[17]
Anticholinesterase tests
Bioautographic method for anticholinesterase activity
Plant extracts were applied on the thin layer chromatography (TLC) plate at concentration of 100 μg/ml and sprayed with 5 mM ATCI and 5 mM DTNB in 50 mM Tris-HCl (pH 8) until saturation of the plate. After 2 min, a solution of 3 U/ml AChE dissolved in 50 mM Tris-HCl, pH 8 was sprayed at 37°C. Appearance of white spots in yellow background indicated the presence of active compounds.[18]
Ellman based colorimetric study of anticholinesterase activity
AChE inhibitory effect of plant extracts was evaluated using Ellman method with some modifications.[19] Tacrine was used as positive control. The percentage of inhibition was calculated as following: %I = Acon − Asam/Acon × 100, where Acon is the absorbance of the control and Asam is the absorbance of the tested sample. The IC50 was calculated by log-probit analysis.
Diphenypicrylhydrazil assay
Assessment of DPPH inhibitory effect of plant extracts was performed using DPPH assay.[20] Butylated hydroxytoluene (BHT) and solvent were used as positive and negative controls, respectively.
Toxicity on PC12 cells
PC12 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS), penicillin 100 unit, and streptomycin 100 mg and maintained at 37°C in incubator 5% CO2. The cells were cultured at a concentration of 104 cells/well with poly-D-lysine (PDL). After 24 h, medium was replaced by plant extract (0.1-200 μg/ml in phosphate buffered saline (PBS)) and incubated for 24 h. Cell survival was evaluated by MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay.
Neuroprotective effect of plant extracts against Aβ peptide toxicity
PC12 cells cultured in 96 microwells coated by PDL and treated with different plant extracts at nontoxic concentrations (0.1, 1, 10, 100, and 200 μg/ml). Aβ peptide (2 μM in distilled water) was added to wells and incubated for 24 h. A stock solution of Aβ peptide (1 mmol Aβ peptide/1 ml distilled water) incubated at 37°C for 3 days to make aggregated form. The viability of cells was checked using MTT assay.[21]
Thioflavine T fluorescence assay
Fluorometric method was used for determination of Aβ peptide aggregation using ThT. Aggregated Aβ peptide forms a complex with ThT which has fluorescence effect at λ exitation of 450 nm and λ extinction of 482 nm. Inhibition of Aβ peptide aggregation causes a decrease in fluorescence intensity.[22] Briefly a stock solution of lyophilized Aβ (5 mg/mL) was sonicated for 15 min. An equal volume of each extract (200 μg/ml PBS) was added to a 50 μM Aβ in PBS (pH 7.4) and incubated at 37°C for 5 days. A control sample containing Aβ with PBS was carried out in parallel. After incubation at 37°C, 50 μL of Aβ solution was added to 3 mL of ThT solution (50 μM). After 30 min, the fluorescence intensity was measured at an excitation wavelength of 450 nm and an emission wavelength of 482 nm with fluorometer. The fluorescence intensity was measured as the average of at least four samples.
Statistical analysis
Each experiment was repeated in triplicate and the results were reported as mean ± standard error of the mean (SEM).
RESULTS
Extraction yield and phytochemical screening
The yield of extraction of T. disciforme and D. multicaule was about 33.2 and 24.7% (g/g), respectively. Results of phytochemical screening indicated the presence of flavonoids, terpenoids, and tannins in both plants.
Bioautographic and colorimetric study of anticholinesterase
Bioautographic study of plant extracts indicated the AChE inhibitory effect of both T. disciforme and D. multicaule extracts in comparison to tacrine. These extracts caused discoloration of the yellow background of the plate as quickly as tacrine.
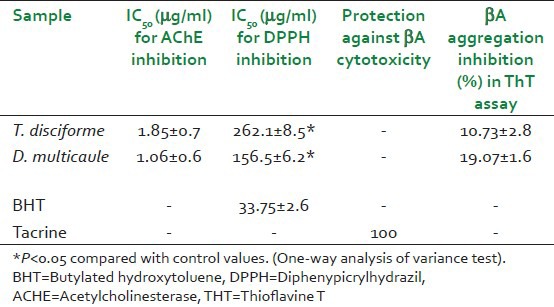
As shown in Figure 1, the results of colorimetric assay show that T. disciforme at concentrations of 1.25, 2.5, and 5 μg/ml and D. multicaule at concentrations of 2.5 and 5 μg/ml significantly in a concentration-dependent route inhibited AChE (P < 0.005). The highest inhibition was shown at 5 μg/ml (71.18 ± 4.9 and 79.06 ± 3.1% by T. disciforme and D. multicaule, respectively) in comparison to tacrine (86.37 ± 3.2% inhibition at concentration of 2 μg/ml). IC50 value of T. disciforme and D. multicaule extracts was calculated from their regression equation and determined as 1.85 ± 0.7 and 1.06 ± 0.6 μg/ml, respectively [Table 1].
Figure 1.
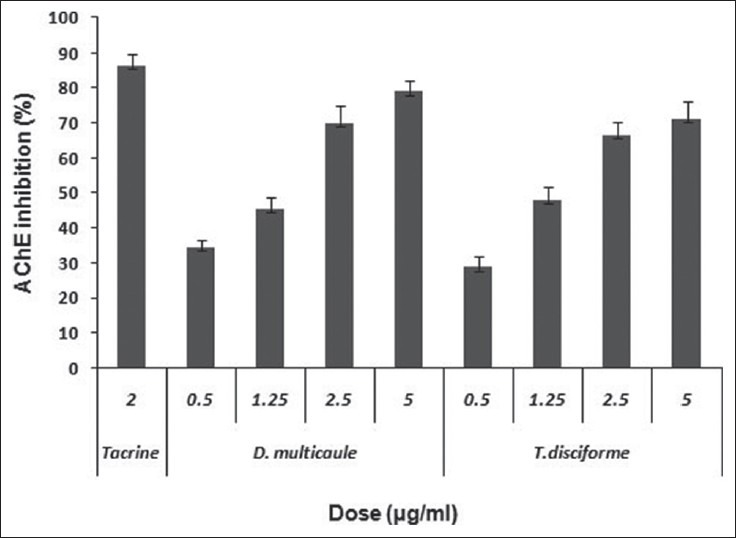
Anticholinesterase effect of different concentrations of Tripleurospermum disciforme and Dracocephalum disciforme extracts in comparison to tacrine (2 μg/ml)
Table 1.
IC50 values of extracts in acetylcholinesterase and diphenypicrylhydrazil inhibition and cytotoxicity and Thioflavine T assay
Antioxidant assay
The results of DPPH inhibition assay show that T. disciforme and D. multicaule extracts inhibited DPPH radical in concentration-dependent manner [Figure 2]. The greatest inhibition occurred at 800 μg/ml (89.04 ± 3.9 and 78.5 ± 3.7%, respectively by T. disciforme and D. multicaule) in comparison to BHT (78.9 ± 4.8% inhibition). IC50 value of T. disciforme and D. multicaule was equal to 262.1 ± 8.5 and 156.5 ± 6.2 μg/ml, respectively [Table 1].
Figure 2.
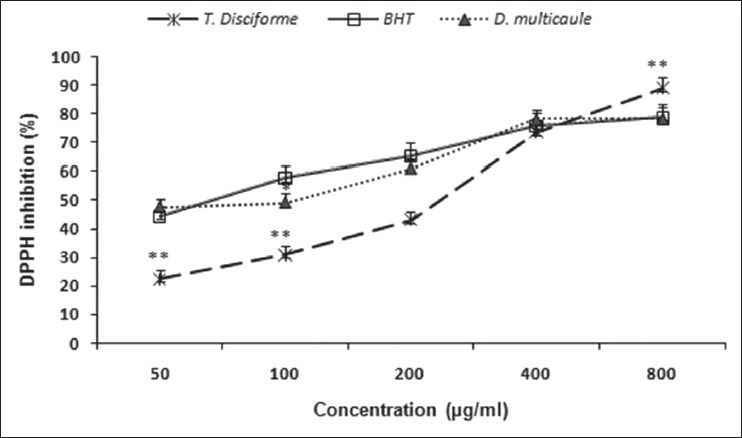
Diphenypicrylhydrazil radical inhibition of different concentrations of Tripleurospermum disciforme and Dracocephalum multicaule extract in comparison to butylated hydroxytoluene
Effect of T. disciforme and D. multicaule on cell viability of PC12 cells
The results of toxicity against PC12 cells shows that T. disciforme and D. multicaule exhibited no cytotoxicity under normal condition after 24 h up to 100 μg/ml [Figure 3].
Figure 3.
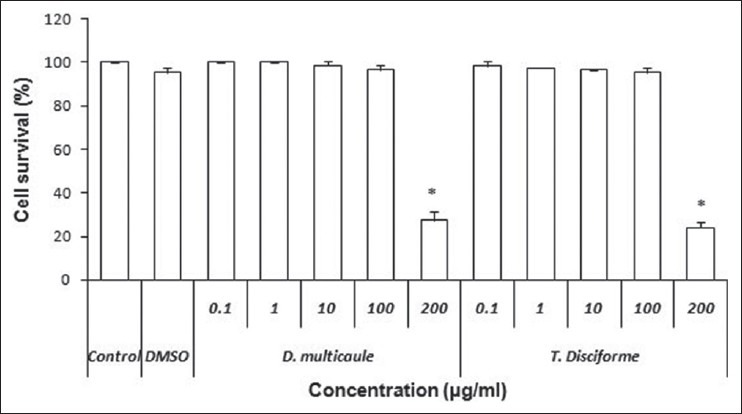
Cytotoxicity effect of different concentrations of Tripleurospermum disciforme and Dracocephalum multicaule extract on PC12 cells using MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. *P < 0.05 compared with control values. (One-way analysis of variance test)
Neuroprotective effect of T. disciforme and D. multicaule on PC12 cells
The incubation of PC12 cells with different concentrations of T. disciforme and D. multicaule (0-100 μg/ml) indicated no protection against Aβ peptide toxicity by these plant extracts. Tacrine at concentration of 1 μM exhibited 100% protection [Table 1].
Fluorometric evaluation of inhibitory effect on Ab peptide aggregation
The results of Tht fluorescence assay of T. disciforme and D. multicaule extracts indicated that these plant extracts could not inhibit aggregation of Aβ peptide at used concentrations. The greatest of inhibition was shown at concentration of 200 μg/ml (10.73 ± 2.8 and 19.07 ± 1.6% by T. disciforme and D. multicaule, respectively) [Table 1].
DISCUSSION
TLC bioautographic study indicated the presence of cholinesterase inhibitory activity of T. disciforme and D. multicaule by formation of well-defined white spots made visible by spraying with DTNB, which gave a yellow background. Even though the TLC assay is a qualitative method, the extracts exhibited white spots at different Rf values which indicated the presence of different compounds with anticholinestease effect in these plants. In colorimetric method, under our study, the highest activity was appeared to be present at 5 μg/ml (71.18 ± 4.9 and 79.06 ± 3.1% AChE inhibition by T. disciforme and D. multicaule, respectively). Phytochemical screening showed the positive results for terpenoids, flavonoids, and tannins in both tested plants, so each or a combination of these metabolites might be responsible for anticholinesterase effect of these plants. Anticholinesterase effect of terpenoids has been reported previously. Terpenoids in tea tree oil were found to possess AChE inhibitory effect individually as well as in the mixed form.[23] The promising anticholinesterase effects have also been reported for some bicyclic monoterpenoides such as α-pinene, and 3-sabinene.[24] Moreover in a study, the presence of hydroxy flavones and methoxy flavonoids has been reported in D. multicaule.[25] A number of flavonoids such as quercetin and macluraxanthone possess a mild inhibitory activity against AChE.[26,27]
In DPPH scavenging assay, T. disciforme as well as D. multicaule exhibited a concentration-dependent DPPH inhibition. The greatest inhibition was 89.04 ± 3.9 and 78.5 ± 3.7% at concentration of 800 μg/ml by T. disciforme and D. multicaule, respectively. Recently antioxidant effect of D. moldavica, has been reported. This plant demonstrated the ability to reduce iron (III) to iron (II) ions. D. moldavica also could scavenge 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonate) diammonium cation free radical (ABTS+) too. This plant potentially inhibited the bleaching of β-carotene by scavenging of ROO radical. These reports show that D. moldavica protected 2-deoxy-D- ribose from oxidative degradation by scavenging OH radical. This plant extract exhibited the ability to scavenge DPPH radical at 1 mg/mL, while the extract of D. multicaule exhibited 50% inhibition of DPPH at 156.5 μg/ml[15,16]
The results of neuroprotective effect of T. disciforme and D. multicaule extracts showed that none of the plant concentrations was active against Aβ toxicity. These extracts also were inactive in fluorometric evaluation of inhibition of Aβ peptide aggregation. With increasing concentrations of 1~100 μg/ml, the protective role of the plants exhibited no protection enhancement. The inactivity of the extracts to inhibit Aβ aggregation and to protect the PC12 cell lines from Aβ does not rule out them as candidates for further studies as anti-AD. Because PC12 is an immortalized cell line and its responses to the therapeutic agents might be different from primary cultures. Furthermore, there is some evidence indicating that therapeutic effect of AChEIs in AD is not direct inhibition of Aβ aggregation. According to the “Cholinergic hypothesis of AD”,[2] it is believed that AChE inhibitors reduce the breakdown of endogenously released ACh, leads to greater activation of postsynaptic ACh receptors that would result to reduction of tau phosphorylation; returning towards normal the secretion of secreted amyloid precursor protein sAPP; reduction of β-amyloid production and returning towards normal glutamatergic neurotransmission. This shows that secretion of Aβ in reduced by AChE inhibitors (AChEIs). There is a hypothesis about the activation of an ‘anti-inflammatory cholinergic pathway’ in response to Aβ.[28] From this one may concluded that AChEIs would attenuate the inflammatory response evoked by Aβ.
CONCLUSION
Although our in vitro experiments are preliminary, from the view of preventive medicine, the results demonstrated the importance of future studies of these plants. Obtained results indicated the antioxidant and anticholinesterase effect of T. disciforme and D. multicaule extracts. There is a need to do further scientific and specific studies and investigate the efficacy of the plants in different AD models. In addition, it is needed to do more studies to find the possibility of a correlation existing between antioxidant and AChE inhibitory activity.
ACKNOWLEDGEMENT
We would like to thank for financial support of Vice Chancellor for Research, Kerman University of Medical Sciences, Kerman, Iran. This article also has been derived from the thesis of Pharm. D student in Kerman University of Medical Sciences, Faculty of Pharmacy, Kerman, Iran.
Footnotes
Source of Support: Vice Chancellor of Research, Kerman University of Medical Sciences, Kerman, Iran,
Conflict of Interest: None declared.
REFERENCES
- 1.Drachman D, Leavitt J. Human memory and the choliner-gic system. A relationship to aging? Arch Neurol. 1974;30:113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 2.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resende R, Moreira PI, Proença T, Deshpande A, Busciglio J, Pereira C, et al. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–7. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Frank B, Gupta S. A review of antioxidants and Alzheimer' s disease. Ann Clin Psychiatry. 2005;17:269–86. doi: 10.1080/10401230500296428. [DOI] [PubMed] [Google Scholar]
- 5.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer's disease: Possible role of periodontal disease. Alzheimer's Dement. 2008;4:242–50. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Rawlins JN, et al. Systematic inflammation induces acute behavioural and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes A, Fernandes E, Lima JL, Mira L, Corvo ML. Molecular mechanism of anti-inflammatory activity mediated by flavonoids. Curr Med Chem. 2008;15:1586–605. doi: 10.2174/092986708784911579. [DOI] [PubMed] [Google Scholar]
- 8.Sharififar F, Mirtajadini M, Azampour MJ, Zamani E. Essential oil and methanolic extract of Zataria multiflora Boiss. With anticholinesterase effect. Pak J Biol Sci. 2012;15:49–53. doi: 10.3923/pjbs.2012.49.53. [DOI] [PubMed] [Google Scholar]
- 9.Sharififar F, Moshafi M, Shafazand E, Koohpayeh A. Acetyl cholinesterase inhibitory, antioxidant and cytotoxic activity of three dietary medicinal plants. Food Chem. 2011;130:20–3. [Google Scholar]
- 10.Gholamhosseinian A, Moradi MN, Sharifi-Far F. Screening the methanol extract of some Iranian plants for acetylcholinesterase inhibitory activity. Res Pharm Sci. 2009;4:105–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Sharififar F, Koohpayeh A, Motaghi MM, Amirkhosravi A, Nasab EP, Khodashenas M. Study the ethnobotany of medicinal plants in Sirjan, Kerman province, Iran. J Herb Drugs. 2010;1:19–28. [Google Scholar]
- 12.Ghassemi-Dehkordi N, Amin G, Rahiminejad R, Salehi M, Jafarpisheh A. Morphological and phytochemical study of Tripleurospermum disciforme (C.A. Mey) Schultz Bip. Pajouhesh Sazandegi. 2003;58:42–6. [Google Scholar]
- 13.Minaiyan M, Ghassemi-Dehkordi N, Mohammadzadeh B. Anti-ulcer effect of Tripleurospermum disciforme (C.A.Mey) Shultz Bip on pylorus ligated (Shay) rats. Res Pharm Sci. 2006;1:15–21. [Google Scholar]
- 14.Zargari A. Vol. 3. Tehran: Tehran University Press; 1990. Medicinal plants. [Google Scholar]
- 15.Dastmalchi K. Helsinki: Helsinki University; 2008. Dracocephalum moldavica L. and Melissa officinalis L.: Chemistry and bioactivities relevant in Alzheimer's disease therapy [dissertation] [Google Scholar]
- 16.Dastmalchi K, Dorman H, Laakso I, Hiltunen R. Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. Food Sci Technol. 2007;49:1655–63. [Google Scholar]
- 17.De S, Dey YN, Ghosh AK. Phytochemical investigation and chromatographic evaluation of the different extracts of tuber of Amorphaphallus paeoniifolius (Araceae) Int J Pharm Biomed Res. 2010;1:150–7. [Google Scholar]
- 18.Rhee IK, van de Meent M, Ingkaninan K, Verpoorte R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J Chromatogr A. 2001;915:217–23. doi: 10.1016/s0021-9673(01)00624-0. [DOI] [PubMed] [Google Scholar]
- 19.SatheeshKumara N, Mukherjee PK, Bhadraa S, Sahaa BP. Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum graecum L and its constituents. Phytomedicine. 2010;17:292–5. doi: 10.1016/j.phymed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Bhat K. DPPH antioxidant assay revisited. Food Chem. 2009;13:1202. [Google Scholar]
- 21.Muthaiyah B, Essa M, Chauhan V, Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res. 2011;36:2096–103. doi: 10.1007/s11064-011-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura Y, Yasuda K, Yamamoto K, Koike M, Nishida Y, Kobayashi K. Inhibition of alzheimer amyloid aggregation with sulfated glycopolymers. Biomacromolecules. 2007;8:2129–34. doi: 10.1021/bm0701402. [DOI] [PubMed] [Google Scholar]
- 23.Miyazawa M, Yamafuji C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. JAgric Food Chem. 2005;53:1765–8. doi: 10.1021/jf040019b. [DOI] [PubMed] [Google Scholar]
- 24.Menichini F, Tundis R, Loizzo MR, Bonesi M, Marrelli M, Statti GA, et al. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from pimpinella anisoides V Brig. (Apiaceae) Fitoterapia. 2009;80:297–300. doi: 10.1016/j.fitote.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Oganesayan G, Mnatsakanyan V. Flavonoids of dracocephalum multicaule. J Arm Khim Zh. 1989;42:717–24. [Google Scholar]
- 26.Orhan I, kartal M, Tosun F, Sener B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z Naturforsch C. 2007;62:829–32. doi: 10.1515/znc-2007-11-1210. [DOI] [PubMed] [Google Scholar]
- 27.Khana M, Orhan I, Senol F, Kartal M, Sener B, Dvorská M, et al. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chemico-Biol Interact. 2009;181:383–9. doi: 10.1016/j.cbi.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Alkalay A, Rabinovici GD, Zimmerman G, Agarwal N, Kaufer D, Miller BL, et al. Plasma acetylcholinesterase activity correlates with intracerebral beta-amyloid load. Curr Alzheimer Res. 2013;10:48–56. [PMC free article] [PubMed] [Google Scholar]


